Abstract
Ethanol (EtOH) promotes GABAergic synaptic transmission in the central nervous system. We have shown that EtOH enhances the frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents less powerfully in hippocampal CA1 pyramidal neurons from adolescent animals compared with those from adults. However, we have also shown that EtOH promotes the firing of hippocampal interneurons, located in stratum lacunosum moleculare (SLM), from adolescent animals more potently than in those from adults. Thus the latter finding would seem to be inconsistent with the former. To understand this apparent inconsistency, we have now assessed the effects of EtOH on a different subpopulation of hippocampal interneurons, those with somata located in the stratum oriens (SO). We found that EtOH-induced enhancement of the frequency of spontaneous action potentials (sAPs) was less in interneurons from adolescent rats compared with those from adults. In addition, EtOH-induced reduction of the afterhyperpolarization decay time constant (τslow) was less pronounced in interneurons from adolescent rats, as was the EtOH-induced increase in the amplitude of the hyperpolarization-activated cation current, Ih. The effect of EtOH on sAP firing frequency was blocked by application of the Ih antagonist 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288). These results indicate that although EtOH promotes the firing of hippocampal interneurons, through promotion of Ih, the developmental expression of this effect differs between interneurons with somata located in the SO and SLM.
Introduction
GABAergic interneurons play a central role in the regulation of hippocampal excitability. A relatively small number of interneurons regulate the excitability of pyramidal cells and synchronize neural activity within the hippocampus through divergent inhibitory connections (Freund and Gulyas, 1997). Based on their firing patterns, molecular expression profiles, and innervation of different subcellular domains of principal cells, more than 20 different subtypes of interneurons have been characterized in hippocampal area CA1 (Klausberger and Somogyi, 2008). Hippocampal CA1 stratum oriens (SO) horizontal interneurons, with recurrent collateral branches to pyramidal cells, form a principal feedback regulator of pyramidal cell activity (Lacaille et al., 1987; Maccaferri 2005). In addition, subpopulations of CA1 interneurons exhibit spontaneous repetitive action potential firing (Lacaille and Williams, 1990; McBain, 1994; Zhang and McBain, 1995; Yan et al., 2009), thus providing a tonic inhibitory input onto their target cells, including principal neurons (McBain and Dingledine, 1993). The hyperpolarization-activated cation current, Ih, regulates spontaneous firing in stratum oriens-alveus interneurons (Maccaferri and McBain, 1996), thus acting as a pacemaker current. Ih was first identified in cardiac sinoatrial cells (Brown and diFrancesco, 1980) and has been subsequently characterized in many different types of neurons (see Pape et al., 1996 for review), including GABAergic hippocampal interneurons (Maccaferri and McBain, 1996; Lupica et al., 2001; Aponte et al., 2006). Ih plays a fundamental role in determining membrane potentials and regulates resting membrane potential, the generation of spontaneous firing, afterhyperpolarizations (AHPs), and GABA release in hippocampal interneurons (Maccaferri and McBain, 1996; Lupica et al., 2001; Aponte et al., 2006; Yan et al., 2009). The channels that carry Ih are assembled from four subunits (HCN1–HCN4), which are expressed in GABAergic interneurons in hippocampus (Notomi and Shigemoto, 2004; Aponte et al., 2006).
Hippocampal GABAA receptor-mediated synaptic function is highly sensitive to EtOH. One manifestation of this sensitivity is that behaviorally relevant concentrations, associated with moderate intoxication, of EtOH increase the frequency of GABAA receptor-mediated spontaneous and miniature inhibitory postsynaptic currents (IPSCs) in hippocampal CA1 (Li et al., 2003, 2006; Ariwodola and Weiner, 2004; Sanna et al., 2004) and CA3 pyramidal cells (Galindo et al., 2005). The EtOH concentrations used in those studies range from those associated with mild to heavy intoxication in humans. Presumably, most pyramidal cell IPSCs are driven by GABA released from local interneurons. Although EtOH is known to inhibit kainite receptor-mediated excitation of CA1 interneurons (Carta et al., 2003), we have shown that it strongly promotes the spontaneous firing of interneurons located near the border of stratum radiatum and stratum lacunosum moleculare (SLM) in CA1, and that the increase in interneuron firing rates is mediated by EtOH-induced promotion of Ih (Yan et al., 2009). This observation is consistent with other reports related to EtOH-induced promotion of firing among dopaminergic neurons in the ventral tegmental area (Brodie and Appel, 1998; Lupica and Brodie, 2006; Okamoto et al., 2006).
