Abstract
Renin, the rate-limiting enzyme in the activation of the renin-angiotensin system (RAS), is synthesized and stored in cardiac mast cells. In ischemia/reperfusion, cardiac sensory nerves release neuropeptides such as substance P that, by degranulating mast cells, might promote renin release, thus activating a local RAS and ultimately inducing cardiac dysfunction. We tested this hypothesis in whole hearts ex vivo, in cardiac nerve terminals in vitro, and in cultured mast cells. We found that substance P-containing nerves are juxtaposed to renin-containing cardiac mast cells. Chemical stimulation of these nerves elicited substance P release that was accompanied by renin release, with the latter being preventable by mast cell stabilization or blockade of substance P receptors. Substance P caused degranulation of mast cells in culture and elicited renin release, and both of these were prevented by substance P receptor blockade. Ischemia/reperfusion in ex vivo hearts caused the release of substance P, which was associated with an increase in renin and norepinephrine overflow and with sustained reperfusion arrhythmias; substance P receptor blockade prevented these changes. Substance P, norepinephrine, and renin were also released by acetaldehyde, a known product of ischemia/reperfusion, from cardiac synaptosomes and cultured mast cells, respectively. Collectively, our findings indicate that an important link exists in the heart between sensory nerves and renin-containing mast cells; substance P released from sensory nerves plays a significant role in the release of mast cell renin in ischemia/reperfusion and in the activation of a local cardiac RAS. This culminates in angiotensin production, norepinephrine release, and arrhythmic cardiac dysfunction.
Introduction
In addition to the traditional circulating renin-angiotensin system (RAS) (Peach, 1977; Campbell, 1987), it is now recognized that a local RAS exists in various organs, including the heart (Dzau, 1987; Dostal and Baker, 1999; Barlucchi et al., 2001; Bader, 2002; Carey and Siragy, 2003). We recently reported that renin, the rate-limiting enzyme of the RAS cascade, is synthesized and stored in cardiac mast cells (Silver et al., 2004). It is released from these cells in active form by exogenous chemicals, such as compound 48/80, by aggregation of mast cell-bound antibodies (Kano et al., 2007; Veerappan et al., 2008), or in the course of ischemia/reperfusion (Mackins et al., 2006) by reactive oxygen species (Koda et al., 2010). Once released, mast cell-derived renin initiates the activation of a local RAS, culminating in angiotensin (ANG II)- and norepinephrine (NE)-mediated arrhythmias (Mackins et al., 2006).
Mast cells are closely apposed to nerves in various tissues (Keith et al., 1995; Domeij et al., 1996; Bauer and Razin, 2000), including the heart (Laine et al., 2000; Silver et al., 2004), an organ that is amply innervated by afferent sensory fibers (Franco-Cereceda, 1988; Zahner et al., 2003; Camici and Pagani, 2006; Ieda and Fukuda, 2009). Stimulation of cardiac sensory nerves, as it occurs in ischemia/reperfusion (Eaton et al., 1999; Trevisani et al., 2007), elicits the release of neuropeptides (CGRP and substance P) (Chiao and Caldwell, 1996; Källner et al., 1998). We hypothesized that by causing degranulation of neighboring mast cells (Imamura et al., 1996; Lorenz et al., 1998), these neurotransmitters might promote renin release, thus activating a local RAS and ultimately inducing cardiac dysfunction. We tested this hypothesis in whole guinea pig hearts ex vivo, in cardiac nerve terminals in vitro, and in cultured mast cells. We show that neuropeptides released from sensory nerve fibers play an important role in the release of mast cell renin in ischemia/reperfusion and in the activation of a local cardiac RAS.
Materials and Methods
Perfusion of Guinea Pig Hearts Ex Vivo.
All experiments were approved by the Institutional Animal Care and Use Committee of Weill Medical College at Cornell University. Male guinea pigs (Charles River Laboratories, Wilmington, MA) weighing 300 to 350 g were anesthetized with CO2 and euthanized by stunning. Hearts were quickly excised and perfused with oxygenated Ringer's solution at 37°C at a constant pressure (40 cm of H2O) via an aortic cannula in a Langendorff apparatus (Radnoti Glass Technology, Monrovia, CA) (Mackins et al., 2006). Spontaneously beating hearts were stabilized for 30 min before experimentation. When used, capsaicin was continuously perfused for 6 min. Normothermic global ischemia was induced by complete cessation of coronary perfusion for 20 min, followed by 30 min of reperfusion. In some cases, hearts were continuously perfused with pharmacological agents 10 min before and during perfusion with capsaicin [i.e., CGRP8–37, CP99994 [(+)-(2S,3S)-3-(2-methoxybenzylamino)-2-phenylpiperidine)], cromolyn, EXP3174 [2-n-butyl-4-chloro-1-((2′-(1H-tetrazol-5-yl)biphenyl-4-yl) methyl)imidazole-5-carboxylic acid], and imetit or before induction of ischemia and during reperfusion (i.e., CGRP8–37 and CP99994). Coronary flow was measured by timed collections of the effluent every 2 min, and all samples were assayed for renin, NE, CGRP, and substance P. Surface electrocardiogram was obtained from leads attached to the left ventricle and the right atrium and analyzed using PowerLab/8SP (ADInstruments, Colorado Springs, CO). Reperfusion arrhythmias were analyzed according to the Lambeth Conventions (Walker et al., 1988).
Cell Culture.
The human mastocytoma cell line (HMC-1) was a gift from Dr. I. Biaggioni (Vanderbilt University, Nashville, TN). Cells were maintained in Iscove's modified Dulbecco's medium supplemented with 25 mM HEPES, 2 mM l-glutamine, 10% fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, and 1.2 mM monothioglycerol at 37°C, 5% CO2 (Silver et al., 2004).
β-Hexosaminidase Assay.
Pooled confluent flasks of HMC-1 cells were pelleted and resuspended in Ringer's solution containing 140 mM NaCl, 5 mM KCl; 10 mM Hepes, 1 mM MgCl2, 2 mM glucose, and 2 mM CaCl2, pH 7.4. HMC-1 cells were incubated with increasing concentrations of substance P or acetaldehyde for 30 min at 37°C. Where appropriate, the cells were incubated for 10 min with CP99994 before and throughout the duration of exposure to substance P. After drug treatment, samples were pelleted by centrifugation at 2000g for 8 min at 4°C, and the levels of β-hexosaminidase were determined using a modification of a previously established method (Schwartz et al., 1979). First, to measure β-hexosaminidase in the releasate, the HMC-1 sample supernatants were placed in a well of a 96-well plate with substrate solution (p-nitrophenyl-N-acetyl-β-d-glucosaminide, 1.3 mg/ml in 0.1 M citrate buffer, pH 4.5) and incubated for 90 min at 37°C. The reaction was stopped with the addition of 0.2 M glycine (pH 10.7). Optical density was read at 405 nm using a plate reader in combination with SoftMax Pro 4.8 (Molecular Devices, Sunnyvale, CA). To measure the total amount of β-hexosaminidase, the samples were lysed with 1× lysis buffer (Cell Signaling Technology, Danvers, MA) for 15 min. The samples were then centrifuged at 13,000 rpm for 10 min at 4°C and the lysate supernatant removed and treated as described for the releasate supernatant.
