Abstract
We investigate the role of M2-muscarinic receptors in maintaining neurogenic bladder contraction during hyperglycemia. Mice were injected with a single dose of streptozotocin (125 mg/kg), and neurogenic contraction of urinary bladder from wild type and M2-muscarinic receptor knockout (M2 KO) mice was measured at 8 to 24 weeks after treatment. In wild-type bladder lacking urothelium, the summation of the cholinergic (64%) and purinergic (56%) components of the electrical-field-stimulated response exceeded 100%, indicating a reserve capacity. Although the cholinergic component was slightly less in the M2 KO mouse, the total electrical-field-stimulated contraction was the same as wild type. The cholinergic and purinergic components of contraction in wild-type bladder were minimally affected by streptozotocin treatment. In M2 KO bladder, streptozotocin treatment reduced both the cholinergic (after 8–9 and 20–24 weeks) and purinergic (after 20–24 weeks only) components. The loss of function was approximately 50 to 70%. Similar results were observed in bladder with intact urothelium. M2 KO bladder was more sensitive to the relaxant effect of isoproterenol compared with wild type, and this difference significantly increased at the early and late time points after streptozotocin treatment. In the presence of urothelium, however, this difference in isoproterenol sensitivity was smaller with streptozotocin treatment, but this trend reversed over time. Our results show that M2 receptors oppose urinary bladder distension in wild-type bladder and inhibit streptozotocin-induced neuropathy.
Introduction
The efferent control of the urinary bladder reservoir is mediated by both sympathetic (hypogastric) and parasympathetic (pelvic) nerves (de Groat and Yoshimura, 2001; Andersson and Arner, 2004; Michel and Barendrecht, 2008). Parasympathetic nerves release ATP and acetylcholine, which act primarily on P2X and M2 and M3 muscarinic receptors, respectively, to contract the bladder reservoir (de Groat and Yoshimura, 2001). Their role in rodent bladder is apparent because neurogenic contractions elicited by electrical-field-stimulation (EFS) are prevented by antagonism of muscarinic and purinergic receptors. The β-adrenergic antagonist propanolol increases the peak contraction to EFS and slows the subsequent rate of relaxation (Giglio et al., 2005), suggesting that norepinephrine released from sympathetic nerves acts on β-adrenoceptors to oppose contraction.
Urinary bladder-voiding dysfunction or cystopathy is commonly reported in diabetes (Freeman, 2005) and is traditionally attributed to diabetic autonomic neuropathy (Faerman et al., 1973; Andersen and Bradley, 1976). A common animal model for studying the influence of diabetes on neurogenic control of urinary bladder makes use of the pancreatic β-cell toxin streptozotocin (STZ) to render animals hyperglycemic. It is still not entirely clear, however, whether neurogenic bladder function is altered in animal models of diabetes.
Studies in Wistar rats have shown that treatment with STZ increases (Benkó et al., 2003) or decreases (Gür and Cinel, 2003) bladder contractions elicited by EFS, depending on whether data are expressed relative to cross-sectional area or maximal KCl contraction, respectively. In Sprague-Dawley rats, STZ treatment has been reported to cause an increase (Liu and Daneshgari, 2005) or no change (Longhurst et al., 2004) in EFS responses. In a study on the mouse urinary bladder, Liu and Lin-Shiau (1996) show decreased contraction to EFS after STZ treatment. The cholinergic component of the EFS contraction in bladder from the STZ-treated Wistar rat increases (Luheshi and Zar, 1991; Benkó et al., 2003), whereas that measured in the Sprague-Dawley rat decreases (Liu and Daneshgari, 2005). After STZ treatment, the purinergic component of EFS contraction was reported to be unchanged in the Sprague-Dawley rat (Liu and Daneshgari, 2005) and slightly increased in the Wistar rat (Benkó et al., 2003).
The more abundant muscarinic receptors in smooth muscle of the urinary bladder and other tissues are of the M2 and M3 subtypes. The M3 receptor acts through Gq to mediate direct contraction in smooth muscle, whereas M2 enhances M3 receptor-mediated contraction and inhibits the relaxant effect of isoproterenol and forskolin on contractions elicited by other contractile receptors, including the M3 (Ehlert et al., 2005, 2007). In the mouse, the M2 receptor also elicits a modest direct contraction of some smooth muscles, but direct M2 contractions of the urinary bladder are quite small (Stengel et al., 2002; Matsui et al., 2003).
Braverman et al. (1998, 1999) and Braverman and Ruggieri (2003) have shown that there is an increase in M2 receptor-mediated contractile function in urinary bladder following denervation or partial outlet obstruction. This increase in M2 function appears to be caused by bladder distension because it is prevented by surgically diverting urine flow from the kidney away from the bladder and into the gastrointestinal tract. Because autonomic neuropathy and urinary bladder distention are associated with diabetes, one might expect a similar increase in M2 function in diabetes. We recently reported an increase in postjunctional M2 receptor contractile function and a corresponding decrease in that of the M3 receptor in the mouse STZ model (Pak et al., 2010).
The up-regulation in M2 receptor function in STZ-induced diabetes suggests that it may have a protective effect, perhaps by enhancing contraction and reducing chronic bladder distention and its associated neuropathy. In the present study, we have investigated this question by monitoring cholinergic and purinergic EFS contractions during the course of the development of STZ-induced diabetes in wild-type and M2 KO mice. Although the direct postjunctional contractile response to the exogenously applied purinergic agonist α,β-methylene ATP (mATP) was maintained in urinary bladder from wild-type and M2 KO mice, there was a large loss of both purinergic and cholinergic EFS-induced contractions in M2 KO urinary bladder but not in wild type. Our results suggest that the M2 receptor inhibits the development of urinary bladder neuropathy in the hyperglycemic state.
Materials and Methods
Animals.
