Abstract
Methamphetamine (METH) abuse is a serious public health issue. Of particular concern are findings that repeated high-dose administrations of METH cause persistent dopaminergic deficits in rodents, nonhuman primates, and humans. Previous studies have also revealed that METH treatment causes alterations in the dopamine transporter (DAT), including the formation of higher molecular mass DAT-associated complexes. The current study extends these findings by examining mechanisms underlying DAT complex formation. The association among DAT complex formation and other METH-induced phenomena, including alterations in vesicular monoamine transporter 2 (VMAT2) immunoreactivity, astrocytic activation [as assessed by increased glial fibrillary acidic protein (GFAP) immunoreactivity], and persistent dopaminergic deficits was also explored. Results revealed that METH-induced DAT complex formation and reductions in VMAT2 immunoreactivity precede increases in GFAP immunoreactivity. Furthermore, and as reported previously for DAT complexes, pretreatment with the D2 receptor antagonist eticlopride [S-(−)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride] attenuated the decrease in VMAT2 immunoreactivity as assessed 24 h after METH treatment. DAT complexes distinct from those present 24 h after METH treatment, decreases in VMAT2 immunoreactivity, and increased GFAP immunoreactivity were present 48 to 72 h after METH treatment. Pretreatment with eticlopride attenuated each of these phenomena. Finally, DAT complexes were present 7 days after METH treatment, a time point at which VMAT2 and DAT monomer immunoreactivity were also reduced. Eticlopride pretreatment attenuated each of these phenomena. These findings provide novel insight into not only receptor-mediated mechanisms underlying the effects of METH but also the interaction among factors that probably are associated with the persistent dopaminergic deficits caused by the stimulant.
Introduction
Methamphetamine (METH) is a highly addictive psychostimulant whose abuse has significant individual and societal costs. One concern associated with METH abuse is the potential for long-term dopaminergic deficits. For example, METH abusers have reduced striatal dopamine (DA) transporter (DAT) densities (Wilson et al., 1996; McCann et al., 1998), an effect that has been associated with motor slowing and memory impairment (Volkow et al., 2001) and may be related to psychiatric symptoms (Sekine et al., 2001).
Studies involving rodents indicate there are many effects caused by repeated high-dose administrations of METH including, but not limited to, oxidative stress (for review, see Brown and Yamamoto, 2003; Krasnova and Cadet, 2009), astrocytic/microglial activation (O'Callaghan and Miller, 1994; LaVoie et al., 2004; Thomas et al., 2004), DAT complex formation (Baucum et al., 2004; Hadlock et al., 2009), and alterations in vesicular monoamine transporter 2 (VMAT2) function (Brown et al., 2000; Eyerman and Yamamoto, 2007; Guillot et al., 2008). However, the relationship among these factors has not been elucidated fully. In addition, an association between METH-induced DAT complex formation and persistent dopaminergic deficits has been suggested (Baucum et al., 2004; Hadlock et al., 2009) but has not been studied specifically. Accordingly, the purpose of this study was to investigate possible associations among three of these phenomena and METH-induced persistent dopaminergic deficits, in particular, METH-induced DAT complex formation, alterations in VMAT2 immunoreactivity, and astrocytic activation. Results revealed that alterations in VMAT2 and DAT immunoreactivity precede increases in glial fibrillary acidic protein (GFAP) immunoreactivity, a marker of astrocytic activation and neuronal damage (Eng et al., 2000). Furthermore, high molecular mass DAT complexes distinct from those apparent 24 h after METH treatment were present 48 to 72 h and 7 days after METH treatment. Finally, D2 receptor activation contributes to each of these phenomena.
Materials and Methods
Animals.
