Abstract
Human pregnane X receptor (PXR) has been implicated in the pathogenesis of inflammatory bowel disease (IBD). Rifaximin, a human PXR activator, is in clinical trials for treatment of IBD and has demonstrated efficacy in Crohn's disease and active ulcerative colitis. In the current study, the protective and therapeutic role of rifaximin in IBD and its respective mechanism were investigated. PXR-humanized (hPXR), wild-type, and Pxr-null mice were treated with rifaximin in the dextran sulfate sodium (DSS)-induced and trinitrobenzene sulfonic acid (TNBS)-induced IBD models to determine the protective function of human PXR activation in IBD. The therapeutic role of rifaximin was further evaluated in DSS-treated hPXR and Pxr-null mice. Results demonstrated that preadministration of rifaximin ameliorated the clinical hallmarks of colitis in DSS- and TNBS-treated hPXR mice as determined by body weight loss and assessment of diarrhea, rectal bleeding, colon length, and histology. In addition, higher survival rates and recovery from colitis symptoms were observed in hPXR mice, but not in Pxr-null mice, when rifaximin was administered after the onset of symptoms. Nuclear factor κB (NF-κB) target genes were markedly down-regulated in hPXR mice by rifaximin treatment. In vitro NF-κB reporter assays demonstrated inhibition of NF-κB activity after rifaximin treatment in colon-derived cell lines expressing hPXR. These findings demonstrated the preventive and therapeutic role of rifaximin on IBD through human PXR-mediated inhibition of the NF-κB signaling cascade, thus suggesting that human PXR may be an effective target for the treatment of IBD.
Introduction
Rifaximin [Rifax; Xifaxan; 4-deoxy-4′-methylpyrido[1′,2′-1,2]imidazo[5,4-c]rifamycin SV], a nonsystemic rifamycin-derived antibiotic that exhibits low gastrointestinal absorption while retaining potent antibacterial activity (Koo and DuPont, 2010), was approved in 2004 for the treatment of traveler's diarrhea (Laustsen and Wimmett, 2005). Clinical trials have also indicated the potential role for rifaximin in inflammatory bowel disease (IBD) (Day and Gearry, 2010). Rifaximin seems to have more efficacy in the therapy of irritable bowel syndrome than do other antibiotics such as neomycin, doxycycline, amoxicillin/clavulanate, and ciprofloxacin(Yang et al., 2008). Other studies revealed that two-thirds of adult patients with Crohn's disease entered remission after rifaximin therapy (Shafran and Burgunder, 2010). A retrospective review revealed a well tolerated and favorable role for rifaximin in pediatric IBD (Muniyappa et al., 2009; Trehan et al., 2009). However, the role of rifaximin in irritable bowel syndrome and IBD therapy and its mechanisms of action are not understood.
A previous study demonstrated that rifaximin is a gut-specific human pregnane X receptor (PXR) agonist (Ma et al., 2007b). PXR is a ligand-activated transcription factor important for its induction of drug transport and metabolism, in particular induction of the cytochrome P450 CYP3A4 involved in the metabolism of many clinically used drugs. Recent data indicate that PXR may be involved in IBD. Gene expression analysis of colon tissues from ulcerative colitis and patients with Crohn's disease demonstrated a significant inhibition of PXR and its target genes compared with normal intestinal samples (Langmann et al., 2004). Specific polymorphisms in the PXR locus that are associated with a decrease in PXR activity are correlated with an increased susceptibility to IBD (Dring et al., 2006). PXR inhibits the proinflammatory transcription factor NF-κB, providing a potential molecular mechanism that links PXR signaling and inflammation (Xie and Tian, 2006; Zhou et al., 2006a). For example, activation of mouse PXR ameliorates dextran sulfate sodium (DSS)-induced colitis via inhibition of NF-κB (Shah et al., 2007). However, PXR ligands are structurally diverse and as noted above display species specificity (Ma et al., 2008). Rifampicin [3-(4-methylpiperazinyliminomethyl) rifamycin SV] is an agonist for human PXR but does not activate rodent PXR, whereas pregnenolone-16α-carbonitrile, a rodent-specific PXR agonist, does not activate human PXR (Zhou et al., 2009). Because of the specific activation of rifaximin on human PXR, the role of this drug in therapy of IBD has been a focus of interest.
In the current study, the preventive and therapeutic role of rifaximin was assessed in DSS- or TNBS-induced IBD models. Rifampicin (Ma et al., 2007b), as another rifamycin-derived antibiotic and human PXR agonist (Bertilsson et al., 1998), was compared with rifaximin. Because rodent models cannot accurately predict inducers and potential drug–drug interactions mediated by human PXR due to different responses to PXR ligands (Jones et al., 2000), PXR-humanized (hPXR) mice were used to investigate the potential role of human PXR activation in the mouse IBD models. The hPXR mice express human PXR in the Pxr-null background and respond only to human PXR-specific ligands (Ma et al., 2007a). Wild-type (WT) and Pxr-null mouse lines were also assessed to distinguish the antibiotic role from its PXR ligand effect. Rifampicin treatment demonstrated no protection against mouse IBD and exacerbated the severity of IBD. However, the hPXR mice treated with rifaximin demonstrated significant protection and a potential therapeutic role of human PXR in IBD. No protection of rifaximin was observed in wild-type and Pxr-null mice, thus demonstrating that rifaximin functions through activating human PXR and not through its gut-specific antibiotic effects. In addition, rifaximin inhibited the NF-κB signaling cascade in a human PXR-dependent manner. These data provide the first mechanistic evidence by which rifaximin and human PXR influence experimental IBD.
Materials and Methods
Animals.
hPXR, wild-type, and Pxr-null male mice were housed in temperature- and light-controlled rooms and given water and pelleted chow ad libitum. The hPXR mice express the human PXR in the Pxr-null background. All animal experiments were carried out in accordance with the Institute of Laboratory Animal Resources guidelines and approved by the National Cancer Institute Animal Care and Use Committee.
