Abstract
The anthracycline doxorubicin (Dox) is an effective antitumor agent. However, its use is limited because of its toxicity in the heart. N-Benzyladriamycin-14-valerate (AD 198) is a modified anthracycline with antitumor efficacy similar to that of Dox, but with significantly less cardiotoxicity and potentially cardioprotective elements. In the present study, we investigated the possibility of in vivo protective effects of low-dose AD 198 against Dox-induced cardiomyopathy. To do this, rats were divided into four groups: vehicle, Dox (20 mg/kg; single injection day 1), AD 198 (0.3 mg/kg per injection; injections on days 1, 2, and 3), or a combination treatment of Dox + AD 198. Seventy-two hours after beginning treatment, hearts from the Dox group had decreased phosphorylation of AMP kinase and troponin I and reduced poly(ADP-ribose) polymerase, β-tubulin, and serum albumin expression. Dox also increased the phosphorylation of phospholamban and expression of inducible nitric-oxide synthase in hearts. Each of these Dox-induced molecular changes was attenuated in the Dox + AD 198 group. In addition, excised hearts from rats treated with Dox had a 25% decrease in left ventricular developed pressure (LVDP) and a higher than normal increase in LVDP when perfused with a high extracellular Ca2+ solution. The Dox-induced decrease in baseline LVDP and hyper-responsiveness to [Ca2+] was not observed in hearts from the Dox + AD 198 group. Thus Dox, with well established and efficient antitumor protocols, in combination with low levels of AD 198, to counter anthracycline cardiotoxicity, may be a promising next step in chemotherapy.
Introduction
The anthracycline doxorubicin (Dox) is a highly effective antineoplastic drug used against many cancers. However, the cumulative dosage of Dox in clinical situations is limited because of serious cardiotoxic side effects. For example, in phase III clinical trials the frequency of congestive heart failure escalated in a dose-dependent manner with an observed 5% frequency at a cumulative anthracylcine dose of 400 mg/m2 body surface area, 26% at 550 mg/m2, and 48% at 700 mg/m2 (Swain et al., 2003). Furthermore, less obvious effects occur with exposure to anthracyclines. For example, chronic left ventricular dysfunction was detected in more than 50% of asymptomatic patients treated with Dox (Gottdiener et al., 1981). These subclinical effects contribute to an inability of the heart to adapt to existing or subsequent cardiovascular disease (Priebe, 2000; Schultz et al., 2003). Because of this cardiotoxicity, a number of therapeutic strategies have been explored to decrease Dox-induced damage to the heart. One such strategy is the use of anthracycline congeners with reduced cardiotoxic potential (Frishman et al., 1996). One promising modified anthracycline we have studied is N-benzyladriamycin-14-valerate, referred to as AD 198.
AD 198 was initially developed to circumvent mechanisms of multidrug resistance that impede successful treatment of cancer (Lothstein et al., 1998; Barrett et al., 2002; Bilyeu et al., 2004). Thus far it has been demonstrated that AD 198 overcomes drug resistance of certain tumors and has comparable tumor cytotoxicity to Dox both in vitro and in vivo (Sweatman and Israel, 1996). Furthermore, AD 198 does not have the very serious cardiotoxic effect of standard anthracyclines and even seems to protect the heart from ischemic-reperfusion injury (Hofmann et al., 2007). These noncardiotoxic and cardioprotective traits are probably caused by the ability of AD 198 to induce activation of PKC-ε (Hofmann et al., 2007). In the present study, we wanted to expand on those studies, determine whether cotherapy of AD 198 + Dox protects the heart from Dox-induced cardiotoxicity, and identify molecular factors that may contribute to any cardioprotective effects of AD 198. The impetus for such a study was the hope that using low-dose AD 198 to counter anthracycline toxicity would prove to be of such apparent benefit that Dox + AD 198 would become the standard of care in treatment for a wide range of cancers.
Materials and Methods
Chemicals and Reagents.
Dox was purchased from Sigma-Aldrich (St. Louis, MO), and AD 198 hydrochloride salt was prepared according to previously described procedures (Lothstein et al., 1998). Dox was formulated in sterile saline, whereas AD 198 was formulated in ethanol and polyethylene glycol. The chemical structure of AD 198 can be found in Hofmann et al., 2007.
Primary antibodies used were phosphorylated AMP kinase (P-AMPK; Cell Signaling Technology, Danvers, MA; 1:1000 dilution), AMP kinase (AMPK; Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:500 dilution), brain creatine kinase (BCK; Santa Cruz Biotechnology, Inc.; 1:500 dilution), inducible nitric-oxide synthase (iNOS; Santa Cruz Biotechnology, Inc.; 1:500 dilution), poly(ADP-ribose) polymerase (PARP; Cell Signaling Technology; 1:1000 dilution), phosphorylated troponin I (P-TnI; Cell Signaling Technology; 1:2000 dilution), TnI (Santa Cruz Biotechnology, Inc.; 1:2000 dilution), β-myosin heavy chain (β-MHC; Millipore Bioscience Research Reagents, Temecula, CA; 1:1000 dilution), phosphorylated phospholamban (P-PLB; Millipore Corporation, Billerica, MA; 1:1000 dilution), PLB (Millipore Corporation; 1:1000 dilution), desmin (Sigma-Aldrich; 1:1000 dilution), and β-tubulin (Sigma-Aldrich; 1:2000 dilution).
In Vivo Animal Models.
All animal procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animal Care. Eight-week-old male Wistar rats were randomly divided into four groups: vehicle, Dox, Dox + AD 198, and AD 198. Dox was administered at a single dose of 20 mg/kg body weight. AD 198 was given in three doses for a total of 1 mg/kg body weight every 24 h over a 72-h period (Table 1). For AD 198 the final ethanol and polyethylene glycol levels were 1 and 2.4%, respectively, in a typical injection volume of 700 μl. All injections were done intraperitoneally.
TABLE 1.