Male Sprague-Dawley rats (290–400 g; Charles River Laboratories, Inc., Raleigh, NC) were maintained under controlled lighting and temperature with constant access to food and water. Rats were housed three to four animals per cage during the experiments. METH-treated rats were maintained at warmer temperatures to ensure METH-induced hyperthermia. Rectal temperatures were assessed at 1-h intervals beginning 30 min before the first saline or METH injection. For experiments involving pretreatment with D1 or D2 receptor antagonists, rats received intraperitoneal injections of the drug treatment or saline vehicle 30 min before each saline or METH injection. Mean temperatures over the course of the experiments were determined. For METH-treated rats, only rats that achieved mean rectal temperatures higher than 38°C over the course of the experiment were used for analysis. Rats were sacrificed by decapitation. All procedures were approved by the University of Utah Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drugs and Chemicals.
S-(−)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride (eticlopride) and R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) were purchased from Sigma-Aldrich (St. Louis, MO). (±) METH hydrochloride was supplied by the Research Triangle Institute (Research Triangle Park, NC). Drugs doses were calculated as the free base. Drugs were dissolved in 0.9% saline vehicle.
Tissue Preparation and Western Blot Analysis.
Synaptosomal tissues were prepared as described previously (Baucum et al., 2004). In brief, striata were dissected, homogenized in 0.32 M ice-cold sucrose, pH 7.4, and centrifuged (800g, 12 min; 4°C). Supernatants were centrifuged (22,000g, 15 min; 4°C), and the resultant pellets were resuspended in ice-cold double-distilled H2O at concentrations of 45 to 55 mg/ml original wet weight. Total protein concentrations were determined as described by Bradford (1976). The samples were then diluted with a nonreducing loading buffer (final concentration: 2.25% SDS, 18% glycerol, 180 mM Tris base, pH 6.8, and bromphenol blue) and frozen at −80°C until Western blot analysis. Equal quantities of total protein (4–10 μg) were loaded onto a 4 to 12% NuPAGE Novex Bis-Tris Midi gradient gel (Invitrogen, Carlsbad, CA) and electrophoresed by using a XCell4 Surelock Midi-Cell (Invitrogen). Samples were then transferred to a polyvinylidene difluoride hybridization transfer membrane (PerkinElmer Life and Analytical Sciences, Waltham, MA). Western blot analysis was performed as described previously (Hadlock et al., 2009). Overall DAT complex immunoreactivity was defined as immunoreactivity more than ∼120 kDa and determined for data presented in Fig. 1. As the majority of DAT complex immunoreactivity shifts to higher molecular masses at 72 h and remains at 7 days after METH treatment, only the highest molecular mass regions of Western blots were used for determining DAT immunoreactivity as presented in Figs. 4 and 6, approximately the top third of the overall DAT complex range, spanning from ∼120 kDa to the top of the gel. DAT was detected by using a rabbit polyclonal N-terminal DAT antibody (generously provided by Dr. Roxanne Vaughan, University of North Dakota, Grand Forks, ND). VMAT2 was detected by using a rabbit polyclonal antibody (AB1767; Millipore Corporation, Billerica, MA), and GFAP was detected by using a mouse monoclonal antibody (556329; BD Biosciences, San Jose, CA).
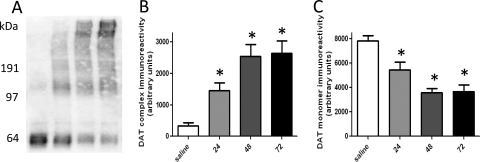
Fig. 1.
Time course of METH-induced DAT complex formation (A and B) and loss of DAT monomer immunoreactivity (A and C). Rats received four injections of METH (7.5 mg/kg s.c. per injection; 2-h intervals) or saline vehicle (1 mg/kg s.c. per injection; 2-h intervals) and were sacrificed 24, 48, or 72 h later. A, a representative blot of samples from saline-treated rats (lane 1) and METH-treated rats 24 h (lane 2), 48 h (lane 3), and 72 h (lane 4) after treatment. Molecular masses (kDa) are indicated adjacent to the representative blot. Columns represent the means and vertical lines indicate 1 S.E.M. determinations in 5 to 10 rats. *, values different from saline-treated controls (p ≤ 0.05).