The promotion of spontaneous interneuron firing by EtOH is also developmentally regulated. We have found that EtOH increases firing rates, shortens AHPs, and enhances Ih more potently in SLM interneurons from adolescent rats than in those from adults (Yan et al., 2009). This finding is significant because it identifies interneurons as a new potential target for understanding the mechani sms underlying the differential effects of EtOH on hippocampal circuit excitability in adolescents and adults (Swartzwelder et al., 1995a,b; Pyapali et al., 1999; Li et al., 2003, 2006; Fleming et al., 2007). However, this developmental sensitivity of SLM interneurons to ethanol also raises questions because it seems counterintuitive in the context of some earlier findings. For example, we have shown that the frequency of spontaneous IPSCs, recorded from CA1 pyramidal cells, is increased more potently by ethanol in hippocampal slices from adult rats than in those from adolescents (Li et al., 2006). Because such IPSCs are presumably driven, at least in part, by the firing of local interneurons, then it would seem more likely that EtOH would increase the firing of interneurons in adults more than it would those in adolescents. However, our findings with SLM interneurons showed the opposite, i.e., a greater firing rate increase in SLM interneurons from adolescents (Yan et al., 2009).
In addition to SLM interneurons, those with somata in SO exert a strong inhibitory influence on pyramidal cells (Klausberger, 2009). In an effort to better understand the relationships between CA1 interneurons in mediating the effects of EtOH in hippocampal excitability in adolescents and adults, we assessed the effects of EtOH on spontaneously firing SO interneurons and the physiological mechanisms that underlie their firing rates.
Materials and Methods
Tissue Preparation.
Hippocampal slices were prepared from male Sprague-Dawley periadolescent [postnatal day (PD) 30–40] and adult (PD 70–80) rats. Although the periadolescent period of development in the rat has been the subject of some controversy, in recent years, based on an accumulating literature, the period between PD 30 and 50 has become an accepted norm for this period in the male rat (see Spear, 2000). The animals were handled and housed according to the guidelines of the National Institutes of Health Committee on Laboratory Animal Resources. All experimental procedures were approved by the Animal Care and Use Committee of Duke University and Durham VA Medical Center. Hippocampal slices from adolescent and adult rats were prepared and maintained as described previously (Yan et al., 2009). The rats were deeply anesthetized with isoflurane and decapitated. The brains were quickly removed from the skulls and placed in ice-cold (<4°C) modified artificial cerebrospinal fluid (aCSF) containing 120 mM NaCl, 3.3 mM KCl, 1.23 mM NaH2PO4, 1 mM MgSO4, 0.2 mM CaCl2, 25 mM NaHCO3, and 10 mM d-glucose with pH 7.3, previously saturated with 95% O2/5% CO2. The tissue was completely submerged into ice-cold modified aCSF and sectioned in 300-μm-thick slices by using a Vibratome series 1000 sectioning system (Vibratome Company, St. Louis, MO). The brain slices were first transferred to aCSF containing 0.5 mM Ca2+ and incubated at room temperature for 20 min to allow gradual adaptation to slightly elevated extracellular Ca2+. The slices were then allowed to equilibrate for at least 1 h, at 35°C, in normal aCSF containing 120 mM NaCl, 3.3 mM KCl, 1.23 mM NaH2PO4, 1 mM MgSO4, 2.0 mM CaCl2, 25 mM NaHCO3, and 10 mM d-glucose, during which time they were continuously bubbled with a mixture of 95% O2/5% CO2 gas. After this period, the brain slices were maintained at room temperature (22–24°C) until recordings were initiated.
Whole-Cell Electrophysiology.
After incubation, one hippocampal slice was transferred to the recording chamber that was connected to a Masterflex C/liter pump superfusion system (Cole-Parmer Instrument Co., Vernon Hills, IL). The slice was held against the bottom of the chamber with silver wires and superfused at a constant rate of 2 ml/min with aCSF, which was bubbled with a mixture of 95% O2/5% CO2 gas. The recording chamber temperature was kept at 29 to 30°C by Chamber System Temperature Controllers (TC-344B; Warner Instruments, Hamden, CT). The slice was visualized with infrared differential interference contrast (Zeiss Axioskope; Carl Zeiss GmbH, Jena, Germany), using an upright microscope, with a 40× water immersion objective, and displayed on a monitor. Interneurons located within the SO of area CA1 were easily distinguishable on visual inspection and then selected for whole-cell recording.