Male C57BL/6 wild-type mice (Harlan Sprague-Dawley, Inc., Indianapolis, IN) and M2-muscarinic receptor knockout (M2 KO) mice (2–3 months old, 19–29 g) (Matsui et al., 2000, 2002) were treated with a single intraperitoneal injection of either vehicle (sodium citrate dihydrate) or 125 mg/kg STZ at 8 to 24 weeks before isolated bladder assays. Mice were fasted for 4 to 6 h and then briefly anesthetized with isoflurane (Phoenix Pharmaceuticals, Inc., St. Joseph, MO) for injection of STZ. To prevent hypoglycemic shock, animals were provided water with 10% sucrose for 48 h after injection. Mice were housed in a 12-h light/dark facility and fed water and food ad libitum. Fasting blood glucose was measured using an Ascensia Contour glucometer (Bayer, Leverkusen, Germany), and diabetic ketoacidosis was assessed by Keto-Diastix (Bayer). All procedures on live mice were approved by the Institutional Laboratory Animal Care and Use Committee at the University of California, Irvine.
Isolated Urinary Bladder.
Whole-urinary bladder was dissected from CO2-asphyxiated mouse and cut in half sagittally. The urothelium was carefully removed from one of the strips (denuded bladder) with forceps under a microscope, whereas the other was left intact. Microscopic examination of the denuded bladder revealed the exposed blood vessels of the compromised suburothelium, confirming the removal of the more superficial urothelium. Each half-bladder strip was mounted in a longitudinal orientation between two platinum electrodes in an organ bath and connected to a force-displacement transducer using silk thread. Tissues were bathed in a Krebs-Ringer-bicarbonate buffer (124 mM NaCl, 5 mM KCl, 1.3 mM MgSO4, 26 mM NaHCO3, 1.2 mM KH2PO4, 1.8 mM CaCl2, and 10 mM glucose) at 37°C and gassed with O2/CO2 (19:1) as described previously (Ehlert et al., 2005). Resting tension was adjusted to a 1-gram load (9.8 mN) during an equilibration period of at least 1 h before stimulating the bladder with two test doses of KCl (50 mM). Tissues were washed and allowed to rest for 10 min after each test dose. Contraction to KCl was calculated as the stable plateau level of contraction after a 3-min period, and subsequent responses were normalized to the larger of the two KCl-induced responses, which were usually similar. All contractile measurements are reported as the total tension minus the resting tension.
EFS Contraction.
Control contractile responses were elicited by EFS (each pulse: 20 Hz, 0.5-ms duration, 40 V/cm) lasting for 5 s before a 2-min resting period. Each control response was calculated as the maximal point of contraction (which was usually a plateau) minus resting tension. A total of nine control responses were elicited, and the last five were averaged for estimation of the control EFS contraction.
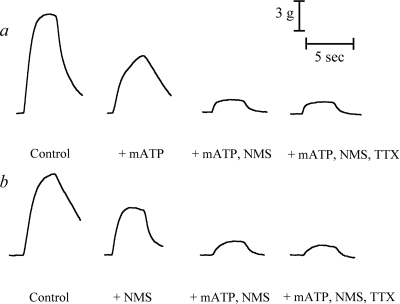
When measuring the effects of mATP, N-methylscopolamine (NMS), and tetrodotoxin (TTX) on EFS contractions, a total of six responses were measured; the average of the last five responses is reported. These agents were added in the following sequence. First, either mATP (100 μM; 4–5 min) or NMS (1 μM; 10 min) was added to the organ bath, and EFS contractions were recorded. Second, the other agent was added so that both NMS and mATP were present together. After 4 to 10 min, EFS contractions were recorded. Finally, the solution was supplemented with TTX (0.1 μM), and EFS contractions were measured 10 min later. The small contractions that persisted in the presence of TTX were assumed to be caused by direct electrical excitation of the muscle because TTX blocks impulse flow through neurons. Representative contractile measurements from these experiments are shown in Fig. 1.
Fig. 1.
Representative traces of EFS contractions in urinary bladder from vehicle-treated wild-type mouse. Control EFS contractions were first inhibited by either mATP (a) or NMS (b) before the additional blockade by the alternative agent and then final supplementation with TTX.
The control EFS response was normalized to that of bladder from vehicle-treated wild-type mouse, and responses elicited after the incubation with mATP, NMS, or TTX were normalized to the respective control contractile response in the same tissue. We define the total contraction as the control EFS contraction minus the residual response after the addition of mATP and NMS. The latter was usually not significantly different from that measured in the presence of TTX (0.1 μM). This result indicates that the neurogenic component of contraction (TTX-sensitive) can be attributed almost entirely to cholinergic and purinergic neurotransmitters. The cholinergic contraction is defined as the EFS contraction measured in the presence of mATP minus that measured in the presence of both mATP and NMS. Finally, the purinergic contraction is defined as the EFS contraction measured in the presence of NMS minus that measured in the presence of both mATP and NMS.
The summation of the cholinergic and purinergic components of contraction often exceeded the total contraction, which indicates a moderate excess of neurotransmitter release. For this reason, we calculated the purinergic and cholinergic components of contraction, as described above, instead of the conventional approach of defining the purinergic and cholinergic contractions as the amount of inhibition caused by purinergic or a muscarinic blockade, respectively.
We also measured the inhibition of EFS contraction by increasing concentrations of isoproterenol. The average of the last five of nine control EFS contractions were calculated as the control contraction. Increasing concentrations of isoproterenol then were added to the bath. After each addition, an EFS contraction was measured approximately 5 min later, and the cycle was repeated with the addition of the next concentration of isoproterenol.
Statistical Analysis.
To determine whether there was a greater loss of EFS contraction in M2 KO bladder relative to wild type after STZ treatment, we first estimated the mean difference (MDSTZ-WT) between the STZ- and vehicle-treated groups, with respect to the difference in EFS contraction in wild-type and M2 KO urinary bladder:
In this equation, CWT-STZ and CM2KO-STZ denote the mean EFS contractions in wild-type and M2 KO urinary bladder after STZ treatment, respectively, and CWT-V and CM2KO-V denote the corresponding values in bladder from vehicle-treated mice. The standard error for the estimate of MDSTZ-WT is:
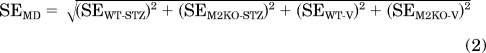 |
In this equation, SEWT-STZ and SEM2KO-STZ denote the standard error of the estimate of the mean EFS contraction in wild-type and M2 KO urinary bladder after STZ treatment, respectively, and SEWT-V and SEM2KO-V denote the corresponding estimates for the vehicle-treated group. To determine whether MDSTZ-WT was significantly different from zero, the following statistic (t) was calculated:
This statistic exhibits a t-distribution with the degrees of freedom equaling the total number of measurements minus four. This test was performed on the total and cholinergic and purinergic components of the EFS contraction.