Experimental Design.
Two- to 3-month-old hPXR, wild-type, and Pxr-null male mice were subjected to DSS- and TNBS-induced IBD models. Mice were placed into four groups (n ≥ 6 per group) in the DSS-induced IBD study: control, DSS, rifaximin or rifampicin, and DSS treatment after rifaximin or rifampicin pretreatment. The control and DSS groups received a control diet for 11 consecutive days, and mice in the rifaximin (Salix Pharmaceuticals, Inc., Morrisville, NC) or rifampicin (Sigma-Aldrich, St. Louis, MO) groups received rifaximin (1 mg/kg/day) or rifampicin (3 mg/kg/day or 10 mg/kg/day), respectively, in the diet for 11 consecutive days. Dosages of rifaximin or rifampicin were calculated based on mouse daily dietary intake. Mice in the rifaximin and DSS or rifampicin and DSS groups received the compounds in the diet for 11 days, and on the 5th day of treatment the mice were administered 2.5% DSS (MP Biomedicals, Solon, OH) in drinking water (wt/v), and all mice were killed 11 days after treatment with the rifaximin or rifampicin diets or the control diet. Serum samples were collected by retro-orbital bleeding. hPXR and Pxr-null mice were used for the therapeutic studies (n ≥ 10 per group) in which mice were treated with 2.5% DSS and control diet for 5 consecutive days, then DSS was withdrawn and rifaximin was administered and compared with vehicle-treated mice. The mice were observed for 7 consecutive days after the onset of rifaximin treatment. The TNBS-induced IBD protocol was also used to confirm the role of rifaximin and human PXR in IBD (n ≥ 6 per group). A 2-mg aliquot of TNBS in 50% ethanol was administered intrarectally to anesthetized mice via a round-bottom (50.8 mm) needle (Popper, Lake Success, NY), followed by control diet or rifaximin diet treatment for 6 consecutive days. The mice were killed 5 days after TNBS administration, and the colons were flushed and resected. All of the above IBD studies were repeated twice. All mice were killed by CO2 asphyxiation at the end of the study, and tissue samples were harvested and stored at −80°C before analysis.
Colitis Evaluation.
Daily changes of body weight, diarrhea, rectal bleeding, and bloody stool were assessed and reported as a score from 0 to 4. For assessment of macroscopic colon damage, the colon was opened longitudinally, flushed with phosphate-buffered saline, and fixed in 10% buffered formalin. Colitis was measured by blinded analysis on a routine hematoxylin and eosin (H&E)-stained section according to the morphological criteria described previously (Cooper et al., 1993).
RNA Analysis.
RNA was isolated and mRNA expression was assessed by quantitative real-time PCR (qPCR) as described previously (Cheng et al., 2009).
Cell Lines and Luciferase Assays.
Human epithelial colorectal adenocarcinoma cells (Caco-2) and human colonic epithelial cells (HT-29) were grown at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA) and 1% penicillin/streptomycin (Invitrogen). Caco-2 and HT-29 cells were seeded at a density of 5 × 104 cells/well in 24-well plates. Expression vectors for human PXR, retinoid X receptor (RXR), and NF-κB luciferase reporters were transfected into cells by using the Fugene transfection reagent (Roche Diagnostics, Indianapolis, IN). Twenty-four hours after transfection, the cells were incubated with DMSO (vehicle) or rifaximin for 24 h, followed by incubation with tumor necrosis factor α (TNFα) (10 ng /ml) for 24 h. A standard dual luciferase assay was used and normalized to a cotransfected control reporter plasmid (Promega, Madison, WI).
Protein Analysis of Human PXR and Microsomal Stearoyl-CoA Desaturase-1.
Fresh colon tissues were collected, and epithelial cells isolated. Nuclear protein was isolated with a NE-PER kit (Thermo Fisher Scientific, Rockford, IL). Liver tissues were collected and homogenized in ice-cold buffer (1.15% KCl, 50 mM Tris-HCl, and 1 mM EDTA, pH 7.4) and microsomes were prepared by differential centrifugation. Pellets were resuspended in 100 mM Tris-HCl (pH 7.4), 0.1 mM EDTA, 0.1 mM dithiothreitol, 0.15 M KCl, and 20% (v/v) glycerol, aliquoted, and stored at −80°C. Primary antibodies to human PXR (H5017) and stearoyl-CoA desaturase-1 (SCD-1) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:1000 with Tris-buffered saline/Tween 20, followed by peroxidase-conjugated anti-mouse IgG diluted 1:10,000 with Tris-buffered saline/Tween 20, were used for Western blot analysis. Nuclear hepatocyte nuclear factor 4 α (HNF4α) and microsomal glyceraldehyde 3-phosphate dehydrogenase were used as loading controls.
Metabolomics Analysis of Serum.
A 1-μl aliquot of diluted serum samples was injected into a Waters (Milford, MA) UPLC-TOFMS system. An Acquity UPLC BEH C18 column (Waters) was used to separate chemical components at 35°C. The mobile-phase flow rate was 0.5 ml/min with an aqueous acetonitrile gradient containing 0.1% formic acid over a 10-min run (0% acetonitrile for 0.5 min to 20% acetonitrile by 5 min to 95% acetonitrile by 9 min, then equilibration at 100% water for 1 min before the next injection). The QTOF Premier mass spectrometer (Waters) was operated in the positive electrospray ionization mode. Capillary voltage and cone voltage were maintained at 3 kV and 20 V, respectively. Source temperature and desolvation temperature were set at 120 and 350°C, respectively. Nitrogen was used as both cone gas (50 l/h) and desolvation gas (600 l/h), and argon was used as collision gas. For accurate mass measurement, the time of flight mass spectrometry was calibrated with sodium formate solution (range m/z 100-1000) and monitored by the intermittent injection of the lock mass sulfadimethoxine ([M + H]+ = 311.0814 m/z) in real time. Mass chromatograms and mass spectral data were acquired and processed by MassLynx software (Waters) in centroid format.