Renin Assay.
Samples of coronary effluent were immediately concentrated 8-fold by centrifugal filtration (Millipore Corporation, Billerica, MA). Concentrated samples were incubated for 18 h with 240 nM human angiotensinogen (EMD Biosciences, San Diego, CA). Samples of HMC-1 releasate were incubated for 1.5 h with 200 nM renin substrate tetradecapeptide (Sigma-Aldrich, St. Louis, MO). Renin activity was determined by Gammacoat Plasma Renin Activity 125I Radioimmunoassay (Diasorin, Stillwater, MN) as described previously (Mackins et al., 2006). The detection limit was approximately 0.01 pmol.
Cardiac Synaptosomes.
Guinea pig hearts were isolated as described for perfusion ex vivo. These spontaneously beating hearts were perfused through the aorta for 15 min at constant pressure (40 cm of H2O) with Ringer's solution at 37°C saturated with 100% O2 to ensure that no traces of blood remained in the coronary circulation. Hearts were then minced in ice-cold 0.32 M sucrose containing 1 mM EGTA, pH 7.4. Minced tissue was digested with 40 mg of Type II collagenase (Worthington Biochemicals, Freehold, NJ) per 10 ml of HEPES-buffered saline solution (HBS) per gram of wet heart weight for 1 h at 37°C. HBS contained 1 mM pargyline to prevent enzymatic destruction of catecholamines. After low-speed centrifugation (10 min at 120g and 4°C), the resulting pellet was suspended in 10 volumes of 0.32 M sucrose and homogenized with a Teflon/glass homogenizer. The homogenate was spun at 650g for 10 min at 4°C, and the pellet re-homogenized and re-spun. The pellet containing cellular debris was discarded, and the supernatants from the last two spins were combined and equally subdivided into seven tubes. Each tube was centrifuged for 20 min at 20,000g at 4°C. This pellet, containing cardiac synaptosomes, was resuspended in HBS to a final volume of 500 μl in the presence or absence of acetaldehyde for a total of 10 min in a water bath at 37°C. Each suspension functioned as an independent sample and was used only once. In each experiment, one sample was untreated (control, basal substance P, and NE release). Controls were incubated for the same length of time without acetaldehyde. At the end of the incubation period, each sample was centrifuged for 20 min (20,000g at 4°C), the supernatant was assayed for NE and substance P content, and the pellet was assayed for protein content by a modified Lowry procedure (Seyedi et al., 1997).
Norepinephrine Assay.
Coronary effluent and synaptosomal supernatants were assayed for NE by HPLC with electrochemical detection, as described previously (Seyedi et al., 2005). The detection limit was approximately 0.2 pmol.
CGRP and Substance P Assays.
Coronary effluent and synaptosomal samples were stored at −20°C for a short period of time (i.e., <2 weeks). The samples were then thawed and assayed for neuropeptide content with the use of commercially available enzyme immunoassay kits, a rat CGRP kit (Cayman Chemical, Ann Arbor, MI), and a substance P kit (Assay Designs, Ann Arbor, MI) according to the manufacturer's protocol. The detection limits of these kits were 1 and 9.76 pg/ml for CGRP and substance P, respectively.
Immunofluorescence.
Rat atrial tissue was fixed with formalin and embedded in paraffin. Atrial sections were deparaffinized, and target retrieval was used (Dako North America, Inc., Carpinteria, CA). Sections were blocked with 10% fetal bovine serum. Primary antibodies were diluted in 1% bovine serum albumin, with 0.5% Tween 20. Secondary antibodies were diluted in phosphate-buffered saline. Rat atrial sections were stained with either anti-renin antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) conjugated to Alexa Fluor 594 or anti-substance P antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) conjugated to Alexa Fluor 594. Mast cells were identified with avidin-fluorescein isothiocyanate. Nuclei were counterstained with 4,6-diamidino-2-phenylindole. Vectashield mounting medium was applied to the tissue sections before the coverslips were mounted. In all immunofluorescence experiments, tissue sections were examined with an Eclipse TE 2000-U inverted fluorescence microscope (Nikon, Melville, NY) interfaced to an electron-multiplying charge-coupled device (Hamamatsu Corporation, Bridgewater, NJ) and processed with MetaMorph software (Molecular Devices).
Reagents.
Acetaldehyde, capsaicin, and cromolyn were purchased from Sigma-Aldrich ; CP99994 was a gift from Pfizer (New York, NY); CGRP8–37 and substance P were purchased from AnaSpec, Inc. (San Jose, CA); EXP3174 was a gift from Merck (Whitehouse Station, NJ); and imetit was purchased from Tocris Bioscience (Ellisville, MO).
Statistics.
The one-way ANOVA test followed by Dunnett's or Bonferroni's Multiple Comparison Tests were used where appropriate and as indicated in the figure legends. P < 0.05 was considered significant.
Results
Renin-Containing Mast Cells Are Located near Substance P-Positive Nerves in the Rat Heart.
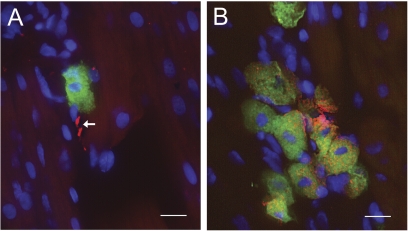
The spatial relationship between mast cells and sensory nerves in rat atria was investigated using immunoscreening (Fig. 1). Mast cells were identified with avidin (conjugated to fluorescein), which selectively binds to mast cell granules (Tharp et al., 1985) (Fig. 1, A and B). Substance P-containing sensory nerves were identified in the same sections by costaining with polyclonal anti-substance P raised in goat against full-length human substance P, conjugated to Alexa Fluor 594 donkey anti-goat IgG (Fig. 1A). Renin-containing mast cells were identified in the same sections by costaining with polyclonal anti-renin raised in goat to human recombinant renin, also conjugated to Alexa Fluor 594 donkey anti-goat IgG (Fig. 1B). Immunohistochemical analysis revealed that sensory nerves containing substance P are closely apposed to renin-containing mast cells in rat atrial tissue.