The analysis of the concentration-response curve of isoproterenol for inhibiting EFS contraction of the urinary bladder was based on the operational model (Black and Leff, 1983):
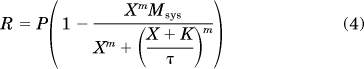 |
In this equation, R denotes contraction, P represents the level of response in the absence of isoproterenol, X is the concentration of isoproterenol, Msys is the maximal response of the system, K is the dissociation constant of isoproterenol, m represents the transducer slope factor, and τ is a parameter proportional to the intrinsic efficacy (ε) of isoproterenol and the sensitivity of its signaling pathway. Specifically, τ is defined as τ = εRT/KE, in which RT denotes the total population of active β-adrenoceptors and KE represents a parameter inversely proportional to the sensitivity of the signaling pathway.
Equation 4 was fitted to the isoproterenol relaxation curve from the M2 KO mouse urinary bladder. In wild-type bladder, both the potency and maximal effect of isoproterenol were lower. These two properties of the concentration-response curve were adequately described by a reduction in the τ parameter of the operational model. Thus, the following equation was fitted to the data from the wild-type bladder:
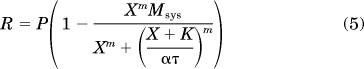 |
This equation is identical to that used to analyze the M2 KO data, with the exception that τ has been multiplied by the scalar α. Thus, α represents the change in τ in the wild-type bladder. When activated by the evoked release of acetylcholine, the M2 receptor in wild-type bladder inhibits the relaxant effect of isoproterenol. This action reduces the sensitivity of the β-adrenoceptor signaling cascade, which is consistent with a reduction in τ. Thus, the reciprocal of α is a measure of the functional role of the M2 receptor in opposing isoproterenol-induced relaxation. In the article, the log of the reciprocal of α is reported (−log α). For a given treatment condition (vehicle or STZ), eqs. 4 and 5 were fitted to the isoproterenol relaxation curves from both M2 KO and wild-type bladder by global nonlinear regression analysis sharing the estimates of Msys, K, m, and τ between the curves and allowing a unique estimate of α for the wild-type data. To determine whether there was a significant difference in α between the vehicle and STZ groups, the significance of the increase in residual sum of squares when the data were analyzed, sharing the estimate of α between the groups, was determined using an F distribution as described previously (Pak et al., 2010). The Student's t test was used to assess statistically significant changes in fasting blood glucose and body weight after vehicle or STZ treatment.
Results
Effect of STZ Treatment on Body Weight Change and Fasting Blood Glucose.
The body weights of wild-type and M2 KO mice injected with STZ (125 mg/kg) significantly decreased compared with that of control (Table 1). Mice treated with STZ exhibited significantly greater fasting blood glucose levels compared with those treated with vehicle (Table 1). Changes in body weight and glucose levels occurred at both early and late time points after STZ treatment.
TABLE 1.
Body weight percentage change and fasting blood glucose levels 8 to 24 weeks after STZ injection
The mean estimate ± S.E.M. are shown. The number or replicates is indicated in parentheses.
| % Body Weight Change |
Fasting Blood Glucose |
||||
|---|---|---|---|---|---|
| Wild Type | M2 KO | Wild Type | M2 KO | ||
| mg/dl | |||||
| 8 to 9 Weeks after treatment | |||||
| Vehicle | 18.0 ± 1.8 (19) | 13.5 ± 2.0 (18) | 112 ± 5 (19) | 113 ± 6 (18) | |
| STZ | −3.8 ± 2.8*** (18) | −0.2 ± 3.6* (17) | 426 ± 32*** (13) | 461 ± 32*** (14) | |
| 20 to 24 Weeks after treatment | |||||
| Vehicle | 38.9 ± 6.3 (8) | 41.7 ± 1.5 (7) | 139 ± 6.5 (8) | 144 ± 8.7 (7) | |
| STZ | 2.1 ± 5.7** (8) | 8.9 ± 4.2*** (4) | 492 ± 42*** (8) | 602 ± 92*** (4) | |
P < 0.05, significantly different from vehicle.
P < 0.001, significantly different from vehicle.
P < 0.0001, significantly different from vehicle.
Effect of STZ Treatment on Bladder Weight.
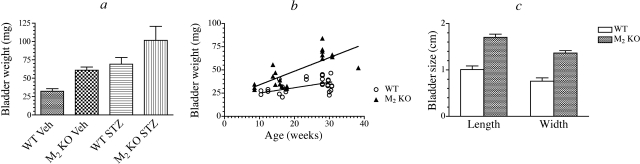
During the course of our experiments, we noted an increase in bladder weight with STZ treatment, particularly in the bladder from M2 KO mice. Figure 2a shows the wet weights of the urinary bladders from some of the animals used in our studies. The average ages of the vehicle-treated wild-type and M2 KO mice and STZ-treated wild-type and M2 KO mice were 21, 33, 30, and 24 weeks, respectively, for the data shown in Fig. 2a. Analysis of variance showed a highly significant difference among the groups (F3,11 = 11.72; P = 0.0009). Neuman Keuls Multiple Comparison Test showed significant differences (P < 0.05) among all of the groups, with the exception of vehicle-treated M2 KO versus STZ-treated wild type. The data show a significant increase in bladder weight with STZ treatment and in the M2 KO mouse relative to wild type.
Fig. 2.
Effect of STZ treatment and age on urinary bladder hypertrophy and distension. a, the effect of STZ treatment on the wet weight of urinary bladders from different treatment groups is shown. Mean values ± S.E.M. are shown. Each group contained three to six mice. b, the weight of urinary bladder from wild-type (n = 27) and M2 KO (n = 22) mice is plotted against age. c, the average length and width of urinary bladders immediately after euthanization of wild-type (n = 5) and M2 KO (n = 6) mice at 7 months of age are shown.