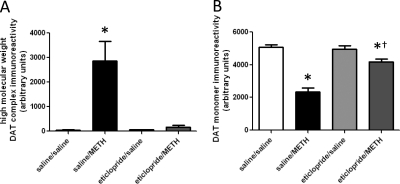
Fig. 4.
Eticlopride pretreatment attenuates METH-induced DAT complex formation (A) and loss of DAT monomer immunoreactivity (B), as assessed 72 h after METH treatment. Rats received four injections of METH (7.5 mg/kg s.c. per injection; 2-h intervals) or saline vehicle (1 ml/kg s.c. per injection; 2-h intervals) and were sacrificed 72 h later. Thirty minutes before each injection, rats were pretreated with eticlopride (0.5 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.). Columns represent the means and vertical lines indicate 1 S.E.M. determinations in 6 to 10 rats. *, values different from saline-treated controls (p ≤ 0.05); †, values different from saline/METH-treated group (p ≤ 0.05).
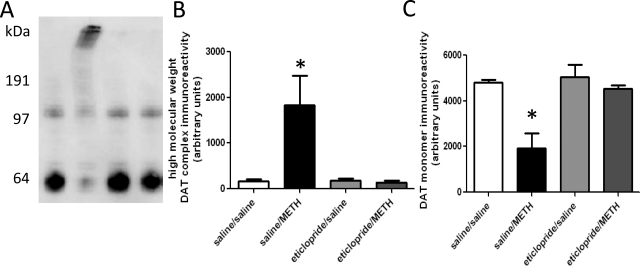
Fig. 6.
Eticlopride pretreatment attenuates METH-induced DAT complex formation (A and B) and loss of DAT monomer immunoreactivity (A and C), as assessed 7 days after METH treatment. Rats received four injections of METH (7.5 mg/kg s.c. per injection; 2-h intervals) or saline vehicle (1 ml/kg s.c. per injection; 2-h intervals) and were sacrificed 7 days later. Thirty minutes before each injection, rats were pretreated with eticlopride (0.5 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.). A, a representative blot of saline/saline-treated (lane 1), saline/METH-treated (lane 2), eticlopride/saline-treated (lane 3), and eticlopride/METH-treated (lane 4) samples. Molecular mass (kDa) is shown adjacent to the representative blot. Columns represent the means and vertical lines indicate 1 S.E.M. determinations in six to eight rats. *, values different from saline-treated controls (p ≤ 0.05).
Data Analysis.
Analysis of variance, followed by Newman-Keuls post hoc test, was performed to determine significant differences among experimental groups. Differences were considered significant if the probability of error was less than or equal to 5%. All statistical analyses were performed with Prism 5 (GraphPad Software Inc., San Diego, CA).
Results
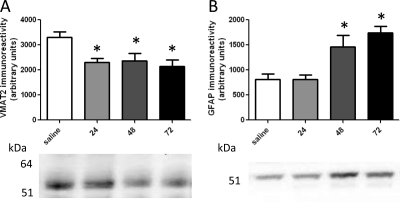
Results presented in Fig. 1 demonstrate that overall DAT complex immunoreactivity was increased (A and B) and DAT monomer immunoreactivity was decreased (A and C), as assessed 24, 48, and 72 h after METH treatment. It is noteworthy that differences in complex immunoreactivity became apparent over time, with the majority of DAT complex immunoreactivity concentrated at the highest molecular masses 48 and 72 h after METH treatment (Fig. 1A). VMAT2 immunoreactivity was decreased 24, 48, and 72 h after METH treatment (Fig. 2A). GFAP immunoreactivity was increased at 48 and 72 h, but not 24 h, after METH treatment (Fig. 2B).
Fig. 2.