Fig. 1.
Renin-containing mast cells are located near substance P-positive nerves in the rat heart. Immunofluorescence of a rat atrial section stained with avidin conjugated to fluorescein (green) to identify mast cells. A, anti-substance P conjugated to Alexa Fluor 594 (red). B, anti-renin conjugated to Alexa Fluor 594 (red). Note in A that the substance P neuronal pattern is shown by the white arrow. Nuclei were counterstained with 4,6-diamidino-2-phenylindole. Scale bar = 10 μm.
Chemical Stimulation of Cardiac Sensory C-Fibers Elicits the Release of Neuropeptides, Mast Cell Renin, and Norepinephrine.
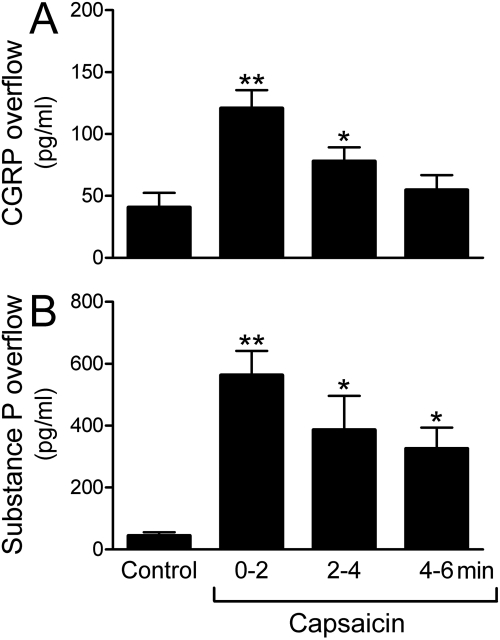
Chemical stimulation of sensory C-fibers with capsaicin in spontaneously beating guinea pig hearts ex vivo elicited the release of the neuropeptides CGRP and substance P. During the first 2 min of capsaicin perfusion (100 nM), the overflow of CGRP and substance P increased approximately 3- and 10-fold, respectively (Fig. 2, A and B). The release of these peptides was accompanied by an approximate 3-fold increase in the overflow of renin and NE (Fig. 3, A and B).
Fig. 2.
Chemical stimulation of sensory C-fibers with capsaicin elicits the release of CGRP (A) and substance P (B) in ex vivo guinea pig hearts. Bars (means ± S.E.M.; n = 6) represent overflows of CGRP and substance P into the coronary effluent. Capsaicin (100 nM) was perfused for 6 min, and the coronary effluent was collected at 2-min intervals. Control bars (means ± S.E.M.; n = 6) represent the basal release of CGRP and substance P before perfusion with capsaicin. *, P < 0.05, **, P < 0.01, from control by one-way ANOVA followed by Dunnett's post-test analysis.
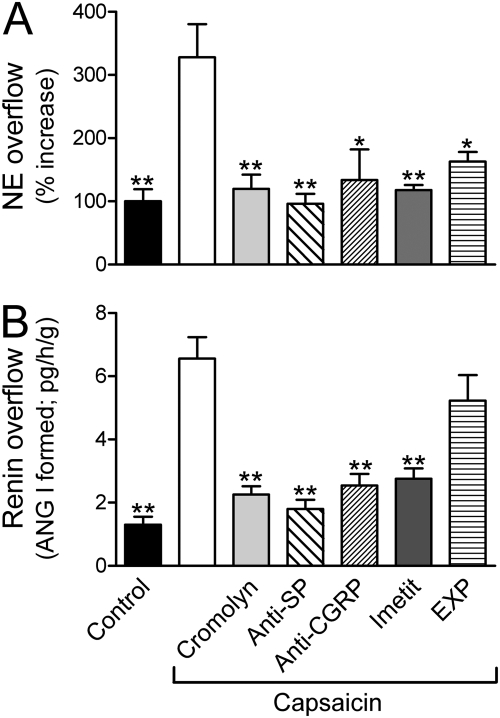
Fig. 3.
Chemical stimulation of sensory C-fibers with capsaicin elicits release of mast cell renin (A), ANG II formation (A and B), and NE release (A) in ex vivo guinea pig hearts. Bars (means ± S.E.M.) represent overflows of renin (angiotensin I formed) and NE into the coronary effluent. Capsaicin (100 nM) was perfused for 6 min alone (n = 8) or in the presence of the mast cell stabilizer cromolyn (300 μM, n = 5), the NK1-receptor antagonist CP99994 [Anti-substance P (anti-SP); 100 nM, n = 5], the CGRP-receptor antagonist CGRP8–37 (Anti-CGRP; 100 nM, n = 3), the H3-receptor agonist imetit (100 nM, n = 6), or the ANG II AT1-receptor antagonist EXP3174 (EXP; 10 nM, n = 4). Control bars (means ± S.E.M.; n = 31). Basal release of NE was 0.69 pmol/g. *, P < 0.05, **, P < 0.01, from capsaicin alone by one-way ANOVA followed by Dunnett's post-test analysis.
Perfusion of guinea pig hearts with the mast cell stabilizer cromolyn (300 μM) attenuated the capsaicin-induced increase in renin and NE overflow by approximately 65% (Fig. 3, A and B), indicating that the renin released into the coronary effluent of capsaicin-perfused hearts originated from mast cells, and suggesting that the release of NE was secondary to the release of mast cell renin. Notably, incubating HMC-1 mast cells in culture with substance P (30 nM–1 μM) resulted in a concentration-dependent release of β-hexosaminidase (an index of their degranulation) and of renin (Fig. 4, A and B). This effect was prevented by preincubation of HMC-1 cells with the specific substance P receptor antagonist CP99994 (100 nM) (Fig. 4, A and B). In contrast, incubation of HMC-1 cells with capsaicin (1 μM) failed to degranulate mast cells (i.e., β-hexosaminidase release increased by 4.36 ± 1.20 and 5.24 ± 1.79% in control and capsaicin-treated conditions, respectively; n = 4). This indicated that capsaicin did not directly degranulate mast cells, but enhanced renin overflow by releasing mast cell-degranulating neuropeptides from sensory C-fibers.
Fig. 4.