Principal Components Analysis of Serum Metabolomic Data.
Chromatographic and spectral data were deconvoluted by MarkerLynx software (Waters). A multivariate data matrix containing information on sample identity, ion identity (retention time and m/z), and ion abundance was generated through centroiding, deisotoping, filtering, peak recognition, and integration. The intensity of each ion was calculated by normalizing the single ion counts versus the total ion counts in the whole chromatogram. The data matrix was further exported into SIMCA-P software (Umetrics, Kinnelon, NJ) and transformed by mean-centering and Pareto scaling, a technique that increases the importance of low abundance ions without significant amplification of noise. Principal components of serum were generated by principal components analysis to represent the major latent variables in the data matrix and were described in a scores scatter plot.
Data Analysis.
Experimental values are expressed as mean ± S.D. Statistical analysis was performed with two-tailed Student's t tests, and percentage of survival was analyzed by Prism 5.0 (GraphPad Software, Inc., San Diego, CA) with survival curve statistical analysis. A p value of < 0.05 was considered statistically significant.
Results
Rifampicin Does Not Protect hPXR Mice in the DSS-Induced Colitis Model.
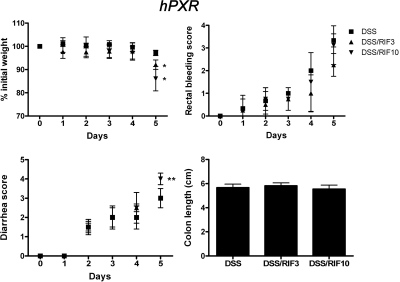
Rifampicin is a derivative antibiotic of rifamycin SV, which is a systemic drug widely studied as a typical human PXR agonist. Long-term treatment in tuberculosis therapy is thought to increase hepatotoxicity (Tostmann et al., 2008). Here, rifampicin was investigated for the treatment of DSS-induced colitis. Effective dosages of 3 mg/kg/day (Xie et al., 2000) and 10 mg/kg/day were selected (Cheng et al., 2009). hPXR mice pretreated with rifampicin followed by DSS treatment demonstrated no protection against DSS-induced colitis compared with DSS treatment alone. No significant changes were observed in control and rifampicin-treated (3 or 10 mg/kg/day) mice in the absence of DSS. It is noteworthy that high doses of rifampicin, combined with DSS, led to more severe colitis compared with coadministration of low dosage of rifampicin with DSS as revealed by decreased body weight and increased diarrhea and rectal bleeding scores (Fig. 1). Expression of hepatic SCD-1, a lipogenic enzyme regulated by PXR (Zhou et al., 2006b), which is protective in IBD (Chen et al., 2008), was repressed at the mRNA and protein levels after DSS alone. Whereas 3 mg/kg/day rifampicin treatment combined with DSS did not alter these low levels, expression was markedly reduced with 10 mg/kg/day of the drug (Supplemental Fig. 1). This reduction of SCD-1 was associated with a reduction of unsaturated lysophosphatidylcholine in plasma. Human PXR target genes in liver and colon were induced and proinflammatory factors were unchanged by rifampicin in the DSS IBD model (Supplemental Fig. 2).
Fig. 1.
Colitis assessment of hPXR mice treated with 2.5% DSS after rifampicin (RIF) pretreatment. hPXR male mice were placed into six groups (n ≥ 6 per group): control group (Cont), DSS treatment alone (DSS), RIF (3 mg/kg/day) treatment alone (RIF3), RIF (10 mg/kg/day) treatment alone (RIF10), DSS treatment after RIF (3 mg/kg/day) pretreatment (DSS/RIF3), and DSS treatment after RIF (10 mg/kg/day) pretreatment (DSS/RIF10). Each bar represents the mean ± S.D. n ≥ 6. *, p < 0.05; **, p < 0.01.
Rifaximin Protects hPXR Mice in the DSS-Induced Colitis Model.
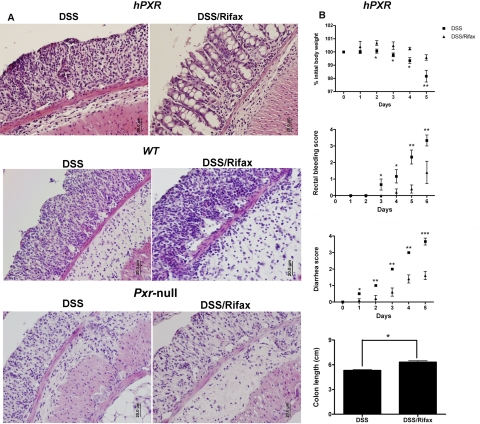
Unlike rifampicin, rifaximin is a nonsystemic antibiotic not significantly absorbed into the circulation. The preventive role of rifaximin was assessed in the DSS-induced colitis model. No significant changes were observed in the colon after rifaximin treatment alone in hPXR, WT, and Pxr-null mice compared with the control groups (Supplemental Fig. 3). In contrast, DSS treatment increased crypt and epithelial cell damage, infiltration of granulocytes and mononuclear immune cells, and tissue edema in hPXR mice. It is noteworthy that rifaximin pretreatment significantly attenuated DSS-induced colon damage in hPXR mice compared with DSS treatment alone (Fig. 2A), as noted by an observed decrease in epithelial loss and mild inflammation. Rifaximin did not protect DSS-induced colitis in wild-type and Pxr-null mice as assessed by colon histology. In addition, several clinical parameters of colitis were statistically improved with rifaximin pretreatment in hPXR mice, such as daily body weight loss, rectal bleeding, diarrhea, and colon length compared with DSS administration alone (Fig. 2B). Meanwhile, there was no protection in WT and Pxr-null mice treated with DSS and rifaximin compared with DSS alone as revealed by clinical indexes of colitis, including body weight, rectal bleeding scores and diarrhea scores, and colon length (Supplemental Fig. 4). These results suggest that the beneficial effects of rifaximin may be attributed to its specific activation of human PXR, high bioavailability in the intestine, and decreased toxicity compared with rifampicin. To ensure that the protective role of rifaximin in acute colitis is not via inducing hepatic SCD-1, Western blot analysis of hepatic microsomal SCD-1 was performed. Rifaximin alone had no effect on SCD-1 expression in hPXR, wild-type, or Pxr-null mice, whereas 2.5% DSS administration clearly repressed SCD-1 expression in hPXR, wild-type, or Pxr-null mice compared with control (Supplemental Fig. 5A). Moreover, serum metabolomics analysis demonstrated that rifaximin-treated hPXR, wild-type, and Pxr-null mice have similar profiles of score plots and loading plots as determined by principal components analysis (Supplemental Fig. 5B), suggesting that rifaximin may be acting locally in the colon and not systemically to decrease the severity of DSS-induced colitis.