Time course of METH-induced alterations in VMAT2 (A) and GFAP immunoreactivity (B). Rats received four injections of METH (7.5 mg/kg s.c. per injection; 2-h intervals) or saline vehicle (1 mg/kg s.c. per injection; 2-h intervals) and were sacrificed 24, 48, or 72 h later. Molecular masses (kDa) are indicated adjacent to the representative blot. Columns represent the means and vertical lines indicate 1 S.E.M. determinations in 5 to 10 rats. *, values different from saline-treated controls (p ≤ 0.05).
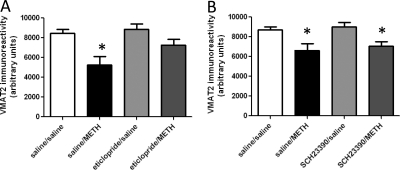
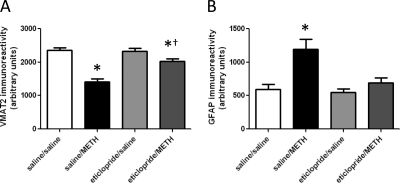
As demonstrated for METH-induced DAT complex formation (Hadlock et al., 2009), pretreatment with the D2 receptor antagonist eticlopride (Fig. 3A), but not the D1 receptor antagonist SCH23390 (Fig. 3B), attenuated the METH-induced decrease in VMAT2 immunoreactivity, as assessed 24 h after treatment. In both experiments, elevated body temperatures were maintained over the course of METH treatment in all METH-treated rats, including those pretreated with eticlopride (mean temperature of 39.4°C ± 0.2 and 39.6°C ± 0.1 for saline/METH-treated and eticlopride/METH-treated rats, respectively) or SCH23390 (mean temperature of 39.3°C ± 0.1 and 39.3°C ± 0.2 for saline/METH-treated and SCH23390/METH-treated rats, respectively). Figure 4 demonstrates that eticlopride pretreatment also attenuated METH-induced DAT complex formation (Fig. 4A) and decreases in DAT monomer immunoreactivity (Fig. 4B), as assessed 72 h after treatment. At this time point, eticlopride pretreatment also attenuated METH-induced decreases in VMAT2 immunoreactivity (Fig. 5A) and increases GFAP immunoreactivity (Fig. 5B). In this experiment, elevated body temperatures were maintained in all METH-treated rats (mean temperature of 39.6 ± 0.1 and 39.4 ± 0.1°C for saline/METH-treated and eticlopride/METH-treated rats, respectively).
Fig. 3.
Eticlopride pretreatment (A), but not SCH23390 pretreatment (B), attenuates METH-induced loss of VMAT2 as assessed 24 h after METH treatment. Rats received four injections of METH (7.5 mg/kg s.c. per injection; 2-h intervals) or saline vehicle (1 ml/kg s.c. per injection; 2-h intervals) and were sacrificed 24 h later. Thirty minutes before each injection, rats were pretreated with eticlopride (A; 0.5 mg/kg i.p.), SCH23390 (B; 0.5 mg/kg i.p.), or saline vehicle (1 ml/kg i.p.). Columns represent the means and vertical lines indicate 1 S.E.M. determinations in six to nine rats. *, values different from saline-treated controls (p ≤ 0.05).
Fig. 5.
Eticlopride pretreatment attenuates METH-induced loss of VMAT2 (A) and increased GFAP immunoreactivity (B), as assessed 72 h after METH treatment. Rats received four injections of METH (7.5 mg/kg s.c. per injection; 2-h intervals) or saline vehicle (1 ml/kg s.c. per injection; 2-h intervals) and were sacrificed 72 h later. Thirty minutes before each injection, rats were pretreated with eticlopride (0.5 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.). Columns represent the means and vertical lines indicate 1 S.E.M. determinations in 6 to 10 rats. *, values different from saline-treated controls (p ≤ 0.05); †, values different from saline/METH-treated group (p ≤ 0.05).