To explore the latter difference more completely, we measured bladder weight in control and vehicle-treated wild-type and M2 KO mice and plotted these data against the age of the mice (Fig. 2b). Linear regression analysis showed a highly significant reduction in residual error (P = 1 × 10−6) when separate linear equations were fitted to each group of data compared with use of a single regression equation. There was no significant increase in residual error when that data were fitted simultaneously, sharing the estimate of the Y intercept (19.3 ± 3.7 mg; F1,45 = 0.463, P = 0.5). Analysis of variance showed that the slope of the line for the M2 KO data (1.47 ± 0.15 mg/week) was significantly greater than that of the wild-type data (0.58 ± 0.09 mg/week) (P = 0.0017). When expressed relative to the Y intercept, the slopes corresponded to 7.6 and 3.0% increases in bladder weight per week for the M2 KO and wild-type mice, respectively. The data suggest that a slowly developing bladder distention occurs in the M2 KO mouse.
To address this question directly, we euthanized 7-month-old wild-type (n = 5) and M2 KO (n = 6) mice at approximately the same time of day (15:00) and immediately measured the length (bladder dome to outlet) and width of the urinary bladder with vernier calipers. These measurements are illustrated in Fig. 2c. Both the length and width were significantly greater in urinary bladder from M2 KO mice compared with wild type (P = 0.00008 and 0.00005, respectively). We also estimated the theoretical surface area of the bladders, assuming an ellipsoid shape with a longitudinal axis equivalent to the length of the bladder. We assumed that a cross-section normal to the longitudinal axis and at its midpoint defined a circle with a diameter equivalent to the width of the bladder. These calculations yielded estimates of the theoretical surface area of urinary bladders from wild-type and M2 KO mice of 2.3 ± 0.33 and 6.92 ± 0.51 cm2, respectively. The difference in theoretical estimates is highly significant (P < 0.00005) and illustrates prominent bladder distention in the M2 KO mouse. Because the average weight of these 7-month-old M2 KO bladders (70 ± 3 mg) was 1.75-fold greater than that of the age-matched wild-type bladders (40 ± 2 mg), yet the corresponding difference in surface area was 3.0-fold, our data suggested greater tension on the M2 KO bladder wall.
The M2 Receptor Inhibits the Development of Impaired Neurogenic Bladder Contraction in STZ-Treated Mice.
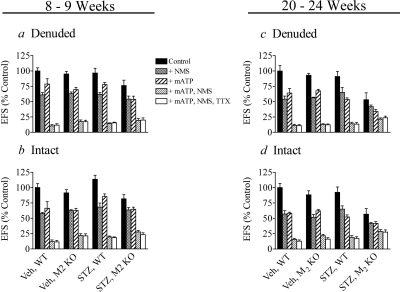
We investigated the effect of STZ treatment on the components of EFS contraction of urinary bladder from wild-type and M2 KO mice. EFS contractions were recorded in the absence (control) and presence of four combinations of inhibitors: 1) NMS, 2) mATP, 3) NMS and mATP, and 4) NMS, mATP, and TTX. When used, the concentrations of NMS, mATP, and TTX were 1 μM, 0.1 mM, and 0.1 μM, respectively. A summary of the data measured at 8 to 9 and 20 to 24 weeks after STZ injection is shown in Fig. 3. For each panel in Fig. 3, the data are expressed relative to the control, vehicle-treated, wild-type condition. There were no significant differences in the mean ± S.E.M. of this control EFS contraction when expressed as a percentage of the KCl-induced contraction for denuded urinary bladder at the 8- to 9- (248 ± 16%) and 20- to 24-week (254 ± 18%) time points and in intact bladder at the same time points (243 ± 13 and 208 ± 18%, respectively) (F3,24 = 1.672; P = 0.20). The residual contraction measured in the presence of both NMS and mATP was approximately the same as that measured in the presence of TTX, indicating that cholinergic and purinergic mechanisms account for the total neurogenic response. The magnitude of the EFS contraction measured in the presence of NMS and mATP was subtracted from that measured 1) under control conditions, 2) in the presence of mATP, and 3) in the presence of NMS. This calculation yielded the 1) total neurogenic contraction and its 2) cholinergic and 3) purinergic components, respectively. These components are displayed in Figs. 4 and 5 for denuded and intact urinary bladders, respectively. In some instances, the summation of the cholinergic and purinergic components in urinary bladder from vehicle-treated wild-type mice exceeded 100%. These data suggest that the signaling pathways of purinergic and muscarinic receptors converge on the same contractile mechanism and that the mechanism exhibits saturation kinetics. That is, when a substantial contractile stimulus has been generated by one type of receptor, the stimulation of a second receptor type leads to less than an additive contraction because contraction is already near maximal.
Fig. 3.
Effects of mATP, NMS, and TTX on EFS contraction in wild-type and M2 KO bladders at 8 to 24 weeks after vehicle or STZ treatment. Inhibition of neurogenic contraction was measured in denuded (a) and intact (b) urinary bladder at 8 to 9 weeks and in denuded (c) and intact (d) bladder at 20 to 24 weeks after treatment. Mean values ± S.E.M. from 3 to 10 experiments are shown.
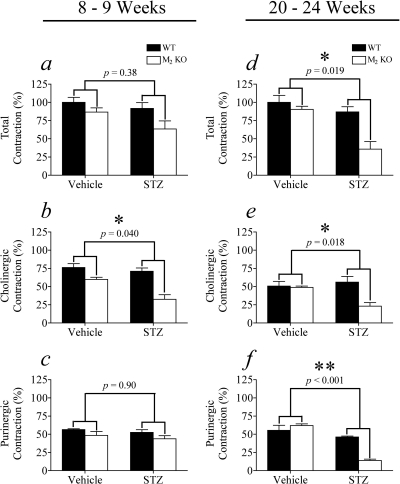
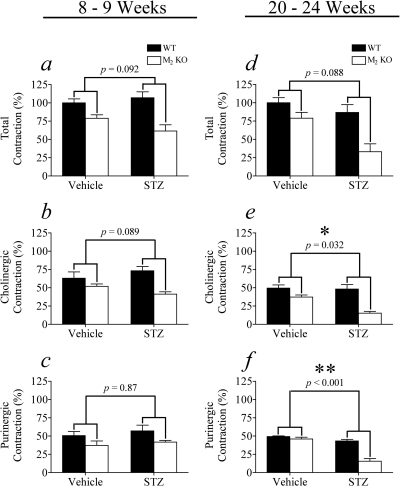
Fig. 4.