Fig. 2.
Rifaximin protects hPXR mice in a DSS-induced colitis model. A, representative H&E-stained colon sections of hPXR, WT, and Pxr-null treated with DSS alone (DSS) or DSS/rifaximin (Rifax). Magnification: 200×. B, colitis assessment of hPXR mice treated with 2.5% DSS (DSS) or 2.5% DSS after rifaximin pretreatment (DSS/Rifax). Each bar represents the mean ± S.D. n ≥ 6. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Rifaximin Protects TNBS-Induced IBD in hPXR mice.
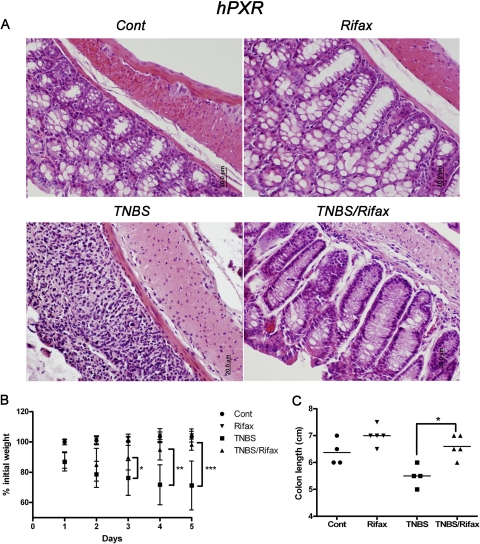
To confirm results from the DSS-induced colitis model, an additional acute colitis model was used. The TNBS-induced IBD model is driven by an initial IL-12 and TNFα response of lymphoid cells (Strober et al., 2009). The TNBS-induced IBD model was initiated in hPXR mice by intrarectal administration of TNBS after rifaximin pretreatment. TNBS triggered weight loss, bloody diarrhea, rectal prolapse, and large bowel wall thickening. Rifaximin significantly improved colon histology (Fig. 3A) and body weight (Fig. 3B) and maintained colon length (Fig. 3C) in hPXR mice, whereas no protection was observed in WT and Pxr-null mice (data not shown). Thus, with a second mouse IBD model, these data further indicate that rifaximin protects against IBD in hPXR mice.
Fig. 3.
Rifaximin protects hPXR mice in a TNBS-induced colitis model. A, representative H&E-stained colon sections of hPXR treated with control (left top), rifaximin (right top), TNBS (left bottom), and TNBS/rifaximin (right bottom). Magnification: 200×. B, body weight loss of hPXR mice treated with control (Cont), rifaximin (Rifax), TNBS, and TNBS/rifaximin (TNBS/Rifax). C, colon length of hPXR mice treated with control (Cont), rifaximin (Rifax), TNBS, and TNBS/rifaximin (TNBS/Rifax). Each bar represents the mean ± S.D. n ≥ 6. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Rifaximin Exerts Therapeutic Efficacy in the DSS-Induced Colitis Model in hPXR Mice.
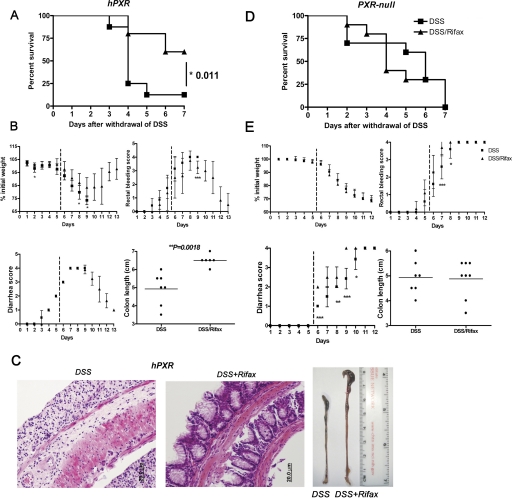
In addition to a preventive role of rifaximin, a therapeutic study was performed in DSS-induced hPXR and Pxr-null mice. Acute colitis was induced by 2.5% DSS treatment for 5 days. Rifaximin (1 mg/kg/day) was administrated to hPXR and Pxr-null mice after the onset of IBD. Survival rates were markedly increased in hPXR mice (P = 0.011) compared with Pxr-null mice (P = 0.730) administered rifaximin after the withdrawal of DSS (Fig. 4A). Clear signs of recovery from colitis were noted in hPXR mice administered rifaximin (Fig. 4B), such as increased colon length (P = 0.002) and increased body weight compared with controls. A significant resolution of colon damage was observed in hPXR mice treated with DSS alone compared with colons from rifaximin-treated hPXR mice (Fig. 4C). No significant protection was afforded in Pxr-null mice treated with rifaximin with respect to survival (Fig. 4D) or improvement of clinical signs (Fig. 4E) compared with hPXR mice.
Fig. 4.