Substance P (SP) causes degranulation and renin release in HMC-1 by activating NK1 receptors. Incubation of HMC-1 cells with substance P for 20 min elicits the release of renin and β-hexosaminidase (an index of mast cell degranulation) in a concentration-dependent manner. Points represent the response to substance P in the absence (closed squares) and presence (open circles) of the NK1-receptor antagonist CP99994 (100 nM). A, points are means (±S.E.M.; n = 8–11) of percentage increases in renin release above basal level (basal renin activity, 1640 pg/h/mg protein ± 295; n = 26). B, points are means (± S.E.M.; n = 4–13) of percentage increases in β-hexosaminidase release above basal level (4.89 ± 0.41; n = 16). *, P < 0.05, **, P < 0.01, from basal by one-way ANOVA followed by Dunnett's post-test analysis. #, P < 0.05, ###, P < 0.001 from substance P by one-way ANOVA followed by Bonferroni's post-test analysis.
Neuropeptides Released from Cardiac Sensory C-Fibers Degranulate Local Mast Cells, Thereby Releasing Renin and Activating a Local Cardiac RAS.
Perfusion of guinea pig hearts with the specific substance P receptor antagonist CP99994 (100 nM) or with the specific CGRP receptor antagonist CGRP8–37 (100 nM) prevented the increases in renin and NE overflows elicited by capsaicin (Fig. 3, A and B). This suggested that substance P and CGRP, released from sensory C-fibers by chemical stimulation with capsaicin, activated NK1 and CGRP receptors on mast cells, eliciting their degranulation and the release of renin, followed by local RAS activation and ANG II-induced release of NE from sympathetic nerves.
Perfusion of guinea pig hearts with the selective H3R agonist imetit (100 nM) prevented the capsaicin-induced increase in renin and NE overflow into the coronary effluent (Fig. 3, A and B). This suggested that the activation of negatively modulatory H3-receptors, which are known to be expressed on sensory C-fibers (Imamura et al., 1996), attenuated the release of neuropeptides from sensory nerves, thus diminishing mast cell degranulation, preventing the release of mast cell renin and local RAS activation, and consequently, diminishing NE release from sympathetic nerves.
In contrast, perfusion of guinea pig hearts with the selective AT1-receptor antagonist EXP3174 (10 nM) prevented only the capsaicin-induced increase in NE overflow but not that of renin (Fig. 3, A and B). This suggested that, although capsaicin still elicited the neuropeptide-induced release of mast cell renin and the consequent activation of a local RAS culminating in ANG II formation, NE failed to be released from sympathetic nerves because of blockade of neuronal AT1-receptors.
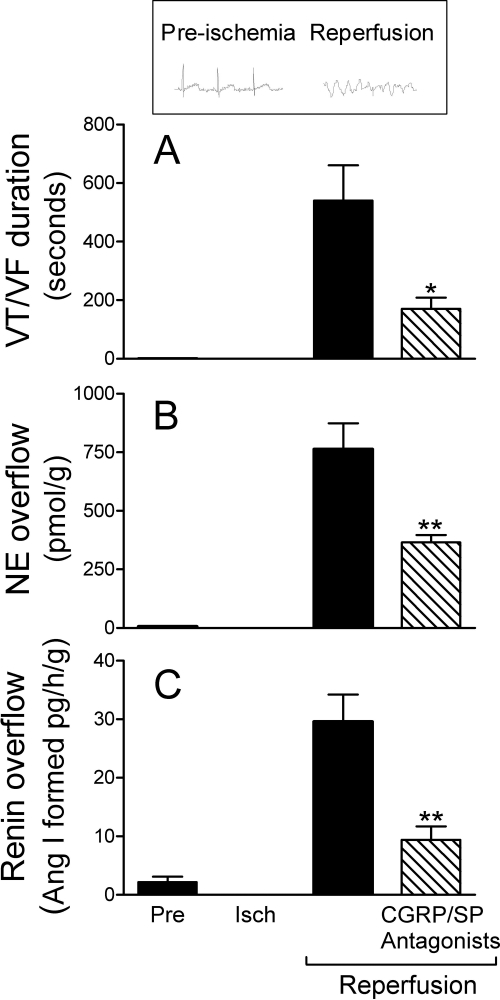
Ischemia/Reperfusion Promotes the Activation of a Local RAS, Culminating in Arrhythmic Cardiac Dysfunction: Relevance of Neuropeptide-Induced Mast Cell Renin Release.
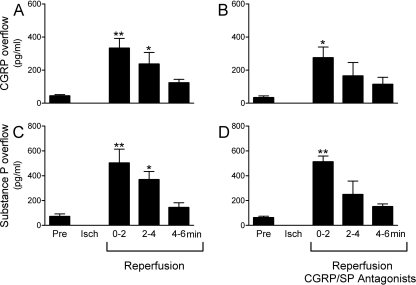
Reactive oxygen species (ROS) generated during ischemia/reperfusion (Vanden Hoek et al., 1996, 1998; Eaton et al., 1999) are known to provoke the release of neuropeptides from sensory nerves (Chiao and Caldwell, 1996; Källner et al., 1998; Trevisani et al., 2007). Hence, we next investigated the coronary overflow of CGRP, substance P, renin, and NE in isolated guinea pig hearts subjected to 20-min global ischemia followed by 30-min reperfusion. During the initial 2 min of reperfusion, the overflow of CGRP and substance P increased approximately 8- to 10-fold above preischemic control (Fig. 5, A and C). Furthermore, the overflows of renin and NE also increased during reperfusion, approximately 10- and 75-fold, respectively (Fig. 6, B and C). Severe arrhythmias, such as ventricular tachycardia (VT) and ventricular fibrillation (VF), invariably occurred during reperfusion. Their incidence was 100%, and their duration was approximately 500 s (Fig. 6A). Other guinea pig hearts were perfused with the CGRP and substance P receptor antagonists CGRP8–37 (100 nM) and CP99994 (100 nM) in combination and then subjected to ischemia/reperfusion. In the presence of the neuropeptide receptor antagonists, renin and NE overflows were reduced by approximately 70 and 50%, respectively, and there was also an approximate 70% decrease in the duration of VT/VF (Fig. 6). In contrast, the neuropeptide receptor antagonists did not affect the overflow of substance P and CGRP (compare Fig. 6, A and C, with 5, B and D), demonstrating that the decreased release of renin and NE was not because of decreased neuropeptide availability. Instead, these findings suggested that CGRP and substance P released from sensory C-fibers during ischemia/reperfusion elicit the release of renin from mast cells, contributing to the activation of a local cardiac RAS and culminating in the formation of ANG II, NE release, and reperfusion arrhythmias.
Fig. 5.