In vivo treatment regime and general characteristics at time of sacrifice
| Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Body Weighta | Heart Weighta |
|---|---|---|---|---|---|---|
| g | g | |||||
| Vehicle | Vehicle for AD 198 | Vehicle for AD 198 | Vehicle for AD 198 | Sacrifice | 275 ± 14 | 0.76 ± 0.03 |
| Dox | 20 mg Dox/kg | Vehicle for AD 198 | Vehicle for AD 198 | Sacrifice | 244 ± 18 | 0.74 ± 0.04 |
| Vehicle for AD 198 | ||||||
| AD 198 | 0.33 mg AD 198/kg | 0.33 mg AD 198/kg | 0.33 mg AD 198/kg | Sacrifice | 287 ± 12 | 0.80 ± 0.01 |
| Dox + AD 198 | 20 mg Dox/kg | 0.33 mg AD 198/kg | 0.33 mg AD 198/kg | Sacrifice | 271 ± 17 | 0.74 ± 0.04 |
| 0.33 mg AD 198/kg |
Body and heart weights had 11 to 13 animals in each group.
Cardiac Myocytes.
Ventricular myocytes were isolated from untreated rats by using the procedure of Leary et al. (2008). Suspended cells were incubated for 20 min in the presence and absence of 1 μM myristoylated PKC-ε V1–2 peptide inhibitor (ENZO Life Science, Plymouth Meeting, PA). Subsequently, vehicle, Dox (1 μM) and/or AD 198 (0.1 μM) were added to the cell suspension. After 60 min Laemmli electrophoretic sample buffer with phosphatase inhibitor cocktail (Sigma-Aldrich) was added to stop any reactions and for cell storage.
Protein Preparation and Western Blots.
For whole animal/heart studies, at sacrifice hearts were minced and homogenized in a buffer containing 50 mM HEPES, 1 mM EDTA, 2 mM dithiothreitol, 10 nM okadaic acid, and 1% protease inhibitor cocktail. Homogenates were then incubated on ice for 30 min and centrifuged twice at 10,000g for 10 min. The supernatant was collected, and the protein concentration was determined by using the Bradford assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein (10 μg/gel lane) were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. For cardiac myocyte studies an equal amount of cells from each group was collected, run on SDS-PAGE, and transferred. Membranes were incubated with primary antibody, washed with Tris-buffered saline and Tween 20, and exposed to secondary peroxidase-coupled antibodies (1:8000 dilution). Signals were detected by using an enhanced chemiluminescence kit (Pierce Chemical, Rockford, IL).
Mass Spectrometry.
Mass spectrometry was done at the Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children's Research Hospital. In brief, after separation on SDS-PAGE the protein of interest was reduced and alkylated, and a tryptic digest was prepared. Mass spectrometric analysis was performed by using matrix-assisted laser desorption/ionization in conjunction with tandem time-of-flight mass analyzers and associated software (model 4700 proteomics analyzer; Applied Biosystems, Forest City, CA). Protein assignments were made on the mass spectrometry and the tandem mass spectrometry spectra with SWISS-Port used for protein identification.
Langendorff-Perfused Heart Preparations.
At sacrifice hearts were excised and cannulated in ice-cold modified Krebs-Henseleit (KH) buffer. KH buffer contained 118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 11 mM glucose, 25 mM NaHCO3, and 1.3 mM CaCl2, pH 7.4. Hearts were then mounted on a Langendorff apparatus and perfused with oxygenated KH buffer at 37°C. After left atriotomy, a cellophane balloon attached to a pressure transducer (BLPR; World Precision Instruments, Inc., Sarasota, FL) was inserted into the left ventricle and inflated to an initial end diastolic pressure (EDP) to maximize left ventricular developed pressure (LVDP). Hearts were paced with electrodes at 300 beats per minute, and pacing voltage was set at two times the threshold value. LVDP and EDP were measured continuously throughout the experiment. Each heart underwent an initial 10-min baseline equilibration (prehigh Ca2+ period) followed by a 45-min perfusion with KH buffer containing 3.5 mM CaCl2 (high Ca2+ period). After the high calcium perfusion, hearts were returned to KH buffer containing 1.3 mM Ca2+ for the final 10 min of perfusion. Prehigh Ca2+ and high Ca2+ LVDP values were averaged over a 1-min period at the end of their perfusion periods to provide values for data normalization.
Statistical Analysis.
Cumulative values are presented as mean ± standard error. Data were analyzed by analysis of variance and Student's t test. Statistical significance was assumed for p < 0.05.
Results
Protein Expression and Phosphorylation from In Vivo Animal Studies.
Body and heart weights were not statistically different between the animal treatment groups at the time of sacrifice (Table 1). However, it is interesting to note the trend in decreased body weight of Dox-treated animals compared with vehicle-treated animals. This is probably caused by the loss of appetite associated with chemotherapy. A trend toward a decrease in body weight is not seen in the comparison of Dox + AD 198-treated versus vehicle-treated animals. However, additional studies are needed to determine whether this is significant.
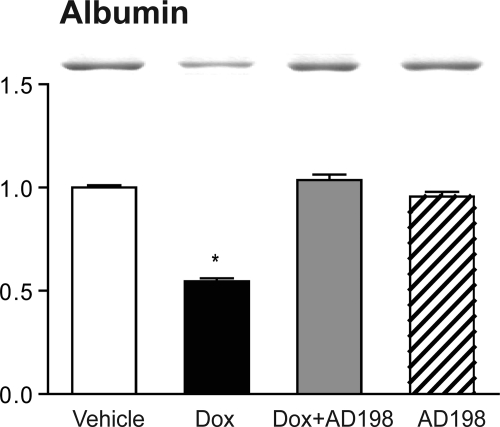
Cardiac lysates from the four treatment groups run on SDS-PAGE consistently showed a Dox-induced reduction in a band at approximately 65 to 70 kDa (data not shown). This protein was identified as serum albumin from rat, with a confidence interval of 100%, using matrix-assisted laser desorption/ionization. Serum albumin is present in our cardiac lysates because the minced hearts were only briefly rinsed of blood before homogenization, i.e., excised hearts did not undergo saline retrograde aortic perfusion to remove all blood. Subsequent analysis of whole-blood samples from the four groups demonstrated albumin expression levels were significantly decreased after Dox treatment, and coadministration of Dox + AD 198 attenuated this Dox-induced decrease in blood albumin (Fig. 1).