Therapeutic role of rifaximin on DSS-induced IBD in hPXR mice. A, survival curve of hPXR mice comparing rifaximin after treatment to no rifaximin treatment. B, colitis assessment of hPXR mice comparing rifaximin after treatment to no rifaximin treatment. C, representative H&E-stained colon sections of hPXR treated with DSS (left) or rifaximin after DSS-induced colitis (center) (magnification: 200×) and macroscopic observation of colon length (left: DSS; right: DSS/Rifax). D, survival curves of Pxr-null mice comparing rifaximin after treatment to no rifaximin treatment. E, colitis assessment of Pxr-null mice comparing rifaximin after treatment to no rifaximin treatment. Each bar represents the mean ± standard deviations. n ≥ 10. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Rifaximin Activates hPXR Target Genes in the Colon.
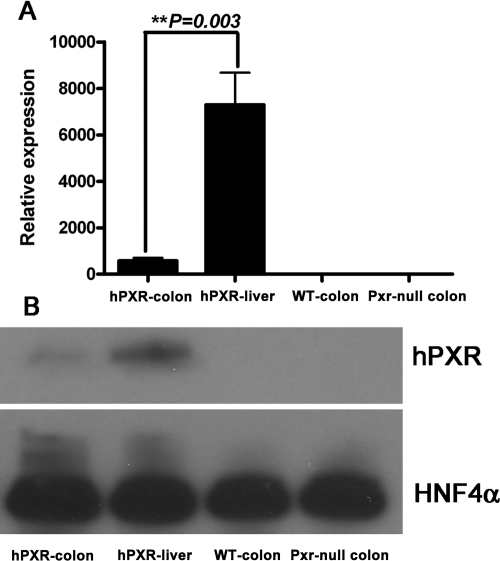
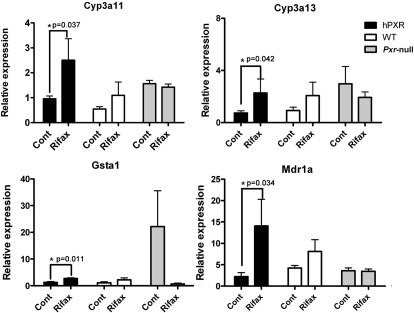
Human PXR expression in colons was measured by qPCR (Fig. 5A) and Western blot (Fig. 5B). Mouse β-actin served as an internal control for qPCR mRNA analysis, and HNF4α served as an internal control for Western blot analysis. Human PXR was expressed in the colons of hPXR mice; however, the expression was significantly less than that observed in the liver. Previous work demonstrated that rifaximin is a human PXR agonist in the small intestine (Ma et al., 2007b). To assess the potential function of rifaximin as a PXR ligand in the colon, qPCR analysis of mouse Cyp3a11, Cyp3a13, Gsta1, and Mdr1a genes in colon was investigated. The expression of Cyp3a11, Cyp3a13, Gsta1, and Mdr1a mRNAs was increased in hPXR mice after rifaximin treatment compared with the control group (Fig. 6), whereas there was no induction of Cyp3a11, Cyp3a13, Gsta1, and Mdr1a mRNAs in wild-type and Pxr-null mice after rifaximin treatment. Together, these data demonstrate that rifaximin is a human PXR agonist in the colon.
Fig. 5.
Basal expression of human PXR in the colon of hPXR mice. A, qPCR analysis of human PXR expression in colon and liver of hPXR mice and in colon of WT and Pxr-null mice. Mouse β-actin mRNA served as an internal control. B, Western blot analysis of human PXR expression in colon and liver of hPXR mice and in colon of WT and Pxr-null mice. Pooled nuclear protein (30 μg) was loaded for each sample (n = 3 per group). The monoclonal antibody against human PXR (H5017) specifically recognizes human PXR but not mouse PXR or other liver proteins. HNF4α was used as a loading control.
Fig. 6.
mRNA analysis of hPXR target genes in colon tissue. Expression of mRNAs encoding Cyp3a11, Cyp3a13, Gsta1, and Mdr1a were determined by qPCR from colon epithelial cells isolated from hPXR, WT, and Pxr-null mice treated with control (Cont) and rifaximin (Rifax). Data were normalized to β-actin. Each bar represents the mean ± standard deviations. n ≥ 6. *, p < 0.05.
Activation of hPXR by Rifaximin Inhibits NF-κB Target Gene Expression.
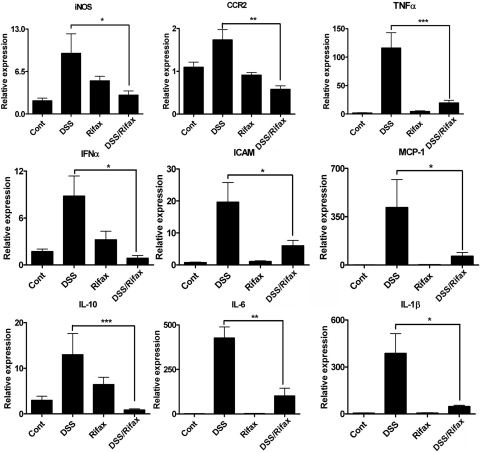
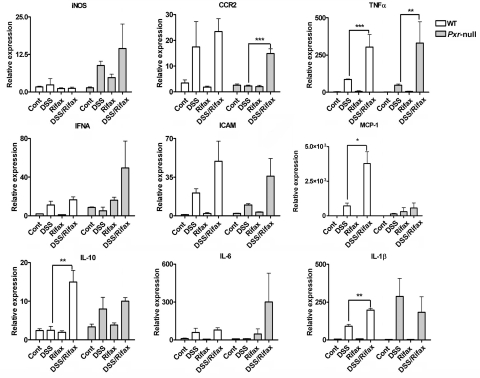
NF-κB is the central transcription factor in the regulation of proinflammatory cytokines and chemokines. Previous studies demonstrated that activation of PXR can attenuate NF-κB signaling (Xie and Tian, 2006). To elucidate the potential function of NF-κB on protection of DSS-induced colitis by rifaximin, qPCR analysis of several NF-κB-regulated proinflammatory cytokines and chemokines in colon were evaluated. The results demonstrated that mRNAs encoding the inducible nitric-oxide synthase (iNOS), chemokine motif receptor 2 (CCR2), TNFα, interferon-α (IFNα), intercellular adhesion molecule 1 (ICAM-1), monocyte chemoattractant protein 1 (MCP-1), and interleukin (IL)-10, IL-6, and IL-1β mRNA were induced in colonic tissue after 5-day DSS treatment of hPXR mice compared with the control group (Fig. 7). The increases in inflammatory mediators (iNOS, CCR2, TNFα, IFNα, ICAM-1, MCP-1, IL-10, IL-6, and IL-1β) after DSS treatment were significantly decreased in hPXR mice pretreated with rifaximin. These data suggest that rifaximin-activated human PXR may protect DSS-induced colitis through inhibiting the NF-κB-mediated proinflammatory response.