Effect of STZ treatment on total, cholinergic, and purinergic EFS contraction in wild-type and M2 KO urinary bladders lacking urothelium. The total (a), cholinergic (b), and purinergic (c) components of EFS contraction were measured at 8 to 9 weeks after STZ treatment and after 20 to 24 weeks (d, e, f, respectively). Mean values ± S.E.M. from 3 to 10 experiments are shown. Significantly different from vehicle (*, P < 0.05, **, P < 0.001).
Fig. 5.
Effect of STZ treatment on total, cholinergic, and purinergic contraction in wild-type and M2 KO intact bladders. Total (a), cholinergic (b), and purinergic (c) responses were measured at 8 to 9 weeks after STZ treatment and after 20 to 24 weeks (d, e, f, respectively). Mean values ± S.E.M. from 3 to 10 experiments are shown. Significantly different from vehicle (*, P < 0.05, **, P < 0.001).
Treatment of wild-type and M2 KO mice with STZ at 8 to 9 weeks before had little effect on the total EFS contraction of the denuded bladder (Fig. 4a). In vehicle-treated mice, the cholinergic component of contraction (Fig. 4b) in the M2 KO mouse was 78% that of wild type. STZ treatment significantly reduced this component to 46% wild type (P = 0.040). In contrast, there was no significant effect of STZ treatment on the purinergic component of contraction (Fig. 4c) in wild-type and M2 KO mice.
At 20 to 24 weeks after STZ treatment, there was a highly significant reduction in the total, cholinergic, and purinergic components of contraction in the M2 KO mouse relative to wild type. This comparative loss of function (M2 KO relative to wild type) in STZ-treated mice was significantly greater than the corresponding difference in vehicle-treated mice in all cases. That is, following STZ treatment, the total (Fig. 4d), cholinergic (Fig. 4e), and purinergic (Fig. 4f) components of contraction in M2 KO mice were only 41 (P = 0.019), 41 (P = 0.018), and 30% (P < 0.001) of wild type, respectively.
Although the purinergic component of EFS contraction decreased at 20 to 24 weeks after STZ treatment, there was no significant difference in the magnitude of the contraction elicited by directly applied mATP (100 μM) among the different treatment groups. The mean mATP-induced contraction expressed relative to that elicited by KCl were: vehicle wild type, 119 ± 7.9%; vehicle M2 KO, 131 ± 8.3%; STZ wild type, 111 ± 9.4%; and STZ M2 KO, 96 ± 7.8% (F3,21 = 2.35; P = 0.102). A similar trend was observed in intact urinary bladder at 8 to 9 weeks after STZ treatment, although the differences between vehicle- and STZ-treated groups were not significant (Fig. 5, a–c).
At 20 to 24 weeks after STZ treatment, EFS contractions in intact urinary bladder were similar to those observed in denuded tissue. That is, there was a highly significant reduction in the total, cholinergic, and purinergic components of contraction in the M2 KO mouse relative to wild type (Fig. 5, d–f). This comparative loss of function (M2 KO relative to wild type) in mice treated with STZ was significantly greater than the corresponding change in vehicle-treated mice in the case of the cholinergic and purinergic components (Fig. 5, e and f, respectively). When expressed relative to wild type, these components in the STZ-treated M2 KO mouse were only 32 (P = 0.032) and 36% (P < 0.001), respectively.
Effects of STZ Treatment on the Relaxant Effect of Isoproterenol on Neurogenic Contraction of the Urinary Bladder.
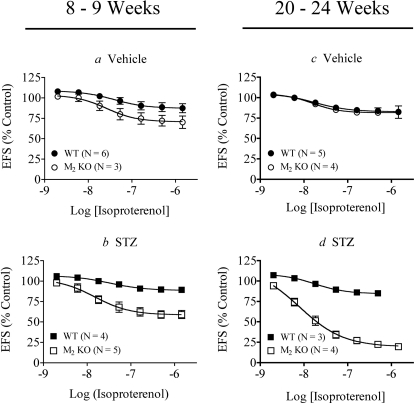
We measured how STZ treatment affected the relaxant action of isoproterenol on EFS contraction of denuded (Fig. 6) and intact (Fig. 7) wild-type and M2 KO urinary bladder. The data were analyzed using global nonlinear regression analysis with eqs. 4 and 5 to quantify the role of the M2 receptor in opposing isoproterenol-induced relaxation as described under Materials and Methods. Denuded bladder from the M2 KO mouse was more sensitive to isoproterenol-mediated relaxation compared with wild-type tissue (Fig. 6a), confirming the role of the M2 receptor in inhibiting isoproterenol-mediated relaxation. This difference was significant at 8 to 9 weeks after STZ treatment (P = 0.046) (Fig. 6b; Table 2) and, to an even greater extent, at 20 to 24 weeks after STZ treatment (P = 1.3 × 10−14) (Fig. 6, c versus d; Table 2). A summary of the Emax and pEC50 values of isoproterenol is given in Table 3.
Fig. 6.
Effect of STZ treatment on isoproterenol-mediated relaxation of EFS contraction in urinary bladder lacking urothelium. Responses were measured at 8 to 9 weeks after mice were treated with vehicle (a) or STZ (b) and after 20 to 24 weeks (c, d, respectively). Mean values ± S.E.M. from three to six experiments are shown. Statistically significant differences are shown in Table 2.
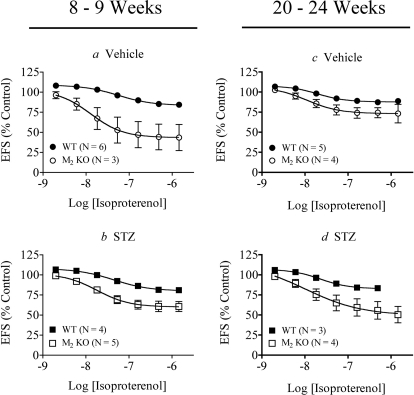
Fig. 7.
Effect of STZ treatment on isoproterenol-mediated relaxation of EFS contraction in intact urinary bladder. Responses were measured at 8 to 9 weeks after mice were treated with vehicle (a) or STZ (b) and after 20 to 24 weeks (c and d, respectively). Mean values ± S.E.M. from three to six experiments are shown. Statistically significant differences are shown in Table 2.
TABLE 2.