Ischemia/reperfusion causes the release of CGRP and substance P from ex vivo guinea pig hearts, and this is unaffected by the blockade of CGRP and NK1 receptors. Bars (means ± S.E.M.) represent overflows of CGRP and substance P into the coronary effluent of isolated guinea pig hearts. Hearts were subjected to ischemia/reperfusion [i.e., 20-min global ischemia (Isch) followed by 30-min reperfusion; n = 6]. A and C, control bars (Pre; means ± S.E.M.; n = 6) represent basal release of CGRP and substance P (SP) preceding ischemia/reperfusion. *, P < 0.05, **, P < 0.01, from Pre by one-way ANOVA followed by Dunnett's post-test analysis. B and D, hearts were perfused with both CGRP8–37 (100 nM) and CP99994 (100 nM) for 10 min preceding ischemia/reperfusion (n = 6). The antagonists remained present throughout reperfusion. Control bars (means ± S.E.M.; n = 6) represent basal release of CGRP and substance P preceding ischemia/reperfusion. *, P < 0.05, **, P < 0.01, from control by one-way ANOVA followed by Dunnett's post-test analysis.
Fig. 6.
Combined CGRP- and NK1-receptor blockade exerts cardioprotective, anti-RAS effects in ex vivo guinea pig hearts subjected to ischemia/reperfusion. Coronary overflow of renin (C) and NE (B) and duration of reperfusion arrhythmias (VT/VF) (A) in isolated guinea pig hearts subjected to ischemia/reperfusion (i.e., 20-min global ischemia + 30-min reperfusion; n = 6). Other hearts were subjected to ischemia/reperfusion preceded by 10-min perfusion with both CGRP and substance P (SP) NK1- receptor antagonists (i.e., CGRP8–37 and CP99994, each at 100 nM; n = 4). Both antagonists remained present throughout the period of reperfusion. Bars are means (± S.E.M.) of renin and NE overflows during the first 6 min of reperfusion and duration of VT/VF. * and **, P < 0.05 and P < 0.01, respectively, from ischemia/reperfusion control by ANOVA followed by Dunnett's multiple comparisons test.
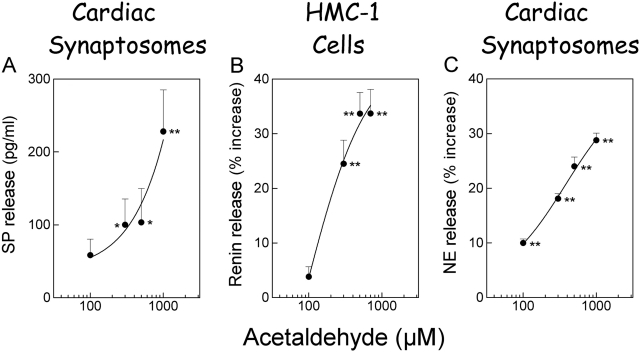
Toxic Aldehydes Generated in Ischemia/Reperfusion Elicit the Release of Substance P from Sensory Nerves, Renin from Mast Cells, and NE from Sympathetic Nerves.
Notably, the amounts of neuropeptides (i.e., substance P and CGRP) released by ischemia/reperfusion in the isolated heart were comparable to the amounts released by capsaicin (compare Fig. 2, A and B, with 5, A and C). However, the amounts of renin and NE released during ischemia/reperfusion were much higher than those released by capsaicin (compare Fig. 3 with 6). This suggested the involvement in ischemia/reperfusion of factors releasing renin and NE directly from mast cells and sympathetic nerve endings, respectively, in addition to a neuropeptide-releasing action on sensory nerve terminals. Accordingly, we tested whether acetaldehyde, one of the toxic aldehydes known to be produced in ischemia/reperfusion (Deyl and Miksik, 1994), might release not only neuropeptides from sensory nerves but also renin from mast cells and NE from sympathetic nerves. We found that acetaldehyde, at concentrations known to be reached in the ischemic/reperfused heart (Cordis et al., 1994), elicited an approximate 4-fold increase in substance P release and an approximate 10 to 30% increase in NE release from cardiac synaptosomes (Fig. 7, A and C), a preparation containing both sensory and sympathetic nerve endings (Seyedi et al., 1999). Acetaldehyde also elicited a 25 to 35% increase in renin release from cultured mast cells (Fig. 7B). Thus, it is likely that, in the context of ischemia/reperfusion, oxygen radicals and toxic aldehydes contribute in various ways to the release of renin and NE, ultimately causing severe arrhythmic dysfunction.
Fig. 7.
Toxic aldehydes elicit the release of substance P (SP) and norepinephrine from cardiac synaptosomes and renin from HMC-1 cells. A, substance P released from cardiac synaptosomes incubated with acetaldehyde for 10 min. Points represent means ± S.E.M. (n = 4–7). Basal release of substance P was 16.36 pg/ml ± 8.92, n = 11). B, renin release from HMC-1 cells incubated with acetaldehyde for 10 min. Points represent mean percentage increase (n = 5–18) above basal renin activity (i.e., 130 pg/h/mg protein ± 17.5; n = 14). C, mean percentage increase in NE release above a basal level of 1.62 ± 0.043 pmol/g in cardiac synaptosomes incubated with acetaldehyde for 10 min (mean ± S.E.M.; n = 30). *, P < 0.05, **, P < 0.01, from control by one-way ANOVA followed by Dunnett's post-test analysis.
Discussion
Our results identify an important interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion that causes the activation of a local cardiac RAS, which culminates in severe arrhythmic dysfunction. This conclusion is based on the following morphological and pharmacological findings. 1) Sensory nerves expressing substance P are juxtaposed to renin-containing cardiac mast cells. 2) Chemical stimulation of sensory C-fibers with capsaicin in the Langendorff-perfused guinea pig heart induces the release of CGRP and substance P. 3) The release of CGRP and substance P is accompanied by an increase in the overflow of renin and NE. 4) The capsaicin-induced release of renin and NE is prevented by mast cell stabilization, by blockade of substance P and CGRP receptors, and by activation of histamine H3-receptors. 5) AT1-receptor blockade prevents the capsaicin-induced release of NE but not the release of renin. 6) Substance P causes degranulation of human mast cells in culture and elicits renin release, an action prevented by NK1-receptor blockade. 7) Ischemia/reperfusion in isolated guinea pig hearts elicits the release of substance P and CGRP, which is accompanied by an increase in the overflow of renin and NE and by sustained reperfusion arrhythmias. 8) Blockade of NK1- and CGRP-receptors markedly reduces the release of renin and NE in the isolated guinea pig heart subjected to ischemia/reperfusion and greatly shortens the duration of reperfusion arrhythmias, without affecting the release of substance P and CGRP.