Fig. 1.
Effects of Dox and AD 198 on albumin expression. Shown are representative Coomassie blue-stained SDS-PAGE gels and cumulative analysis of albumin content in whole-blood samples. Values are expressed relative to vehicle and are the mean ± standard error for three rats. *, significance compared with vehicle.
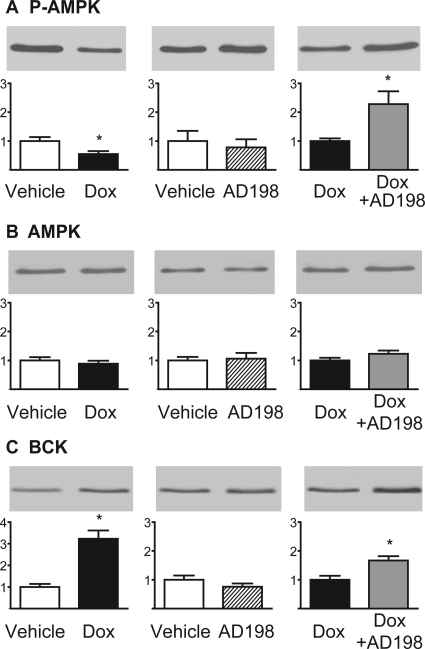
Cardiac lysates from the four treatment groups were used to measure P-AMPK, AMPK, and BCK (Fig. 2). These kinases have been associated with energy balance in the heart. Using the phosphosite-specific antibody against phospho-Thr172 of P-AMPK, we observed a 45% decrease in P-AMPK induced by Dox that was reversed by coadministration of AD 198. AMPK expression was not altered by any treatments. Dox also induced an approximate 3-fold increase in BCK, and the coadministration of Dox + AD 198 further increased BCK expression. AD 198 administered alone did not alter phosphorylation of AMPK or AMPK and BCK expression.
Fig. 2.
Effects of Dox and AD 198 on enzymes associated with energy balance in the heart. Shown are representative Western blots and cumulative data of P-AMPK (A), AMPK (B), and BCK (C) in myocardial lysates. Values are expressed relative to the first treatment in each pairing and are the mean ± standard error for three hearts. *, significance compared with the first treatment in each pairing.
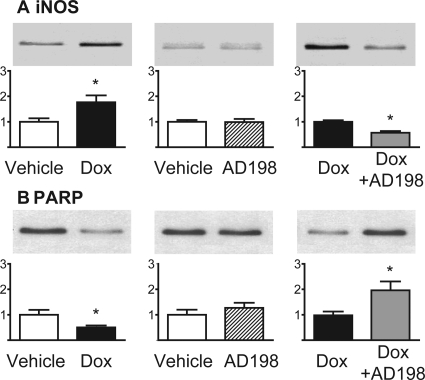
Inducible NOS and PARP content were also examined in cardiac lysates (Fig. 3). These enzymes have been associated with oxidative damage and apoptosis. Dox treatment markedly up-regulated myocardial expression of iNOS, and this effect was attenuated by AD 198 coadministration. Dox also induced a decrease in PARP, which was reversed by AD 198 coadministration. AD 198 alone did not alter iNOS or PARP content in cardiac lysates.
Fig. 3.
Effects of Dox and AD 198 on enzymes associated with oxidative damage and programmed cell death in the heart. Shown are representative Western blots and cumulative date of iNOS (A) and PARP (B) in myocardial lysates. Values are expressed relative to the first treatment in each pairing and are the mean ± standard error for three hearts. *, significance compared with the first treatment in each pairing.
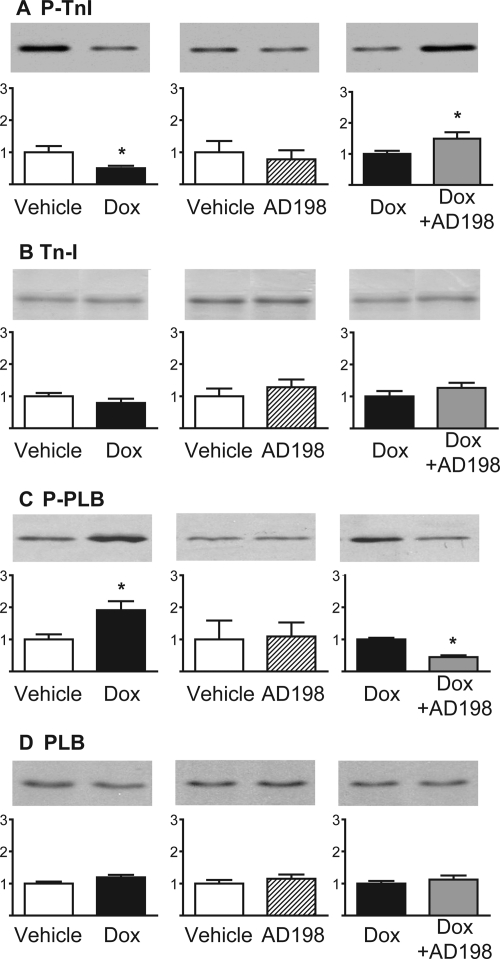
Expression levels and phosphorylation of TnI and PLB, proteins associated with Ca2+ homeostasis, were measured in cardiac lysates (Fig. 4). TnI and PLB expression did not change in any of the groups. However, P-TnI was reduced by Dox, and this effect was blocked and/or reversed by coadministration of AD 198. P-PLB was increased by Dox treatment, whereas Dox + AD 198 administration reversed this effect.