Fig. 7.
mRNA analysis of proinflammatory mediators in colon tissue from hPXR mice. Colon RNA was isolated from hPXR mice treated with control (Cont), DSS, rifaximin (Rifax), and DSS/rifaximin (DSS/Rifax). Expression of mRNAs encoding iNOS, CCR2, TNFα, IFNα, ICAM-1, MCP-1, IL-10, IL-6, and IL-1β was determined by qPCR. Data were normalized to β-actin. Each bar represents the mean ± standard deviations. n ≥ 6. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
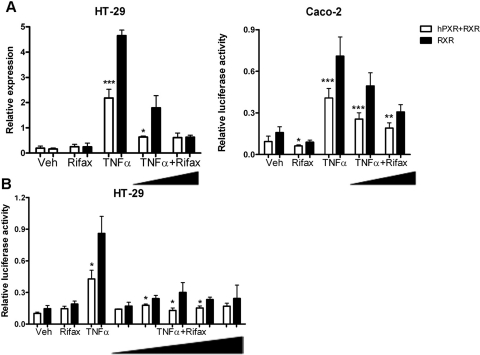
Direct Inhibition of NF-κB by Rifaximin.
To determine the direct role of human PXR in the repression of NF-κB signaling by rifaximin, luciferase activities of an NF-κB reporter construct were measured. In HT-29 and Caco-2 cells, TNFα (10 ng/ml) induced NF-κB activation, which was significantly attenuated after cotransfection with human PXR and RXR constructs. These data revealed that human PXR could inhibit TNFα-induced inflammation. The addition of 1 and 100 μM rifaximin potentiated the repression of NF-κB activation after TNFα treatment (Fig. 8A). The significant inhibition of NF-κB activity after rifaximin treatment in the absence of human PXR transfection might be caused by basal expression of human PXR in HT-29 and Caco-2 cells as assessed by Western blot analysis (data not shown). Moreover, a dose response of rifaximin on NF-κB inhibition was evaluated in HT-29 cells. These results showed that the optimal inhibition dosage of rifaximin was between 0.01 and 1 μM (Fig. 8B). Consistent with the in vivo results, the in vitro luciferase assay indicated that rifaximin protected hPXR mice from acute colitis through NF-κB inhibition via gut-specific human PXR activation.
Fig. 8.
Rifaximin inhibited TNFα-activated NF-κB luciferase reporter through human PXR. A, HT-29 cells and Caco-2 cells (5 × 104 cells/well) were cotransfected with NF-κB luciferase reporter (0.2 mg/well) and mouse RXR (0.04 μg/well) or cotransfected with NF-κB luciferase reporter (0.2 μg/ml/well), mouse RXR (0.04 μg/well), and hPXR (0.04 μg/well). Twenty-four hours after transfection cells were incubated with vehicle (Veh, DMSO), Rifax (1 or 100 μM, dissolved in DMSO), or TNFα (10 ng/ml) or coincubated with TNFα and rifaximin for 24 h (TNFα+ Rifax). B, HT-29 cells were cotransfected with NF-κB luciferase reporter (0.2 μg/ml/well) and mouse RXR (0.04 μg/well) or cotransfected with NF-κB luciferase reporter (0.2 μg/ml/well), mouse RXR (0.04 μg/well), and hPXR (0.04 μg/well). Twenty-four hours after transfection cells were incubated with vehicle (Veh), Rifax (100 μM), and TNFα (10 ng/ml) or coincubated with rifaximin (0.001, 0.01, 0.1, 1, or 100 μM) and TNFα (10 ng/ml) for 24 h (TNFα+ Rifax). Standard dual luciferase assays were performed on cell extracts. Each bar represents the mean ± standard deviation. n ≥ 6. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Rifaximin Inhibits NF-κB Target Genes through a hPXR-Mediated Mechanism.
NF-κB target genes iNOS, CCR2, TNFα, IFNα, ICAM-1, MCP-1, IL-10, IL-6, and IL-1β mRNAs were measured in wild-type and Pxr-null mice after DSS treatment. A significant induction of all proinflammatory mediators was observed in wild-type and Pxr-null mice compared with the control group. However, rifaximin did not decrease inflammatory mediators after DSS treatment (Fig. 9), but actually increased TNFα and CCR2 mRNAs in Pxr-null mice and TNFα, IL-1β, IL-10, and MCP-1 in wild-type mice compared with DSS and rifaximin cotreatment to DSS treated alone, which might result from a differential inflammation response of the above factors in Pxr-null and wild-type mice. These data demonstrate that rifaximin affords protection mainly through a gut-specific hPXR-dependent mechanism and not through its antibiotic properties.