We had previously demonstrated the presence of renin in cardiac mast cells and its release in active form, either by chemical stimulation with compound 48/80 or by exposing the heart to ischemia/reperfusion (Silver et al., 2004; Mackins et al., 2006). Once released, mast cell-derived renin acts on angiotensinogen present in the cardiac interstitial fluid to form ANG I, which is then converted to ANG II by interstitial angiotensin-converting enzyme (Mackins et al., 2006). ANG II activates AT1-receptors on sympathetic nerve endings eliciting the release of NE, which amplifies the arrhythmogenic effects of ANG II (Mackins et al., 2006).
Oxidative stress during ischemia/reperfusion is likely to stimulate cardiac sensory C-fibers (Fu and Longhurst, 2009), thus releasing neuropeptides such as CGRP and substance P (Chiao and Caldwell, 1996; Källner et al., 1998), which are known to activate mast cells (Lorenz et al., 1998) (see Fig. 4). Because cardiac mast cells contain renin (Silver et al., 2004) that can be released in active form in ischemia/reperfusion (Mackins et al., 2006) and cardiac mast cells are closely associated with neuronal terminals (Silver et al., 2004), we hypothesized that neuropeptides released from sensory C-fibers could play a role in the release of mast cell renin in ischemia/reperfusion and the consequent activation of a local RAS. We found that when the release of neuropeptides was prevented by activating heteroinhibitory histamine H3-receptors on sensory C-fiber endings (Imamura et al., 1996), chemical stimulation of sensory C-fibers with capsaicin failed to cause a surge in renin overflow into the coronary effluent. Likewise, stabilization of cardiac mast cells with cromolyn also prevented the increase in renin overflow. Hence, it appears that stimulation of sensory fibers in turn results in mast cell degranulation. Neuropeptides, such as CGRP and substance P, are probably the means by which sensory fibers communicate with mast cells. Indeed, the neuropeptide-releasing effect of capsaicin, which we previously found to be inhibited by H3-receptor activation (Imamura et al., 1996), is associated with the release of cardiac renin, an effect again blocked by H3-receptor activation. Notably, the capsaicin-induced release of renin was prevented not only by pharmacological mast cell stabilization with cromolyn but also by precluding the effects of CGRP and substance P via blockade of their receptors. Collectively, these findings indicate that the release of cardiac renin via sensory C-fiber stimulation entails an intermediate step, represented by renin-containing mast cells degranulated by C-fiber-derived neuropeptides. This notion is supported by the finding that substance P elicited degranulation of isolated mast cells in culture that was associated with renin release and was prevented by substance P receptor blockade.
Chemical stimulation of cardiac sensory C-fibers with capsaicin resulted also in the release of NE. We had previously demonstrated that neuropeptides liberated by stimulation of sensory nerves can elicit the release of NE from cardiac sympathetic nerves by activating neuronal CGRP and NK1 receptors (Seyedi et al., 1999). We find now that an additional pathway initiated by stimulation of sensory nerves is likely to play an important role in the release of NE. This notion is supported by the finding that the selective AT1-receptor antagonist EXP3174 inhibited the release of NE associated with capsaicin-induced stimulation of sensory nerves, implicating an ANG II-mediated component in this effect. Previous findings indicate that ANG II is formed when a local cardiac RAS is activated upon release of renin from mast cells (Mackins et al., 2006; Veerappan et al., 2008), as was the case here when renin was released by neuropeptides liberated by capsaicin from sensory nerves. Indeed, EXP3174 blocked the release of NE but not that of renin, indicating that ANG II was formed by mast cell-derived renin; however, EXP3174 prevented the downstream activation of AT1 receptors and, thus, NE release from sympathetic nerves. In contrast, pharmacologic blockade of neuropeptide receptors on mast cells prevented both renin and NE release, because this upstream effect prevented mast cell degranulation and, thus, RAS activation.
ROS and toxic aldehydes generated during ischemia/reperfusion (Vanden Hoek et al., 1996, 1998; Eaton et al., 1999) are known to elicit substance P and CGRP release from sensory nerves (Chiao and Caldwell, 1996; Källner et al., 1998; Trevisani et al., 2007). Having established that chemical stimulation of sensory C-fibers with capsaicin elicits the release of CGRP and substance P from sensory nerves, which then promote mast cell renin release and the activation of a local RAS, we next questioned whether the same series of events would occur in ischemia/reperfusion. We found that the overflow of CGRP and substance P was markedly increased at reperfusion after a period of ischemia, and this was associated with an approximate 15- and 100-fold increase in renin and NE overflow, respectively, and sustained VT/VF. Notably, blockade of CGRP- and NK1-receptors with CGRP8–37 and CP99994 markedly reduced the overflow of renin and NE and significantly shortened the duration of VT/VF in the ischemia/reperfusion hearts. However, CGRP- and substance P-receptor antagonists in combination did not modify the release of CGRP and substance P, clearly demonstrating that the protective effect of the neuropeptide antagonists resulted from blockade of the effects of peptides at their receptor sites rather than a modulation of their release. We had previously demonstrated that, similar to the effect of neuropeptide receptor blockade seen here, mast cell stabilization inhibits renin and NE release and curtails reperfusion arrhythmias in the same ischemia/reperfusion model (Mackins et al., 2006). Thus, it is very likely that the cardioprotective effects of neuropeptide antagonists resulted from an inhibition of mast cell degranulation similar to that seen with cromolyn in the ex vivo guinea pig heart.
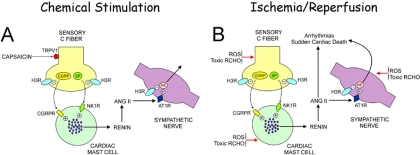
Based on the findings of the present study and previous evidence from other laboratories (Chiao and Caldwell, 1996; Trevisani et al., 2007), we propose that analogous to chemical stimulation with capsaicin (Fig. 8A), ROS and reactive aldehydes generated during ischemia/reperfusion evoke neuropeptide release from sensory C-fibers (Fig. 8B). These neuropeptides activate CGRP- and NK1-receptors on adjacent mast cells, evoking their degranulation and thus renin release, RAS activation, and local ANG II formation. ANG II then activates AT1-receptors on sympathetic nerve terminals eliciting NE release. Sudden cardiac death-type arrhythmias are generated by the combined arrhythmogenic actions of NE and ANG II. The fact that VT/VF was seen only at reperfusion of ischemic hearts, but not in hearts administered capsaicin, is most likely related to the fact that hearts subjected to ischemia/reperfusion released approximately 5- and 2.5-fold more renin and NE, respectively, than capsaicin-treated hearts. We interpret these findings as an indication that ROS and toxic aldehydes generated in ischemia/reperfusion, in addition to releasing mast cell-degranulating neuropeptides from sensory nerves (Chiao and Caldwell, 1996; Källner et al., 1998; Trevisani et al., 2007) as capsaicin does, degranulate mast cell directly (Henderson et al., 1980; Mannaioni et al., 1988; Yoshimaru et al., 2006) and also evoke NE release from sympathetic nerve terminals (Chahine et al., 1991), as demonstrated in Fig. 7. It is also important to consider that ANG II (Weiss et al., 2001; Raizada et al., 2007) and monoamine oxidases (Kaludercic et al., 2010) represent additional sources of ROS and thus may contribute to the overall release of neuropeptides, renin, and NE.