Fig. 4.
Effects of Dox and AD 198 on proteins associated with Ca2+ sensitivity and Ca2+ handling in the heart. Shown are representative Western blots and cumulative data of P-TnI (A), TnI (B), P-PLB (C), and PLB (D) in myocardial lysates. Values are expressed relative to the first treatment in each pairing and are the mean ± standard error for three hearts. *, significance compared with the first treatment in each pairing.
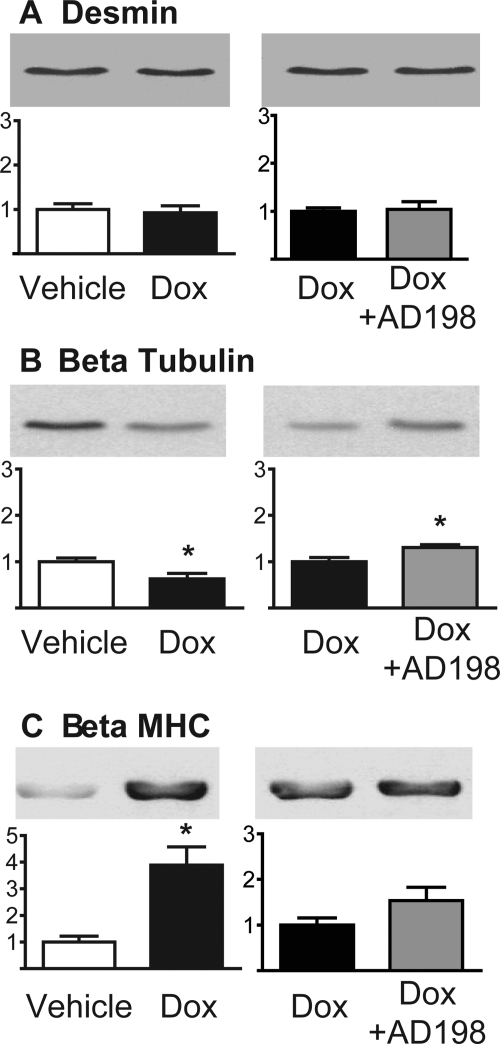
Cytoskeletal proteins, desmin and β-tubulin, and the contractile protein β-MHC were also examined in cardiac lysates. There was no change in desmin expression with Dox (Fig. 5). However, Dox induced a decrease in β-tubulin and an increase in β-MHC expression. The Dox-induced decrease in β-tubulin was attenuated by coadministration of AD 198, whereas coadministration of Dox + AD 198 did not alter the Dox-induced increase in β-MHC.
Fig. 5.
Effects of Dox and AD 198 on proteins associated with cytoskeletal structure and contractile function in the heart. Shown are representative Western blots and cumulative data of desmin (A), β-tubulin (B), and β-MHC (C) in myocardial lysates. Values are expressed relative to the first treatment in each pairing and are the mean ± standard error for three hearts. *, significance compared with the first treatment in each pairing.
PKC-ε Inhibition in Cardiac Myocytes.
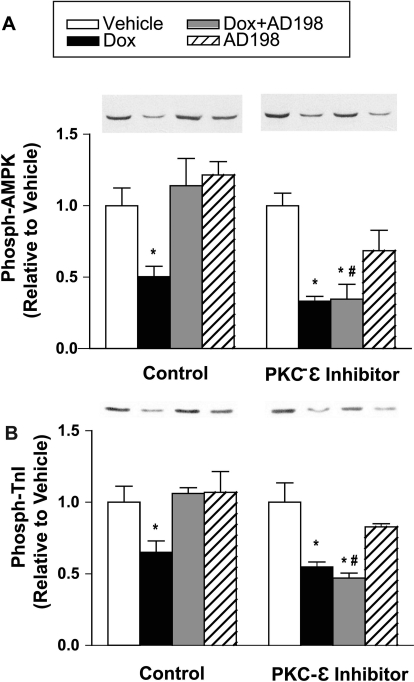
Isolated ventricular myocytes from untreated rats were preincubated in a saline solution with and without a PKC-ε peptide inhibitor, and then acutely exposed to vehicle, Dox, and/or AD 198. Expression levels of AMPK and TnI did not change in any of the groups (data not shown). In the absence of the PKC-ε inhibitor Dox induced a decrease in phosphorylation of AMPK and TnI that was blocked and/or reversed by coadministration of AD 198 (Fig. 6). These observations are similar to those seen in the in vivo studies (Figs. 2 and 4). With preincubation with the PKC-ε inhibitor Dox continued to reduce phosphorylation of AMPK and TnI, but coadministration of AD 198 did not attenuate the Dox effects (Fig. 6).
Fig. 6.
Effect of acute PKC-ε inhibition on the ability of AD 198 to reverse the Dox-induced decreases in phosphorylated AMPK and TnI. Isolated ventricular myocytes from untreated rats were preincubated in the presence and absence of a PKC-ε peptide inhibitor and exposed to Dox and/or AD 198. Shown are representative Western blots and cumulative data of phosphorylated AMPK (A) and TnI (B). Cumulative values are expressed relative to vehicle and are the mean ± standard error for three cell isolations. *, significance compared with vehicle; #, significance compared with AD 198 alone.
Myocardial Function from In Vivo Animal Studies.