Fig. 9.
mRNA analysis of proinflammatory factors in colon tissue from WT and Pxr-null mice. Colon RNA was isolated from WT and Pxr-null mice treated with control (Cont), DSS, Rifax, and DSS/Rifax. Expression of mRNAs encoding iNOS, CCR2, TNFα, IFNα, ICAM-1, MCP-1, IL-10, IL-6, and IL-1β was determined by qPCR. Data were normalized to β-actin. Each bar represents the mean ± standard deviations. n ≥ 6. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
The etiology of IBD is still not completely understood; however, it is widely accepted that the development of IBD is associated with the interplay of genetic, bacterial, and environmental factors and dysregulation of the intestinal immune system (Kaser et al., 2010). Among the IBD models, chemically induced animal models are commonly used, particularly with DSS colitis and hapten-induced colitis, using either DNBS/TNBS or oxazolone. These two models produce robust and immediate inflammation in the colon and have high reproducibility (Hoffmann et al., 2002). In the present study, acute colitis was induced by DSS and TNBS administration. Despite the acute nature of these models, they clearly show the impact of human PXR and rifaximin and suggest its clinical utility.
The treatment of IBD is highly individualized; however, in most cases the first-line therapy uses immune-suppressing drugs, which act systemically. Rifaximin, an efficient gut-specific antibiotic because of its zwitterionic property, which inhibits absorption into the systemic circulation (Marchi et al., 1985), has been shown to be beneficial in specific cases of IBD (Gionchetti et al., 2006). Using a mouse model that accurately expresses human PXR, the present study clearly demonstrates that rifaximin protects through a PXR-dependent mechanism and not as a general antibiotic in both DSS- and TNBS-induced IBD. In the therapeutic study, no mice in the DSS group survived, whereas rifaximin administered after treatment with DSS resolved the colitis symptoms and led to 60% survival. The resolution of inflammation in acute models of colitis is not completely understood; however, these data provide evidence that rifaximin and hPXR might be worthwhile for the treatment of human IBD.
It is noteworthy that a well known human PXR agonist, rifampicin, which is a systemic antibiotic used to treat tuberculosis, demonstrated an increase in hepatotoxicity when administered before treatment with DSS and provided no significant protection of hPXR mice in the DSS-induced colitis model. Rifampicin treatment in the colitis model actually slightly increased the severity of symptoms such as body weight loss and diarrhea score. This was quite unexpected because pregnenolone-16α-carbonitrile, which also induces PXR target genes in liver and colon, protects against DSS-induced IBD (Shah et al., 2007). The reason for the differences between the protective effect of rifaximin and rifampicin in experimental IBD is not understood. Because both drugs can induce PXR target genes in the intestine, the difference is not caused by the increase in the availability of rifaximin in the gut compared with rifampicin. Further experimentation is required to determine the reason for the lack of effect of rifampicin on DSS-induced colitis in the hPXR mouse. It was reported that rifampicin led to several pseudomembranous colitis cases (Akbar et al., 2003; Mazokopakis et al., 2008; Chen et al., 2009) and has been associated with adverse effects in patients with chronic inflammatory diseases (Yuhas et al., 2009). It is noteworthy that rifampicin administered with DSS markedly lowered the already suppressed SCD-1 protein and the serum levels of unsaturated fatty acids compared with DSS alone. The current results in the mouse IBD model suggest that these clinical observations could be the result of the lowering of SCD-1 expression and associated anti-inflammatory unsaturated fatty acids (Chen et al., 2008).
Previous studies have shown that PXR is remarkably divergent across mammalian species with the ligand binding domains sharing 70 to 80% identity compared with the 90% typically exhibited by other nuclear receptors (Zhou et al., 2009). Because mouse PXR was reported to ameliorate DSS-induced IBD by NF-κB inhibition, it was critical to investigate the mechanism by which human PXR was protective in an acute colitis model. In vivo data demonstrated several NF-κB target genes to be significantly attenuated in hPXR mice after rifaximin pretreatment and DSS treatment compared with DSS treatment alone, and the attenuation of NF-κB signaling by rifaximin was not observed in wild-type and Pxr-null mice. Consistent with the in vivo data, in vitro evaluation of rifaximin on NF-κB inhibition suggested a direct role for human PXR in the inhibition of NF-κB signaling. Analysis of NF-κB response in colon-derived cancer cells using the NF-κB response element demonstrated a significant induction with TNFα treatment, which was inhibited after overexpression of human PXR, and the inhibition was further potentiated in the presence of rifaximin. It was assumed that transrepression of NF-κB target genes might be mediated by the SUMOylation of PXR (Zhou et al., 2006b), and, NF-κB disrupted binding of the PXR–RXR complex to its binding motif (Gu et al., 2006).
In summary, the hPXR mice were used to demonstrate the beneficial effects of rifaximin in acute models of colitis through the activation of human PXR, and a critical role for PXR in IBD. In addition, these data provide a mechanistic basis for novel therapies targeting human PXR in the treatment of inflammatory diseases of the gut.
Supplementary Material
Acknowledgments
We thank John Buckley for assistance with the animal studies and Salix Pharmaceuticals, Inc., for supplying rifaximin.
This work was supported by the Intramural Research Program of the National Institutes of Health National Cancer Institute.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.170225.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- PXR
- pregnane X receptor
- Rifax
- rifaximin, 4-deoxy-4′-methylpyrido[1′,2′-1,2]imidazo[5,4-c]rifamycin SV
- RIF
- rifampicin, 3-(4-methylpiperazinyliminomethyl)rifamycin SV
- IBD
- inflammatory bowel disease
- DSS
- dextran sulfate sodium
- TNBS
- trinitrobenzene sulfonic acid
- NF-κB
- nuclear factor κB
- hPXR
- PXR-humanized
- WT
- wild type
- DSS/RIF
- coadministration of rifampicin and DSS
- DSS/Rifax
- coadministration of rifaximin and DSS
- TNBS/Rifax
- coadministration of rifaximin and TNBS
- iNOS
- inducible nitric-oxide synthase
- TNF
- tumor necrosis factor
- CCR2
- chemokine motif receptor 2
- IFN
- interferon
- ICAM-1
- intercellular adhesion molecule 1
- MCP-1
- monocyte chemoattractant protein 1
- IL
- interleukin
- qPCR
- quantitative real-time PCR
- HNF4α
- hepatocyte nuclear factor 4 α
- SCD-1
- stearoyl-CoA desaturase-1
- DMSO
- dimethyl sulfoxide
- RXR
- retinoid X receptor
- H&E
- hematoxylin and eosin.