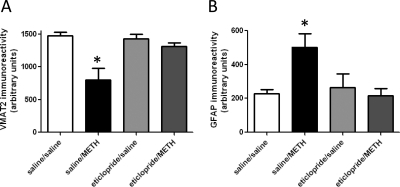
Results presented in Fig. 6, A and B reveal that DAT complexes were present 7 days after METH treatment and their formation was attenuated by eticlopride pretreatment. It is noteworthy that after 48 and 72 h (Fig. 1A) the majority of overall DAT complex immunoreactivity was concentrated in the highest molecular mass regions of the Western blots. At this time point, there were also decreases in DAT monomer immunoreactivity (Fig. 6C), decreases in VMAT2 immunoreactivity (Fig. 7A), and increases in GFAP immunoreactivity (Fig. 7B). All of these effects were attenuated by eticlopride pretreatment. In this experiment, elevated body temperatures were maintained in all METH-treated rats (mean temperature of 39.1 ± 0.2 and 38.9 ± 0.1°C for saline/METH-treated and eticlopride/METH-treated rats, respectively).
Fig. 7.
Eticlopride pretreatment attenuates METH-induced loss of VMAT2 (A) and increase in GFAP immunoreactivity (B), as assessed 7 days after METH treatment. Rats received four injections of METH (7.5 mg/kg s.c. per injection; 2-h intervals) or saline vehicle (1 ml/kg s.c. per injection; 2-h intervals) and were sacrificed 7 days later. Thirty minutes before each injection, rats were pretreated with eticlopride (0.5 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.). Columns represent the means and vertical lines indicate 1 S.E.M. determinations in six to eight rats. *, values different from saline-treated controls (p ≤ 0.05).
Discussion
Previous studies have revealed that METH treatment causes alterations in monoaminergic transporters. For example, multiple high-dose administrations of METH rapidly (within 1 h) decrease VMAT2 activity (Brown et al., 2000), an effect that may be caused by a rapid redistribution of VMAT2 to a location that is not retained in the preparation of synaptosomes (Riddle et al., 2002) and oxidation of VMAT2 (Eyerman and Yamamoto, 2007). The decrease in VMAT2 function and a loss of VMAT2 immunoreactivity persist 24 h after treatment (Eyerman and Yamamoto, 2005; Chu et al., 2008). The present studies extend this work by demonstrating that the loss of VMAT2 immunoreactivity after 24 h is attenuated by pretreatment with the D2 receptor antagonist eticlopride. Likewise, pretreatment with eticlopride attenuates the reduction in VMAT2 immunoreactivity after 72 h. These results are of significance because several studies have indicated that aberrant VMAT2 function contributes to the monoaminergic deficits caused by METH. For example, pretreatment with the VMAT2 inhibitor reserpine worsens the dopaminergic deficits caused by METH (Wagner et al., 1983; Thomas et al., 2008). In addition, heterozygous VMAT2 knockout mice exhibit increased METH-induced dopaminergic deficits (Fumagalli et al., 1999). It is noteworthy that treatment of mice with pituitary adenylyl cyclase-activating polypeptide 38 increases the expression and function of VMAT2 and attenuates METH-associated astrocytic activation (Guillot et al., 2008a).
The involvement of D2 receptor activation in the effects of METH is not restricted to VMAT2. In particular, both the METH-induced increase in DAT complex formation and decrease in DAT activity observed after 24 h (Hadlock et al., 2009) and 72 h (Fig. 4) are attenuated by D2 antagonist pretreatment. Thus, D2 receptor-mediated mechanisms underlie both the METH-induced alterations in DAT and VMAT2 24 to 72 h after treatment.
Because oxidative stress probably contributes to DAT complex formation (Baucum et al., 2004; Hadlock et al., 2009) and METH-induced alterations in VMAT2 may contribute to oxidative stress (for review, see Fleckenstein et al., 2009), it is reasonable to speculate that the alterations in VMAT2 contribute to DAT complex formation. This may occur because METH redistributes vesicular DA into the cytosol and causes DA-related oxidative stress (Cubells et al., 1994; for review, see Brown and Yamamoto, 2003; Krasnova and Cadet, 2009). A reduction in VMAT2 function/protein would promote this oxidative stress, because less DA would be sequestered. Indeed, reduced vesicular DA sequestration exacerbates METH-induced dopaminergic deficits (Wagner et al., 1983; Fumagalli et al., 1999; Guillot et al., 2008b; Thomas et al., 2008).