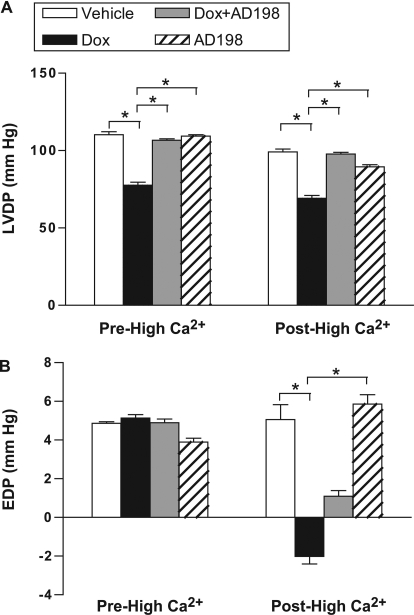
To determine whether AD 198 can restore myocardial functional effects induced by Dox in vivo, we measured LVDP and EDP in excised and perfused hearts of rats pretreated as shown in Table 1. Specifically, ex vivo hearts were subjected to a normal extracellular [Ca2+] for 10 min (prehigh Ca2+ of 1.3 mM), switched to a high [Ca2+] perfusion for 45 min (high Ca2+ of 3.5 mM), and returned to the normal extracellular [Ca2+] for 10 min (posthigh Ca2+ of 1.3 mM). A high [Ca2+] challenge was imposed to better quantify Dox-induced effects on Ca2+ homeostasis and uncover any effect coadministration of AD 198 had on Dox-induced changes in Ca2+ handling.
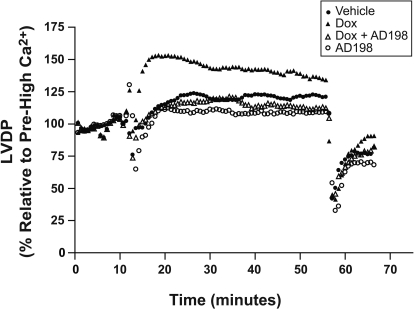
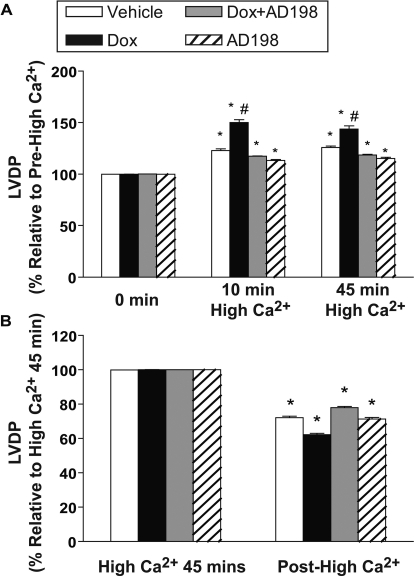
Before high-Ca2+ exposure LVDP was depressed in rats treated with Dox. This decrease was attenuated by coadministration of AD 198 (Fig. 6A). During the period of high [Ca2+] those hearts from rats pretreated with Dox demonstrated an exaggerated response to high extracellular Ca2+. This effect was attenuated by Dox + AD 198 pretreatment (Figs. 7 and 8A). After high [Ca2+], LVDP continued to be lower in hearts from Dox-treated rats (Fig. 6A), and all hearts showed a significant and similar reduction in relative LVDP posthigh [Ca2+] (Fig. 8B). It is noteworthy that EDP after posthigh [Ca2+] perfusion was significantly different between groups (Fig. 6B).
Fig. 7.
LVDP (mm Hg) and EDP in hearts from rats pretreated as indicated. Data are from hearts before a high [Ca2+] challenge (Pre-High Ca2+) and after the high [Ca2+] challenge (Post-High Ca2+). Values are means ± standard error; n = 10 per group.
Fig. 8.
Effects of Dox and AD 198 on relative LVDP in representative hearts from rats pretreated as indicated. Excised hearts were initially perfused for 10 min in a Krebs-Henseleit buffer containing 1.3 mM CaCl2 (Pre-High Ca2+), and then switched to Krebs-Henseleit containing 3.5 mM CaCl2 (high Ca2+). High Ca2+ perfusion of the heart continued for 45 min, and perfusion was then returned to Krebs-Henseleit containing 1.3 mM CaCl2 for the final 10 min (posthigh Ca2+). LVDP is expressed as percentage change from prehigh Ca2+ LVDP baseline.
Discussion
In the present study we found many, but not all, of the Dox-induced changes in select myocardial proteins were blocked or reversed by cotreatment with AD 198 (Table 2). AD 198 attenuation of Dox effects seems, in part, to be caused by its ability to activate PKC-ε. In addition, we found that both the Dox-induced 1) decrease in left ventricular developed pressure and 2) exaggerated responsiveness to Ca2+ overload in excised hearts were not present in hearts from rats that had been cotreated with AD 198 (Figs. 7–9). Thus, our studies support the idea that some of the underlying molecular and functional changes induced in hearts that have been exposed to Dox can be blocked or reversed by low-dose cotreatment with the modified anthracycline AD 198.
TABLE 2.
Summary of effects on myocardial proteins
| Marker | Doxa Hearts | [Dox + AD 198]b Hearts |
|---|---|---|
| P-AMPK | Decrease | Blocked/reversed* |
| AMPK | No change | No change |
| BCK | Increase | Additional increase |
| iNOS | Increase | Blocked/reversed |
| PARP | Decrease | Blocked/reversed |
| P-TnI | Decrease | Blocked/reversed* |
| TnI | No change | No change |
| P-PLB | Increase | Blocked/reversed |
| PLB | No change | No change |
| Desmin | No change | No change |
| β-Tubulin | Decrease | Blocked/reversed |
| β-MHC | Increase | Not blocked/reversed |
| Albumin | Decrease | Blocked/reversed |
Compared with avehicle or
Dox alone.
PKC-ε dependent mechanism implicated.
Fig. 9.
Relative LVDP in hearts from rats pretreated as indicated. Prehigh and posthigh Ca2+ used a Krebs-Henseleit buffer containing 1.3 mM CaCl2, whereas high Ca2+ was Krebs-Henseleit buffer containing 3.5 mM CaCl2. Data are expressed relative to the prehigh Ca2+ LVDP (A) and at high Ca2+ for 45 min LVDP (B). *, significance compared with the same treatment prehigh Ca2+ (A) or high Ca2+ for 45 min (B). #, significance comparing Dox with all other groups at that time point.