Effect of STZ treatment on isoproterenol-mediated inhibition of EFS contraction in urinary bladder from wild-type and M2 KO mice
The parameter -log α is a measure of the role of the M2 receptor in opposing isoproterenol-induced relaxation. The parameters were estimated from the data in Figs. 6 and 7. Mean estimates ± S.E.M. are shown, and the number of replicates is indicated within parentheses.
| M2 Receptor Disinhibitory Effect |
||
|---|---|---|
| Denuded Bladder (−log α) | Intact Bladder (−log α) | |
| 8 to 9 Weeks after treatment | ||
| Vehicle | 0.74 ± 0.18 (3–6) | 1.24 ± 0.098 (3–6) |
| STZ | 1.01 ± 0.071* (4–5) | 0.860 ± 0.051** (4–5) |
| 20 to 24 Weeks after treatment | ||
| Vehicle | 0.076 ± 0.046 (4–5) | 0.53 ± 0.098 (4–5) |
| STZ | 0.88 ± 0.10*** (3–4) | 0.69 ± 0.050 (3–4) |
P < 0.05, significantly different from vehicle.
P < 0.01, significantly different from vehicle.
P < 0.0001, significantly different from vehicle.
TABLE 3.
Summary of pEC50 and Emax values after isoproterenol-mediated inhibition of EFS contraction
The parameters were estimated from the data in Figs. 6 and 7. Mean values ± S.E.M. are shown, and the number of replicates is indicated within parentheses.
| Denuded Bladder |
Intact Bladder |
|||||||
|---|---|---|---|---|---|---|---|---|
| pEC50 |
Emax (% Control) |
pEC50 |
Emax (% Control) |
|||||
| WT | M2 KO | WT | M2 KO | WT | M2 KO | WT | M2 KO | |
| 8 to 9 Weeks | ||||||||
| Vehicle | 7.41 ± 0.32 (6) | 7.58 ± 0.30 (3) | 87 ± 4.0 (6) | 71 ± 4.8 (3) | 7.26 ± 0.16 (6) | 7.89 ± 0.56 (3) | 84 ± 2.7 (6) | 43 ± 9.9 (3) |
| STZ | 7.49 ± 0.21 (4) | 7.83 ± 0.31 (5) | 89 ± 2.0 (4) | 59 ± 4.2 (5) | 7.35 ± 0.16 (4) | 7.74 ± 0.24 (5) | 80 ± 2.7 (4) | 60 ± 3.9 (5) |
| 20 to 24 Weeks | ||||||||
| Vehicle | 7.78 ± 0.32 (5) | 7.79 ± 0.15 (4) | 83 ± 2.9 (5) | 82 ± 1.6 (4) | 7.71 ± 0.19 (5) | 7.96 ± 0.46 (4) | 88 ± 1.9 (5) | 73 ± 4.5 (4) |
| STZ | 7.75 ± 0.25 (3) | 8.11 ± 0.28 (4) | 85 ± 3.4 (3) | 19 ± 4.1 (4) | 7.64 ± 0.13 (3) | 7.98 ± 1.3 (4) | 83 ± 2.1 (3) | 49 ± 15 (4) |
Although the magnitude of the EFS contraction in the absence of isoproterenol was slightly lower in tissue from STZ-treated M2 KO mice at the late time point, one-way analysis of variance showed that the difference was insignificant (F3,12 = 1.06, P = 0.403). It might be argued that decreased neurotransmitter release in the M2 KO mouse after STZ treatment reduces the contractile stimulus in the urinary bladder, making it more susceptible to the relaxant action of isoproterenol. While this seems likely to at least some extent, we found that the pEC50 and Emax values of isoproterenol for inhibiting the contractions elicited by EC50 (Emax, 52% inhibition; pEC50, 7.9 ± 0.086) and EC90 (Emax, 47% inhibition; pEC50, 8.3 ± 0.083) concentrations of the muscarinic agonist oxotremorine-M were similar. In other words, the relaxant effect of isoproterenol did not change when the concentration of muscarinic agonist decreased. Therefore, these results provide little support for a decrease in the release of endogenous acetylcholine as the cause of the increased relaxant effect of isoproterenol in the M2 KO mouse bladder after STZ-treatment.
In urinary bladder with intact urothelium, the difference in isoproterenol sensitivity between wild type and M2 KO bladders was decreased with STZ treatment (P = 0.0036) at the early time point (Fig. 7, a versus b; Table 2). The trend reverses by 20 to 24 weeks when STZ treatment increases isoproterenol sensitivity in M2 KO urinary bladder (Fig. 7, c versus d; Table 2). However, the latter change was not significant. A summary of the Emax and pEC50 values of these experiments is given in Table 3.
Discussion
We used a single injection of STZ (125 mg/kg) to induce hyperglycemia. This dose causes substantial increases in blood glucose levels with minimal mortality (data not shown). Others (Tesch and Nikolic-Paterson, 2006; Tesch and Allen, 2007) have shown that a similar dose produces minimal acute renal cytotoxicity while inducing chronic renal damage akin to human nephropathy. Urinalysis of our STZ-treated mice showed no diabetic ketoacidosis as reflected by undetectable, or very rarely, trace (5 mg/dl) amounts of acetoacetic acid (data not shown).
We found that STZ treatment caused a loss of EFS cholinergic contraction in urinary bladder lacking the M2-muscarinic receptor at 8 to 9 weeks after STZ injection (Fig. 4b). This result is consistent with prior work (Pak et al., 2010) showing that urinary bladder lacking the M2 receptor exhibits a greater loss of responsiveness to directly applied muscarinic agonist following STZ treatment.
At 20 to 24 weeks after STZ treatment, there was a large neurogenic deficit in both the cholinergic and purinergic components of contraction in denuded M2 KO urinary bladder (Fig. 4, d–f, respectively). The purinergic deficit cannot be attributed to a postjunctional loss of function because there was no significant difference in the contraction elicited by directly applied mATP (100 μM) among the different treatment groups. We previously reported little difference in muscarinic agonist-induced contractions of isolated urinary bladder from STZ-treated wild-type and M2 KO mice at this time point (Pak et al., 2010). Thus, the loss of neurogenic cholinergic and purinergic contraction in urinary bladder lacking the M2 receptor indicates a loss of nerve function. Our data suggest that the presence of the M2 receptor throughout the course of hyperglycemia prevents this nerve damage in wild-type urinary bladder from STZ-treated animals. We cannot rule out the possibility, however, that the congenital lack of the M2 receptor before STZ treatment has an influence on the development of neuropathy in the M2 KO mouse.