Astrocytes and microglia are activated after neuronal insults (Whitney et al., 2009), including METH treatment (O'Callaghan and Miller, 1994; LaVoie et al., 2004; Thomas et al., 2004). Consistent with these findings, results revealed that METH treatment increased GFAP immunoreactivity as assessed 48 and 72 h after treatment. It is noteworthy that the onset of this increase occurred after METH-induced DAT complex formation and loss of VMAT2 immunoreactivity. Like the effects on DAT and VMAT2, pretreatment with eticlopride attenuated this phenomenon as assessed 72 h after treatment. These results permit speculation that the earlier D2 receptor-mediated alterations and deficits in VMAT2 and DAT, described above, may contribute to astrocytic activation.
DAT complexes are present within 24 h and remain 7 days after METH treatment. However, a novel finding of the present study is that the nature of the DAT complexes changes over time. Specifically, the DAT complexes present at 48 and 72 h are different from the DAT complexes at 24 h as evidenced by findings that the majority of overall immunoreactivity is concentrated in the highest molecular mass regions of the Western blots. Furthermore, DAT complexes present at 7 days also have a much higher molecular mass than those observed 24 h after METH treatment. There are a number of possible explanations for these phenomena. For instance, the lower molecular mass DAT complexes present at 24 h may be preferentially degraded such that the higher molecular mass DAT complexes predominate at 48 to 72 h and at 7 days. Alternatively, the lower molecular mass DAT complexes present at 24 h may continue to increase in molecular mass to create the high molecular mass DAT complexes observed at 48 to 72 h and 7 days. It is noteworthy that DAT monomer immunoreactivity levels do not change significantly between 24 and 72 h, suggesting that alterations in DAT complex immunoreactivity may be caused primarily by DAT protein that has already formed complexes. Previous studies have suggested that DAT complex formation occurs through an oxidative mechanism (Baucum et al., 2004; Hadlock et al., 2009). The broad molecular mass range of the complexes observed in this and previous studies may be caused by numerous additional modifications and/or protein–protein interactions. Although further studies are needed to determine the precise composition of the DAT complexes, it is reasonable to postulate that an oxidative mechanism also contributes to the increase in molecular mass of DAT complexes seen at 48 h, 72 h, and 7 days after METH treatment. This prolonged oxidative stress may be caused by numerous mechanisms involved in METH-induced neurotoxicity that cause oxidative stress including, but not limited to, microglial activation, astrocytic activation, and excitotoxicity (Quinton and Yamamoto, 2006; for review, see Krasnova and Cadet, 2009).
Previous studies have suggested that DAT complex formation may be associated with persistent dopaminergic deficits (Baucum et al., 2004; Hadlock et al., 2009). This is supported by evidence that prevention of METH-induced hyperthermia or prior treatment with the DA-depleting agent α-methyl-p-tyrosine attenuates both DAT complex formation (Baucum et al., 2004) and the persistent dopaminergic deficits caused by the stimulant (Schmidt et al., 1985; Bowyer et al., 1992). In addition, METH-induced DAT complex formation does not occur in the nucleus accumbens (Hadlock et al., 2009), a brain region that is refractory to the METH-induced persistent dopaminergic deficits (Eisch et al., 1992; Cass, 1997; but see also Broening et al., 1997; Haughey et al., 1999; Thomas et al., 2009). The present findings further suggest an association between DAT complex formation and persistent METH-induced dopaminergic deficits because both phenomena are attenuated by D2 receptor antagonist pretreatment, even when hyperthermia was maintained in the eticlopride-pretreated rats (see also Broening et al., 2005).