Albumin content was reduced in whole-blood samples of animals that were Dox-treated (Fig. 1). This effect has been documented previously (Iliskovic et al., 1998). Hypoalbuminemia is associated with reduced survival in patients with heart failure (Horwich et al., 2008). Mechanisms that may contribute to this include increased atrial fibrillation, a reduction in colloid oncotic pressure leading to pulmonary edema, and/or a reduction in the ability of albumin to be protective in an antioxidant or antiapoptic capacity (Horwich et al., 2008; Böhm et al., 2009). We found AD 198 cotreatment reversed the Dox effect on albumin concentration. This is consistent with the idea that cotreatment with low levels of AD 198 may protect hearts that have been exposed to Dox.
Dox also reduced AMPK phosphorylation and increased BCK expression (Fig. 2). This is consistent with observations by Tokarska-Schlattner et al. (2006). AMPK plays a key role as an energy sensor and regulator of energy substrate utilization (recently reviewed in Wong et al., 2009). Reduced phosphorylation of AMPK reduces ATP availability (Wong et al., 2009) and contributes to the negative energy balance seen in Dox-treated hearts. It has been hypothesized that increased expression of brain-type CK helps to maintain creatine kinase activity in the face of loss of muscle-type CK in the Dox-exposed heart (Tokarska-Schlattner et al., 2006). Thus, the Dox-induced increase in BCK expression may be a compensatory effect to maintain energy availability. We found AD 198 cotreatment prevented the Dox-induced decrease in phosphorylation of AMPK and further increased BCK expression. Both of these effects are consistent with the idea that cotreatment of Dox plus low levels of AD 198 would lead to hearts that have improved energy balance compared with hearts from animals that receive only Dox treatment.
Dox increased iNOS expression and decreased PARP content (Fig. 3). These observations are consistent with studies by Andreadou et al. (2007), Liu et al. (2006), and Zhu et al. (2009). The Dox-induced increase in iNOS would increase NO and increase the reaction of NO and superoxide anion that leads to the synthesis of peroxynitrate. Peroxynitrate is a potent cellular oxidant that damages the heart. Increased PARP cleavage, via caspases 3 and 7, is a marker of increased apoptosis (Gobeil et al., 2001). We found AD 198 cotreatment reduced the Dox-induced increase in iNOS and reduced PARP cleavage. Both of these effects are consistent with the idea that cotreatment of Dox plus low levels of AD 198 would protect hearts through reduced oxidative damage and a decreased rate of apoptosis compared with hearts from animals that receive only Dox treatment.
Dox decreased phosphorylation of TnI and increased phosphorylation of PLB (Fig. 4). The Dox-induced decrease in phosphorylation of TnI is consistent with the work of Maejima et al. (2008). Reduced TnI phosphorylation has been shown to increase the Ca2+ sensitivity of tension and may slow myocardial relaxation. On the other hand, increased phosphorylation of PLB would increase the rate of Ca2+ uptake of the Ca2+-ATPase on the sarcoplasmic reticulum. This would lead to an increase in the rate of relaxation and increased Ca2+ load within the sarcoplasmic reticulum. We found AD 198 cotreatment reversed the Dox effects such that phospho-TnI was increased and phospho-PLB decreased. Both of these effects are consistent with the idea that cotreatment of Dox plus low levels of AD 198 would produce hearts that have a Ca2+ sensitivity and Ca2+ handling close to that of hearts that have not been exposed to Dox.
Dox had no effect on desmin expression, but reduced β-tubulin levels and increased β-MHC content (Fig. 5). Previously, Dox has been shown to reduce desmin (Fisher et al., 2005) and β-tubulin (Dudnakova et al., 2003) and increase β-MHC (de Beer et al., 2002) content in the heart. The Dox-induced decrease in β-tubulin would alter microtubules that are 1) key to intracellular trafficking of proteins such as connexin 43 and the sodium channel (Casini et al., 2010; Smyth et al., 2010) and 2) have been implicated as mediators of stress/strain effects in the heart (Iribe et al., 2009). Microtubule disruption in the heart is associated with cardiac arrhythmias and altered Ca2+ handling (Iribe et al., 2009; Casini et al., 2010; Smyth et al., 2010). Increased β-MHC is consistent with the onset of a hypertrophic phenotype being induced by Dox. We found AD 198 cotreatment reduced the Dox-induced decrease in β-tubulin, but did not block/reverse the increase in β-MHC. The restoration of β-tubulin to more control-like levels is consistent with the idea that cotreatment of Dox plus low levels of AD 198 would produce hearts with protected microtubules compared with hearts from animals that received only Dox treatment. The failure of AD 198 to reverse the Dox-induced increase in β-MHC, at the time point studied, suggests Dox + AD 198 hearts are not fully protected or restored from Dox-induced cardiotoxic effects.
Dox also reduces left ventricular developed pressure in hearts perfused with solutions containing a physiologic level of extracellular Ca2+, and these same hearts had an exaggerated response to a perfusion solution containing high extracellular Ca2+ (Figs. 7–9). These effects were blocked and/or reversed by cotreatment of animals with AD 198. This supports a conclusion that AD 198 can attenuate Dox-induced functional changes in the heart.
Dox did not alter end diastolic pressure in hearts perfused with solutions containing a physiologic level of extracellular Ca2+. This is in contrast to data from human patients in which cumulative doses of Dox typically leads to an increase in end diastolic pressure. However, work by others suggests a Dox-induced increase in end diastolic pressure is a function of time such that at 4 weeks after Dox injection there is no change, whereas at 12 weeks after injection there is an increase in left ventricular diastole diameter (Teraoka et al., 2000). Thus, caution should be used comparing our results, using analysis at 3 days after injection from a single dose of Dox in rats, to humans. Furthermore, we should also note end diastolic pressure after high Ca2+ was reduced in the Dox and Dox + AD 198 groups. Although the mechanism and consequences for such an effect is not clear, it seems AD 198 does not reverse this Dox-induced effect.