References
- Akbar DH, Al-Shehri HZ, Al-Huzali AM, Falatah HI. (2003) A case of rifampicin induced pseudomembraneous colitis. Saudi Med J 24:1391–1393 [PubMed] [Google Scholar]
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Bäckman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. (1998) Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA 95:12208–12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Shah YM, Morimura K, Krausz KW, Miyazaki M, Richardson TA, Morgan ET, Ntambi JM, Idle JR, Gonzalez FJ. (2008) Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 down-regulation exacerbates inflammation and acute colitis. Cell Metab 7:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TC, Lu PL, Lin WR, Lin CY, Wu JY, Chen YH. (2009) Rifampin-associated pseudomembranous colitis. Am J Med Sci 338:156–158 [DOI] [PubMed] [Google Scholar]
- Cheng J, Ma X, Krausz KW, Idle JR, Gonzalez FJ. (2009) Rifampicin-activated human pregnane X receptor and CYP3A4 induction enhance acetaminophen-induced toxicity. Drug Metab Dispos 37:1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69:238–249 [PubMed] [Google Scholar]
- Day AS, Gearry RB. (2010) Rifaximin and Crohn's disease: a new solution to an old problem? Dig Dis Sci 55:877–879 [DOI] [PubMed] [Google Scholar]
- Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, Smyth CM, Keeling PW, O'Donoghue D, O'Sullivan M, et al. (2006) The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology 130:341–348; quiz 592. [DOI] [PubMed] [Google Scholar]
- Gionchetti P, Rizzello F, Lammers KM, Morselli C, Tambasco R, Campieri M. (2006) Antimicrobials in the management of inflammatory bowel disease. Digestion 73 (Suppl 1):77–85 [DOI] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. (2006) Role of NF-κB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem 281:17882–17889 [DOI] [PubMed] [Google Scholar]
- Hoffmann JC, Pawlowski NN, Kühl AA, Höhne W, Zeitz M. (2002) Animal models of inflammatory bowel disease: an overview. Pathobiology 70:121–130 [DOI] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. (2000) The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27–39 [DOI] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. (2010) Inflammatory bowel disease. Annu Rev Immunol 28:573–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HL, DuPont HL. (2010) Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol 26:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. (2004) Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology 127:26–40 [DOI] [PubMed] [Google Scholar]
- Laustsen G, Wimmett L. (2005) 2004 drug approval highlights: FDA update. Nurse Pract 30:14–29; quiz 29–31 [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Gonzalez FJ. (2008) The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol 4:895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. (2007a) The pregnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos 35:194–200 [DOI] [PubMed] [Google Scholar]
- Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, Gonzalez FJ. (2007b) Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther 322:391–398 [DOI] [PubMed] [Google Scholar]
- Marchi E, Montecchi L, Venturini AP, Mascellani G, Brufani M, Cellai L. (1985) 4-Deoxypyrido[1′,2′:1,2]imidazo[5,4-c]rifamycin SV derivatives. A new series of semisynthetic rifamycins with high antibacterial activity and low gastroenteric absorption. J Med Chem 28:960–963 [DOI] [PubMed] [Google Scholar]
- Mazokopakis EE, Giannakopoulos TG, Christias EG. (2008) Acute brucellosis as a cause of infective colitis. Mil Med 173:1145–1147 [DOI] [PubMed] [Google Scholar]
- Muniyappa P, Gulati R, Mohr F, Hupertz V. (2009) Use and safety of rifaximin in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 49:400–404 [DOI] [PubMed] [Google Scholar]
- Shafran I, Burgunder P. (2010) Adjunctive antibiotic therapy with rifaximin may help reduce Crohn's disease activity. Dig Dis Sci 55:1079–1084 [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. (2007) Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-κB target gene expression. Am J Physiol Gastrointest Liver Physiol 292:G1114–G1122 [DOI] [PubMed] [Google Scholar]
- Strober W, Kitani A, Fichtner-Feigl S, Fuss IJ. (2009) The signaling function of the IL-13Rα2 receptor in the development of gastrointestinal fibrosis and cancer surveillance. Curr Mol Med 9:740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. (2008) Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 23:192–202 [DOI] [PubMed] [Google Scholar]
- Trehan I, Shulman RJ, Ou CN, Maleta K, Manary MJ. (2009) A randomized, double-blind, placebo-controlled trial of rifaximin, a nonabsorbable antibiotic, in the treatment of tropical enteropathy. Am J Gastroenterol 104:2326–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Tian Y. (2006) Xenobiotic receptor meets NF-κB, a collision in the small bowel. Cell Metab 4:177–178 [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. (2000) Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406:435–439 [DOI] [PubMed] [Google Scholar]
- Yang J, Lee HR, Low K, Chatterjee S, Pimentel M. (2008) Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci 53:169–174 [DOI] [PubMed] [Google Scholar]
- Yuhas Y, Berent E, Cohen R, Ashkenazi S. (2009) Roles of NF-κB activation and peroxisome proliferator-activated receptor γ inhibition in the effect of rifampin on inducible nitric oxide synthase transcription in human lung epithelial cells. Antimicrob Agents Chemother 53:1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, et al. (2006a) Mutual repression between steroid and xenobiotic receptor and NF-κB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Verma S, Blumberg B. (2009) The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal 7:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. (2006b) A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 281:15013–15020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.