Fig. 8.
Proposed functional link between sensory C-fibers and cardiac mast cells and its role in renin release and local RAS activation. A, chemical stimulation of cardiac sensory C-fibers with capsaicin elicits the release of neuropeptides (CGRP and substance P), which activate their respective receptors on cardiac mast cells, sequentially promoting their degranulation, renin release, RAS activation, ANG II formation, stimulation of AT1-receptors on sympathetic nerves, and thus, NE release. B, similarly to capsaicin, ROS and toxic aldehydes (RCHO) generated during ischemia/reperfusion act upon sensory C-fibers, eliciting the release of neuropeptides that in turn causes degranulation of juxtaposed mast cells, evoking the release of renin, RAS activation, ANG II formation, and NE release from sympathetic nerves. ANG II and NE contribute to the generation of sudden cardiac death-type arrhythmias. ROS and RCHO can further amplify the arrhythmogenic threat by directly eliciting renin and NE release from mast cells and sympathetic nerve terminals, respectively.
Interestingly, infusion of exogenous CGRP in dogs results in a positive inotropic effect attributable to the accumulation of cardiac interstitial NE (Katori et al., 2005). In light of our findings, it is conceivable that by eliciting the release of mast cell renin, CGRP activated a local RAS leading to an ANG II-induced release of NE, its accumulation in the interstitium, and the development of a positive inotropic response. On the other hand, it is also plausible that by activating mast cells, neuropeptides may contribute to the onset and/or progression of heart failure (Stewart et al., 2003; Reid et al., 2007).
In conclusion, we present data supporting the hypothesis that an important link exists in the heart between sensory C-fibers and renin-containing mast cells. In ischemia/reperfusion, toxic aldehydes and ROS elicit the release of mast cell-degranulating neuropeptides from sensory nerves, thus provoking renin release and activating a local cardiac RAS. This ultimately produces ANG II, which potentiates NE release, leading to potentially lethal arrhythmias. The deleterious effects deriving from the activation of this novel pathway can potentially be enhanced by the direct mast cell-degranulating and NE-releasing effects of ROS and toxic aldehydes generated in ischemia/reperfusion (see Fig. 8B). Furthermore, by activating mast cells, neuropeptides may contribute to the process of remodeling in heart failure. Hence, the translational aspects of these findings include the prospective beneficial effects derived from targeting the sensory nerve–mast cell link and its pathophysiological consequences.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL34415, HL73400, T32-HL007423].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172262.
- RAS
- renin-angiotensin system
- ANG I
- angiotensin I
- ANG II
- angiotensin II
- HBS
- HEPES-buffered saline solution
- HMC-1
- human mastocytoma cell line
- NE
- norepinephrine
- ROS
- reactive oxygen species
- VT/VF
- ventricular tachycardia/ventricular fibrillation
- ANOVA
- analysis of variance
- CGRP
- calcitonin gene-related peptide
- CP99994
- (+)-(2S,3S)-3-(2-methoxybenzylamino)-2-phenylpiperidine)
- EXP3174
- 2-n-butyl-4-chloro-1-((2′-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)imidazole-5-carboxylic acid.
References
- Bader M. (2002) Role of the local renin-angiotensin system in cardiac damage: a minireview focussing on transgenic animal models. J Mol Cell Cardiol 34:1455–1462 [DOI] [PubMed] [Google Scholar]
- Barlucchi L, Leri A, Dostal DE, Fiordaliso F, Tada H, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. (2001) Canine ventricular myocytes possess a renin-angiotensin system that is upregulated with heart failure. Circ Res 88:298–304 [DOI] [PubMed] [Google Scholar]
- Bauer O, Razin E. (2000) Mast cell-nerve interactions. News Physiol Sci 15:213–218 [DOI] [PubMed] [Google Scholar]
- Camici PG, Pagani M. (2006) Cardiac nociception. Circulation 114:2309–2312 [DOI] [PubMed] [Google Scholar]
- Campbell DJ. (1987) Circulating and tissue angiotensin systems. J Clin Invest 79:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Siragy HM. (2003) Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24:261–271 [DOI] [PubMed] [Google Scholar]
- Chahine R, Chen X, Yamaguchi N, de Champlain J, Nadeau R. (1991) Myocardial dysfunction and norepinephrine release in the isolated rat heart injured by electrolysis-induced oxygen free radicals. J Mol Cell Cardiol 23:279–286 [DOI] [PubMed] [Google Scholar]
- Chiao H, Caldwell RW. (1996) The role of substance P in myocardial dysfunction during ischemia and reperfusion. Naunyn Schmiedebergs Arch Pharmacol 353:400–407 [DOI] [PubMed] [Google Scholar]
- Cordis GA, Bagchi D, Maulik N, Das DK. (1994) High-performance liquid chromatographic method for the simultaneous detection of malonaldehyde, acetaldehyde, formaldehyde, acetone and propionaldehyde to monitor the oxidative stress in heart. J Chromatogr A 661:181–191 [DOI] [PubMed] [Google Scholar]
- Deyl Z, Miksík I. (1994) Age- and feeding-dependent production of carbonyl compounds in hypoxic heart. The role of carbonyls produced in connective tissue modification. Mech Ageing Dev 73:47–55 [DOI] [PubMed] [Google Scholar]
- Domeij S, Dahlqvist A, Eriksson A, Forsgren S. (1996) Similar distribution of mast cells and substance P- and calcitonin gene-related peptide-immunoreactive nerve fibers in the adult human larynx. Ann Otol Rhinol Laryngol 105:825–831 [DOI] [PubMed] [Google Scholar]
- Dostal DE, Baker KM. (1999) The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res 85:643–650 [DOI] [PubMed] [Google Scholar]
- Dzau VJ. (1987) Implications of local angiotensin production in cardiovascular physiology and pharmacology. Am J Cardiol 59:59A–65A [DOI] [PubMed] [Google Scholar]
- Eaton P, Li JM, Hearse DJ, Shattock MJ. (1999) Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol Heart Circ Physiol 276:H935–H943 [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A. (1988) Calcitonin gene-related peptide and tachykinins in relation to local sensory control of cardiac contractility and coronary vascular tone. Acta Physiol Scand Suppl 569:1–63 [PubMed] [Google Scholar]