Multiple mechanisms and targets of Dox contribute to its cardiotoxicity. However, underlying much of the Dox-induced toxicity is its ability to increase reactive oxygen species (ROS; Minotti et al., 2004). The chemical modifications of Dox that produce AD 198 creates a domain within AD 198 that has a structural similarity to diacyglycerol, an endogenous ligand for protein kinase C (Roaten et al., 2001). We have shown previously AD 198 binds to PKC at its diacyglycerol binding site (Roaten et al., 2002) and this activates PKC-ε in cardiomyocytes (Hofmann et al., 2007). PKC-ε activation is thought to be cardioprotective through its ability to: 1) prime sarcolemma KATP channels to reduce action potential duration and, as such, reduce Ca2+ entry and Ca2+ overload, 2) open mitochondrial KATP channels to prevent Ca2+ overload and volume overload of the mitochondria and reduced mitochondrial ROS production, 3) inhibit the mitochondrial permeability transition pore opening to inhibit mitochondrial swelling, cytochrome C release, and the uncoupling of oxidative phosphorylation, 4) activate protein phosphatase 1, which decreases phospho-PLB to decrease Ca2+ content and Ca2+ cycling, thus attenuating any rise in cytosolic [Ca2+], and 5) activate transcription factors that increase antioxidant expression to reduce ROS overload (Bolli et al., 2007; Budas et al., 2007). In a previous study we demonstrated that AD 198 protects from ischemia-reperfusion induced cardiac dysfunction, and this benefit is not observed in PKC-ε knockout mice (Hofmann et al., 2007). In the present study we show PKC-ε inhibition blocks the ability of AD 198 to reverse Dox-induced changes in phosphorylation of AMPK and TnI (Fig. 6). These data support an AD 198–PKC-ε-dependent protective pathway of action. However, other current findings, such as the AD 198-induced reversal in hypoalbuminemia (Fig. 1), are hard to envision as attributable to PKC-ε activation and require additional investigation. Studies are also needed to specifically look at Dox pharmacokinetic/distribution in the heart in the presence of AD 198. Although it is unlikely AD 198 is cardioprotective based solely on a mechanism in which AD 198 displaces Dox binding to targets given Dox, cytotoxicity of leukemia cells is not reduced by low doses of AD 198 (unpublished observation). Thus, one mechanism, but not necessarily the only mechanism, of AD 198 effects is through PKC-ε activation.
Finally, consideration of the doses used in the in vivo studies should be considered. A past study demonstrated a single 3 mg/kg dose of AD 198 in rats resulted in an ∼20% decrease in mean white blood cell (WBC) count, whereas a single 10 mg/kg dose of DOX produced an ∼85% decrease in mean WBC count (Sweatman et al., 1999). With both doses, the rat WBC count returned to normal levels. Thus in the present studies AD 198 is cardioprotective at a dose that has a limited hematotoxicity. This is therapeutically important in considering coadministration of both agents to protect from DOX-induced damage to the heart.
In summary, the present studies support the conclusion that in vivo cardiotoxicity induced by Dox can be substantially countered, molecularly and functionally, through cotherapy with the modified anthracycline AD 198. Much, but not all, of this cardioprotection is caused by the ability of AD 198 to activate PKC-ε. Given the extent and frequency of acute and chronic heart disease attributable to antitumor treatment with Dox, the combination therapy of Dox plus the cardioprotective anthracylcine AD 198 represents a promising next step in treatment for a wide range of cancers.
This work was supported by the American Heart Association [Grant 0855263E] (to P.A.H.); the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL-74001] (to R.R.M.); and the American Lebanese Syrian Associated Charities.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.167965.
- Dox
- doxorubicin
- AD 198
- N-benzyladriamycin-14-valerate
- LVDP
- left ventricular developed pressure
- EDP
- end diastolic pressure
- AMPK
- AMP kinase
- P-AMPK
- phosphorylated AMPK
- CK
- creatine kinase
- BCK
- brain CK
- iNOS
- inducible nitric-oxide synthase
- PARP
- poly(ADP-ribose) polymerase
- TnI
- troponin I
- P-TnI
- phosphorylated TnI
- β-MHC
- β-myosin heavy chain
- PLB
- phospholamban
- P-PLB
- phosphorylated PLB
- PKC
- protein kinase C
- PAGE
- polyacrylamide gel electrophoresis
- KH
- Krebs-Henseleit
- ROS
- reactive oxygen species
- WBC
- white blood cell.