In conclusion, the present study suggests an association between METH-induced DAT complex formation, decreases in VMAT2 immunoreactivity, astrocytic activation, and persistent dopaminergic deficits because each is prevented by D2 antagonist pretreatment. Although these results do not prove causal relationships among these events, they allow speculation that early (within 24 h) alterations in DAT and/or VMAT2 contribute to astrocytic activation and that each contributes to the persistent dopaminergic deficits caused by the stimulant. These findings provide novel insight into not only receptor-mediated mechanisms underlying the effects of METH, but also the interaction among factors that are probably associated with the persistent dopaminergic deficits caused by the stimulant.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA00869, DA04222, DA13367, DA11389, DA019447, DA00378] and a fellowship from the Pharmaceutical Research and Manufacturers of America Foundation (to G.C.H.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.166660.
- METH
- methamphetamine
- DA
- dopamine
- DAT
- DA transporter
- eticlopride
- S-(−)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl) methyl]-6-hydroxy-2-methoxybenzamide hydrochloride
- GFAP
- glial fibrillary acidic protein
- SCH23390
- R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- VMAT2
- vesicular monoamine transporter 2.
References
- Baucum AJ, 2nd, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. (2004) Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci 24:3436–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. (1992) The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther 260:817–824 [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Vorhees CV. (2005) Interactions of dopamine D1 and D2 receptor antagonists with d-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse 56:84–93 [DOI] [PubMed] [Google Scholar]
- Broening HW, Pu C, Vorhees CV. (1997) Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse 27:153–160 [DOI] [PubMed] [Google Scholar]
- Brown JM, Yamamoto BK. (2003) Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacol Ther 99:45–53 [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. (2000) Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem 74:2221–2223 [DOI] [PubMed] [Google Scholar]
- Cass WA. (1997) Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther 280:105–113 [PubMed] [Google Scholar]
- Chu PW, Seferian KS, Birdsall E, Truong JG, Riordan JA, Metcalf CS, Hanson GR, Fleckenstein AE. (2008) Differential regional effects of methamphetamine on dopamine transport. Eur J Pharmacol 590:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. (1994) Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci 14:2260–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O'Dell SJ, Marshall JF. (1992) Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res 598:321–326 [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25:1439–1451 [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. (2005) Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J Pharmacol Exp Ther 312:160–169 [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. (2007) A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem 103:1219–1227 [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. (2009) Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology 56:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. (1999) Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci 19:2424–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Richardson JR, Wang MZ, Li YJ, Taylor TN, Ciliax BJ, Zachrisson O, Mercer A, Miller GW. (2008a) PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides 42:423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Shepherd KR, Richardson JR, Wang MZ, Li Y, Emson PC, Miller GW. (2008b) Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J Neurochem 106:2205–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Baucum AJ, 2nd, King JL, Horner KA, Cook GA, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. (2009) Mechanisms underlying methamphetamine-induced dopamine transporter complex formation. J Pharmacol Exp Ther 329:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Hanson GR. (1999) Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem 72:661–668 [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev 60:379–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. (2004) Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol 187:47–57 [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. (1998) Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinon users: evidence from positron emission tomography studies with [11C] WIN-35,428. J Neurosci 18:8417–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:741–751 [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. (2006) Causes and consequences of methamphetamine and MDMA toxicity. AAPS J 8:E337–E347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Topham MK, Haycock JW, Hanson GR, Fleckenstein AE. (2002) Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur J Pharmacol 449:71–74 [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. (1985) Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther 233:539–544 [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. (2001) Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry 158:1206–1214 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. (2008) The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem 105:605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. (2009) Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem 109:1745–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311:1–7 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, et al. (2001) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382 [DOI] [PubMed] [Google Scholar]
- Wagner GC, Lucot JB, Schuster CR, Seiden LS. (1983) α-Methyltyrosine attenuates and reserpine increases methamphetamine induced neuronal changes. Brain Res 270:285–288 [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. (2009) Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem 108:1343–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703 [DOI] [PubMed] [Google Scholar]