In intact bladder, STZ treatment was without effect on neurogenic contraction (Fig. 5,a–c) at the 8- to 9-week time point. At the late time point (20–24 weeks, Fig. 5, d–f), however, the presence of the M2 receptor was critical in maintaining function in STZ-treated animals, as both cholinergic and purinergic contractions were impaired in its absence (Fig. 5, e and f). Thus, the M2 receptor protects the urinary bladder from STZ-induced neuropathy as assessed in both the absence and presence of urothelium.
Male M3 KO mice rapidly develop distended urinary bladders and a complete loss of EFS bladder contraction, including the purinergic component, approximately 3 to 4 months of age (Matsui et al., 2000; Ehlert et al., 2007), presumably because of the near complete loss of postjunctional muscarinic receptor mediated-contraction. We have shown that the M2 receptor causes a low-potency enhancement of M3 receptor-mediated contractions of the urinary bladder (Ehlert et al., 2005). It is conceivable that the absence of this M2 contractile function during the micturition reflex causes incomplete voiding and a gradual bladder distention (see Fig. 2).
We found no significant effect of STZ treatment on EFS contraction in urinary bladder from wild-type animals and a huge loss of EFS contraction in M2 KO bladder, which could not be attributed to a loss of postjunctional mechanisms during the EFS measurement. Perhaps the susceptibility of the M2 KO mouse to bladder distention coupled with the neuropathic effects of STZ-induced hyperglycemia leads to more prominent bladder distension in the M2 KO mouse. This may put greater tension on the nerves in the bladder wall leading to a cycle of more neuropathy and more distension. Thus, the deficit in M2 contractile activity may ultimately lead to greater STZ-induced neuropathy. It is also possible that the lack of the M2 receptor in the urothelium may lead to a diminished afferent input for the micturition reflex, and hence, greater bladder distention, and ultimately, neuropathy. Finally, it is possible that a lack of the M2 receptor elsewhere in the body may ultimately cause enhanced STZ-induced neuropathy in the M2 KO mouse.
The susceptibility of the M2 KO mouse to STZ-induced neuropathy of the urinary bladder seems to be a phenotype of this mouse strain. It seems likely that the loss of function can be attributed to the loss of M2 receptors, as described above, and not to changes in other mechanisms. We have previously reported no change in the sensitivity of the M2 KO mouse urinary bladder to the contractile effects of mATP and prostaglandin F2α and the relaxant effect of isoproterenol against KCl- and mATP-induced contractions (Ehlert et al., 2005, 2007). Ito et al. (2009) reported 35 and 34% decreases in total muscarinic receptors in bladder from M4 and M5 KO mice, suggesting that a loss of one subtype in a single KO mouse causes a loss of other subtypes. The authors suggested that a loss of prejunctional M4 receptors might cause a postjunctional down-regulation of receptors. Regardless, the loss of muscarinic receptors in the M2 KO mouse (82%) is commensurate with the high proportion of this receptor in wild-type bladder.
We also investigated the effect of STZ treatment on the ability of the M2 receptor to inhibit the relaxant effects of isoproterenol on EFS contraction of the urinary bladder. Isoproterenol was slightly more effective in relaxing EFS contractions in M2 KO bladder compared with wild-type tissue (Fig. 6a), indicating that neuronally released acetylcholine acts on the M2 receptor to oppose relaxant responses, as described previously, with other stimulation parameters (Ehlert et al., 2007). STZ treatment enhanced this effect (Fig. 6b), suggesting a greater role for this M2 mechanism in diabetic bladder. This role was greater at 20 to 24 weeks after STZ treatment (Fig. 6, c versus d). At the late time point, deletion of the M2 receptor had a very robust effect in STZ-treated animals (Fig. 6d) versus those injected with vehicle (Fig. 6c). Our results suggest that the urinary bladder reservoir may become more susceptible to β-adrenoceptor-mediated relaxation over the development of STZ-induced diabetes and that the M2 receptor opposes this susceptibility, although part of the enhanced effect of isoproterenol may be caused by changes in the release of norepinephrine, acetylcholine, and ATP.
In intact urinary bladder, the ability of the M2 receptor to oppose isoproterenol-mediated relaxation was greater in vehicle-treated (Fig. 7a) rather than STZ-treated (Fig. 7b) mice at the 8- to 9-week time point. At the later time point, this trend was reversed, albeit without reaching statistical significance. The known effect of muscarinic receptor activation on the release of an inhibitory factor from the urothelium (Hawthorn et al., 2000) and the loss of this release following STZ treatment (Kosan et al., 2005) might account for these data. The M2 receptor in the urothelium inhibits the release of an inhibitory factor whose initial release is stimulated by a muscarinic receptor other than the M2 subtype (Pak et al., 2010). The relaxant effect of isoproterenol against EFS contraction of urinary bladder from vehicle-treated mice, therefore, may be greater in M2 KO bladder because of a lack of M2 receptor-mediated inhibition of the release of the inhibitory factor. STZ treatment causes a loss of the urothelial inhibitory factor, and hence, the relaxant effect of isoproterenol would not be enhanced by the inhibitory factor in M2 KO bladder. This explanation may account for the reduced relaxant effect of isoproterenol at the early time point after STZ treatment in intact bladder (Fig. 7, a versus b). At the later time point (Fig. 7, c versus d), however, the increased role of the postjunctional M2 receptor in opposing isoproterenol-induced relaxation probably increases, similar to that observed in denuded bladder (Fig. 6, c versus d). This change may lead to a reversal in the magnitude of role of the M2 receptor between the vehicle- and STZ-treated groups (Fig. 7, c versus d). The effect of β-adrenoceptor agonists on the release of mediators from urothelial tissue after STZ treatment (Birder et al., 2002) may also make interpretation of these results difficult.