References
- Andreadou I, Sigala F, Iliodromitis EK, Papaefthimiou M, Sigalas C, Aligiannis N, Savvari P, Gorgoulis V, Papalabros E, Kremastinos DT. (2007) Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol 42:549–558 [DOI] [PubMed] [Google Scholar]
- Barrett CM, Lewis FL, Roaten JB, Sweatman TW, Israel M, Cleveland JL, Lothstein L. (2002) Novel extranuclear-targeted anthracyclines override the antiapoptotic functions of Bcl-2 and target protein kinase C pathways to induce apoptosis. Mol Cancer Ther 1:469–481 [PubMed] [Google Scholar]
- Bilyeu JD, Panta GR, Cavin LG, Barrett CM, Turner EJ, Sweatman TW, Israel M, Lothstein L, Arsura M. (2004) Circumvention of nuclear factor κB-induced chemoresistance by cytoplasmic-targeted anthracyclines. Mol Pharmacol 65:1038–1047 [DOI] [PubMed] [Google Scholar]
- Böhm M, Thoenes M, Neuberger HR, Gräber S, Reil JC, Bramlage P, Volpe M. (2009) Atrial fibrillation and heart rate independently correlate to microalbuminuria in hypertensive patients. Eur Heart J 30:1364–1371 [DOI] [PubMed] [Google Scholar]
- Bolli R, Li QH, Tang XL, Guo Y, Xuan YT, Rokosh G, Dawn B. (2007) The late phase of preconditioning and its natural clinical application–gene therapy. Heart Fail Rev 12:189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Mochly-Rosen D. (2007) Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res 55:523–536 [DOI] [PubMed] [Google Scholar]
- Casini S, Tan HL, Demirayak I, Remme CA, Amin AS, Scicluna BP, Chatyan H, Ruijter JM, Bezzina CR, van Ginneken AC, et al. (2010) Tubulin polymerization modifies cardiac sodium channel expression and gating. Cardiovasc Res 85:691–700 [DOI] [PubMed] [Google Scholar]
- de Beer EL, Bottone AE, van Rijk MC, van der Velden J, Voest EE. (2002) Dexrazoxane pre-treatment protects skinned rat cardiac trabeculae against delayed doxorubicin-induced impairment of crossbridge kinetics. Br J Pharmacol 135:1707–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudnakova TV, Lakomkin VL, Tsyplenkova VG, Shekhonin BV, Shirinsky VP, Kapelko VI. (2003) Alterations in myocardial cytoskeletal and regulatory protein expression following a single doxorubicin injection. J Cardiovasc Pharmacol 41:788–794 [DOI] [PubMed] [Google Scholar]
- Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. (2005) Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111:1601–1610 [DOI] [PubMed] [Google Scholar]
- Frishman WH, Sung HM, Yee HC, Liu LL, Einzig AI, Dutcher J, Keefe D. (1996) Cardiovascular toxicity with cancer chemotherapy. Curr Probl Cardiol 21:225–286 [PubMed] [Google Scholar]
- Gobeil S, Boucher CC, Nadeau D, Poirier GG. (2001) Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ 8:588–594 [DOI] [PubMed] [Google Scholar]
- Gottdiener JS, Mathisen DJ, Borer JS, Bonow RO, Myers CE, Barr LH, Schwartz DE, Bacharach SL, Green MV, Rosenberg SA. (1981) Doxorubicin cardiotoxicity: assessment of late left ventricular dysfunction by radionuclide cineangiography. Ann Intern Med 94:430–435 [DOI] [PubMed] [Google Scholar]
- Hofmann PA, Israel M, Koseki Y, Laskin J, Gray J, Janik A, Sweatman TW, Lothstein L. (2007) N-benzyladriamycin-14-valerate (AD 198): a noncardiotoxic anthracycline that is cardioprotective through PKC-ε activation. J Pharmacol Exp Ther 323:658–664 [DOI] [PubMed] [Google Scholar]
- Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. (2008) Albumin levels predict survival in patients with systolic heart failure. Am Heart J 155:883–889 [DOI] [PubMed] [Google Scholar]
- Iliskovic N, Li T, Khaper N, Palace V, Singal PK. (1998) Modulation of adriamycin-induced changes in serum free fatty acids, albumin and cardiac oxidative stress. Mol Cell Biochem 188:161–166 [PubMed] [Google Scholar]
- Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, et al. (2009) Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res 104:787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary PJ, Rajasekaran S, Morrison RR, Tuomanen EI, Chin TK, Hofmann PA. (2008) A cardioprotective role for platelet-activating factor through NOS-dependent S-nitrosylation. Am J Physiol Heart Circ Physiol 294:H2775–H2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Li H, Qu H, Sun B. (2006) Nitric oxide synthase expressions in ADR-induced cardiomyopathy in rats. J Biochem Mol Biol 39:759–765 [DOI] [PubMed] [Google Scholar]
- Lothstein L, Rodrigues PJ, Sweatman TW, Israel M. (1998) Cytotoxicity and intracellular biotransformation of N-benzyladriamycin-14-valerate (AD 198) are modulated by changes in 14-O-acyl chain length. Anti-Cancer Drugs 9:58–66 [DOI] [PubMed] [Google Scholar]
- Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. (2008) Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell 7:125–136 [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229 [DOI] [PubMed] [Google Scholar]
- Priebe HJ. (2000) The aged cardiovascular risk patient. Br J Anaesth 85:763–778 [DOI] [PubMed] [Google Scholar]
- Roaten JB, Kazanietz MG, Caloca MJ, Bertics PJ, Lothstein L, Parrill AL, Israel M, Sweatman TW. (2002) Interaction of the novel anthracycline antitumor agent N-benzyladriamycin-14-valerate with the C1-regulatory domain of protein kinase C: structural requirements, isoform specificity, and correlation with drug cytotoxicity. Mol Cancer Ther 1:483–492 [PubMed] [Google Scholar]
- Roaten JB, Kazanietz MG, Sweatman TW, Lothstein L, Israel M, Parrill AL. (2001) Molecular models of by N-benzyladriamycin-14-valerate (AD 198) in complex with the phorbol ester-binding C1b domain of protein kinase C-δ. J Med Chem 44:1028–1034 [DOI] [PubMed] [Google Scholar]
- Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R. (2003) Health profiles in 5836 long-term cancer survivors. Int J Cancer 104:488–495 [DOI] [PubMed] [Google Scholar]
- Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. (2010) Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest 120:266–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Whaley FS, Ewer MS. (2003) Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97:2869–2879 [DOI] [PubMed] [Google Scholar]
- Sweatman TW, Israel M. (1996) Anthracyclines, in Cancer Therapeutics: Experimental and Clinical Agents (Teicher BA. ed) pp 113–136, Humana Press Inc, Totowa, NJ [Google Scholar]
- Sweatman TW, Seshadri R, Israel M. (1999) Pharmacology of N-benzyladriamycin-14-valerate in the rat. Cancer Chemother Pharmacol 43:419–426 [DOI] [PubMed] [Google Scholar]
- Teraoka K, Hirano M, Yamaguchi K, Yamashina A. (2000) Progressive cardiac dysfunction in adriamycin-induced cardiomyopathy rats. Eur J Heart Fail 2:373–378 [DOI] [PubMed] [Google Scholar]
- Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. (2006) New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol 41:389–405 [DOI] [PubMed] [Google Scholar]
- Wong AK, Howie J, Petrie JR, Lang CC. (2009) AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 116:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, Caldwell RL, Shou W, Field LJ. (2009) Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 119:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]