- Fu LW, Longhurst JC. (2009) Regulation of cardiac afferent excitability in ischemia. Handb Exp Pharmacol 194:185–225 [DOI] [PubMed] [Google Scholar]
- Henderson WR, Chi EY, Klebanoff SJ. (1980) Eosinophil peroxidase-induced mast cell secretion. J Exp Med 152:265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fukuda K. (2009) Cardiac innervation and sudden cardiac death. Curr Cardiol Rev 5:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M, Smith NC, Garbarg M, Levi R. (1996) Histamine H3-receptor-mediated inhibition of calcitonin gene-related peptide release from cardiac C fibers. A regulatory negative-feedback loop. Circ Res 78:863–869 [DOI] [PubMed] [Google Scholar]
- Källner G, Gonon A, Franco-Cereceda A. (1998) Calcitonin gene-related peptide in myocardial ischaemia and reperfusion in the pig. Cardiovasc Res 38:493–499 [DOI] [PubMed] [Google Scholar]
- Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, et al. (2010) Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano S, Tyler E, Salazar-Rodriguez M, Estephan R, Mackins CJ, Veerappan A, Reid AC, Silver RB, Levi R. (2008) Immediate hypersensitivity elicits renin release from cardiac mast cells. Int Arch Allergy Immunol 146:71–75 [DOI] [PubMed] [Google Scholar]
- Katori T, Hoover DB, Ardell JL, Helm RH, Belardi DF, Tocchetti CG, Forfia PR, Kass DA, Paolocci N. (2005) Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res 96:234–243 [DOI] [PubMed] [Google Scholar]
- Keith IM, Jin J, Saban R. (1995) Nerve-mast cell interaction in normal guinea pig urinary bladder. J Comp Neurol 363:28–36 [DOI] [PubMed] [Google Scholar]
- Koda K, Salazar-Rodriguez M, Corti F, Chan NY-K, Estephan R, Silver RB, Mochly-Rosen D, Levi R. (2010) Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation 122:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine P, Naukkarinen A, Heikkilä L, Penttilä A, Kovanen PT. (2000) Adventitial mast cells connect with sensory nerve fibers in atherosclerotic coronary arteries. Circulation 101:1665–1669 [DOI] [PubMed] [Google Scholar]
- Lorenz D, Wiesner B, Zipper J, Winkler A, Krause E, Beyermann M, Lindau M, Bienert M. (1998) Mechanism of peptide-induced mast cell degranulation. Translocation and patch-clamp studies. J Gen Physiol 112:577–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackins CJ, Kano S, Seyedi N, Schäfer U, Reid AC, Machida T, Silver RB, Levi R. (2006) Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 116:1063–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaioni PF, Giannella E, Palmerani B, Pistelli A, Gambassi F, Bani-Sacchi T, Bianchi S, Masini E. (1988) Free radicals as endogenous histamine releasers. Agents Actions 23:129–142 [DOI] [PubMed] [Google Scholar]
- Peach MJ. (1977) Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 57:313–370 [DOI] [PubMed] [Google Scholar]
- Raizada V, Skipper B, Luo W, Griffith J. (2007) Intracardiac and intrarenal renin-angiotensin systems: mechanisms of cardiovascular and renal effects. J Investig Med 55:341–359 [DOI] [PubMed] [Google Scholar]
- Reid AC, Silver RB, Levi R. (2007) Renin: at the heart of the mast cell. Immunol Rev 217:123–140 [DOI] [PubMed] [Google Scholar]
- Schwartz LB, Austen KF, Wasserman SI. (1979) Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J Immunol 123:1445–1450 [PubMed] [Google Scholar]
- Seyedi N, Mackins CJ, Machida T, Reid AC, Silver RB, Levi R. (2005) Histamine H3-receptor-induced attenuation of norepinephrine exocytosis: a decreased protein kinase a activity mediates a reduction in intracellular calcium. J Pharmacol Exp Ther 312:272–280 [DOI] [PubMed] [Google Scholar]
- Seyedi N, Maruyama R, Levi R. (1999) Bradykinin activates a cross-signaling pathway between sensory and adrenergic nerve endings in the heart: a novel mechanism of ischemic norepinephrine release? J Pharmacol Exp Ther 290:656–663 [PubMed] [Google Scholar]
- Seyedi N, Win T, Lander HM, Levi R. (1997) Bradykinin B2-receptor activation augments norepinephrine exocytosis from cardiac sympathetic nerve endings. Mediation by autocrine/paracrine mechanisms. Circ Res 81:774–784 [DOI] [PubMed] [Google Scholar]
- Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R. (2004) Mast cells: a unique source of renin. Proc Natl Acad Sci USA 101:13607–13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JA, Jr., Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell'Italia LJ. (2003) Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol 35:311–319 [DOI] [PubMed] [Google Scholar]
- Tharp MD, Seelig LL, Jr., Tigelaar RE, Bergstresser PR. (1985) Conjugated avidin binds to mast cell granules. J Histochem Cytochem 33:27–32 [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, et al. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. PNAS 104:13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. (1998) Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273:18092–18098 [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Shao ZH, Li CQ, Zak R, Schumacker PT, Becker LB. (1996) Reperfusion injury in cardiac myocytes after simulated ischemia. Am J Physiol Heart Circ Physiol 270:H1334–H1341 [DOI] [PubMed] [Google Scholar]
- Veerappan A, Reid AC, Estephan R, O'Connor N, Thadani-Mulero M, Salazar-Rodriguez M, Levi R, Silver RB. (2008) Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci USA 105:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DW. (1988) The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res 22:447–455 [DOI] [PubMed] [Google Scholar]
- Weiss D, Sorescu D, Taylor WR. (2001) Angiotensin II and atherosclerosis. Am J Cardiol 87 (Suppl 1):25C–32C [DOI] [PubMed] [Google Scholar]
- Yoshimaru T, Suzuki Y, Inoue T, Niide O, Ra C. (2006) Silver activates mast cells through reactive oxygen species production and a thiol-sensitive store-independent Ca2+ influx. Free Radic Biol Med 40:1949–1959 [DOI] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Chen SR, Pan HL. (2003) Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol 551:515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]