Our research demonstrates that, during STZ-induced hyperglycemia, the M2-muscarinic receptor inhibits the development of neuropathy of the urinary bladder. These results suggest that the use of anticholinergics in the treatment of overactive bladder during the early stages of diabetic incontinence may enhance the rate of progression to bladder neuropathy, which is common in the later stages of the disease (see Daneshgari et al. (2009) for the progression of urinary bladder dysfunction in diabetes). In humans, the cholinergic component to EFS contraction of the urinary bladder is 95 to 100% (Sibley, 1984; Burnstock, 2002), suggesting that inhibition of muscarinic receptor function would be more likely to promote neuropathy in the human than in the mouse.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant F30-DK081289]; the University of Tennessee Federal Flow-Through Funds from the National Institutes of Health National Heart, Lung and Blood Institute [Grant UTN-37775]; the National Institutes of Health National Institutes of General Medical Sciences [Grant R01-GM069829]; Arnold and Mabel Beckman Foundation and Achievement Rewards for College Scientists Foundation; and the University of California Irvine Medical Scientist Training Program.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.169995.
- EFS
- electrical-field-stimulated
- STZ
- streptozotocin
- KO
- knockout
- mATP
- α,β-methylene ATP
- NMS
- N-methylscopolamine
- TTX
- tetrodotoxin.
References
- Andersen JT, Bradley WE. (1976) Abnormalities of bladder innervation in diabetes mellitus. Urology 7:442–448 [DOI] [PubMed] [Google Scholar]
- Andersson KE, Arner A. (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986 [DOI] [PubMed] [Google Scholar]
- Benkó R, Lázár Z, Pórszász R, Somogyi GT, Barthó L. (2003) Effect of experimental diabetes on cholinergic, purinergic and peptidergic motor responses of the isolated rat bladder to electrical field stimulation or capsaicin. Eur J Pharmacol 478:73–80 [DOI] [PubMed] [Google Scholar]
- Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. (2002) Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22:8063–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW, Leff P. (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162 [DOI] [PubMed] [Google Scholar]
- Braverman A, Legos J, Young W, Luthin G, Ruggieri M. (1999) M2 receptors in genito-urinary smooth muscle pathology. Life Sci 64:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman AS, Luthin GR, Ruggieri MR. (1998) M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol 275:R1654–R1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman AS, Ruggieri MR., Sr (2003) Hypertrophy changes the muscarinic receptor subtype mediating bladder contraction from M3 toward M2. Am J Physiol Regul Integr Comp Physiol 285:R701–R708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. (2002) Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin Med 2:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. (2009) Diabetic bladder dysfunction: current translational knowledge. J Urol 182:S18–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. (2001) Pharmacology of the lower urinary tract. Ann Rev Pharmacol Toxicol 41:691–721 [DOI] [PubMed] [Google Scholar]
- Ehlert FJ, Ahn S, Pak KJ, Park GJ, Sangnil MS, Tran JA, Matsui M. (2007) Neuronally released acetylcholine acts on the M2 muscarinic receptor to oppose the relaxant effect of isoproterenol on cholinergic contractions in mouse urinary bladder. J Pharmacol Exp Ther 322:631–637 [DOI] [PubMed] [Google Scholar]
- Ehlert FJ, Griffin MT, Abe DM, Vo TH, Taketo MM, Manabe T, Matsui M. (2005) The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol Exp Ther 313:368–378 [DOI] [PubMed] [Google Scholar]
- Faerman I, Glocer L, Celener D, Jadzinsky M, Fox D, Maler M, Alvarez E. (1973) Autonomic nervous system and diabetes. Histological and histochemical study of the autonomic nerve fibers of the urinary bladder in diabetic patients. Diabetes 22:225–237 [DOI] [PubMed] [Google Scholar]
- Freeman R. (2005) Autonomic peripheral neuropathy. The Lancet 365:1259–1270 [DOI] [PubMed] [Google Scholar]
- Giglio D, Delbro DS, Tobin G. (2005) Postjunctional modulation by muscarinic M2 receptors of responses to electrical field stimulation of rat detrusor muscle preparations. Auton Autacoid Pharmacol 25:113–120 [DOI] [PubMed] [Google Scholar]
- Gür S, Cinel I. (2003) Sodium selenate partially corrects impaired functional responses in detrusor muscle in streptozotocin-induced diabetic rats. Biol Trace Elem Res 93:171–188 [DOI] [PubMed] [Google Scholar]
- Ito Y, Oyunzul L, Seki M, Fujino Oki T, Matsui M, Yamada S. (2009) Quantitative analysis of the loss of muscarinic receptors in various peripheral tissues in M1–M5 receptor single knockout mice. Br J Pharmacol 156:1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Daneshgari F. (2005) Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol 288:F1220–F1226 [DOI] [PubMed] [Google Scholar]
- Liu S-H, Lin-Shiau SY. (1996) The effects of uranyl ions on neuromuscular transmission in the urinary bladder of the normal and streptozotocin-diabetic mouse. Naunyn Schmiedebergs Arch Pharmacol 354:773–778 [DOI] [PubMed] [Google Scholar]
- Longhurst PA, Levendusky MC, Bezuijen MW. (2004) Diabetes mellitus increases the rate of development of decompensation in rats with outlet obstruction. J Urol 171:933–937 [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Zar MA. (1991) The effect of streptozotocin-induced diabetes on cholinergic motor transmission in the rat urinary bladder. Br J Pharmacol 103:1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Griffin MT, Shehnaz D, Taketo MM, Ehlert FJ. (2003) Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M2 receptor knockout mice. J Pharmacol Exp Ther 305:106–113 [DOI] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. (2002) Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22:10627–10632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. (2000) Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA 97:9579–9584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Barendrecht MM. (2008) Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol Ther 117:297–312 [DOI] [PubMed] [Google Scholar]
- Pak KJ, Ostrom RS, Matsui M, Ehlert FJ. (2010) Impaired M3 and enhanced M2 muscarinic receptor contractile function in a streptozotocin model of mouse diabetic urinary bladder. Naunyn Schmiedebergs Arch Pharmacol 381:441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley GN. (1984) A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol 354:431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel PW, Yamada M, Wess J, Cohen ML. (2002) M(3)-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol 282:R1443–R1449 [DOI] [PubMed] [Google Scholar]
- Tesch GH, Allen TJ. (2007) Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology 12:261–266 [DOI] [PubMed] [Google Scholar]
- Tesch GH, Nikolic-Paterson DJ. (2006) Recent insights into experimental mouse models of diabetic nephropathy. Nephron Exp Nephrol 104:e57–62 [DOI] [PubMed] [Google Scholar]