Recordings were made by using standard whole-cell patch recording techniques. Patch pipettes were borosilicate glass capillaries (o.d. 1.5 mm; i.d. 0.86 mm; Sutter Instrument Company, Novato, CA), pulled on a Flaming/Brown Micropipette Puller (model P-97; Sutter Instrument Company) to produce electrodes with 3- to 5-MΩ resistance. The pipette solution for current-clamp experiments consisted of 130 mM potassium gluconate, 5 mM KCl, 1 mM MgCl2, 0.5 mM EGTA, 10 mM HEPES, 4 mM Mg-ATP, 0.5 mM Tris-GTP, and 10 mM phosphocreatine (pH 7.3, 290 mOsm). For voltage-clamp experiments (Ih), the patch pipettes were filled with 125 mM KMeSO4, 5 mM KCl, 5 mM NaCl, 1 mM MgCl2, 11 mM HEPES, 0.02 mM EGTA, 4 mM Mg-ATP, 0.5 mM Na-GTP, and 10 mM phosphocreatine (pH 7.3, 290 mOsm). Tight seals (>1 GΩ) were formed on cell bodies, and whole-cell recordings were made by rupturing the cell membrane with negative pressure. Axopatch 200B (Molecular Devices, Sunnyvale, CA) was used for current- and voltage-clamp recordings that were low-pass filtered (2 or 5 kHz, Bessel filter). Output signals were d.c.-coupled to a digital oscilloscope (model 410; Thermo Fisher Scientific, Madison, WI), and data acquisition was performed by using pCLAMP 10 (Molecular Devices), with an interface DigiData 1440A (Molecular Devices) coupled with a computer. Resting membrane potential was directly measured in current-clamp mode after membrane rupture (range −72 to −44 mV), and only cells with a resting membrane potential more negative than −50 mV were studied. In spontaneously firing neurons, the resting membrane potential was not stable and varied slightly around the initial baseline measure that we acquired. Therefore, we also retrospectively measured the baseline resting membrane potential before each action potential, using simple visual inspection and Clampfit 10.0 software (Molecular Devices). The liquid junction potential was estimated to be 15.4 mV for the current-clamp solution and was not corrected. Input resistance was calculated from membrane voltage deflection, evoked by 600-ms hyperpolarizing current injections (0 to −300 pA in steps of 50 pA). To measure cell capacitance, interneurons were depolarized by applying 5 mV at a holding potential of −70 mV, and cell capacitance was measured from the change in membrane charge, determined from the integrated capacity transients. Series resistance was approximately 15 MΩ and was monitored by small (depolarizing 5 mV, 150 ms) voltage steps during voltage-clamp recording or current steps (hyperpolarizing 25 pA, 50 ms) during current-clamp recording. Cells were rejected from analysis if the series resistance changed by >15%. The effects of EtOH on action potentials were assessed only in cells that manifested spontaneous action potential firing.
Identification of Recorded Cells.
We initially visualized and selected interneurons for recording based on somatic shape and electrophysiological properties as described previously (Lacaille et al., 1987; Yan et al., 2009). We used several electrophysiological criteria to identify interneurons, including the response to depolarizing current injections, and the observation of short-duration and fast spike action potentials that were followed by large afterhyperpolarizing potentials (AHPs) (Schwartzkroin and Mathers, 1978). In contrast, CA1 pyramidal neurons generated action potentials that accommodated during maintained depolarization (Madison and Nicoll, 1984). The recorded cells were also filled with Alexa Fluor 568 hydrazide (50–80 μM; Invitrogen, Carlsbad, CA) to reveal their morphological characteristics for post hoc analysis. At the end of recordings, the fluorescence-filled slices were fixed with 4% paraformaldehyde for 20 to 30 min and rinsed three times by using a 0.2 M phosphate buffer. The slices were then mounted on gelatin-coated slides with Prolong Gold Antifade (Invitrogen) and visualized with a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems, Inc., Deerfield, IL).
Pharmacology.
To isolate Ih, 2 mM tetraethyl-ammonium chloride, 1 μM tetrodotoxin (TTX), and 1 mM BaCl2 were substituted for equimolar NaCl to block unwanted potassium, sodium, and inward rectifier potassium currents, respectively. TTX and 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288) were purchased from Tocris Bioscience (Ellisville, MO), and the other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). The drugs were dissolved in distilled water or dimethyl sulfoxide to make stock solutions. The stock solutions were stored frozen in 1-ml aliquots, and before each experiment they were diluted in aCSF to their final concentrations. The drugs were infused into the recording chamber by using a standard perfusion system. After the establishment of stable baseline recordings, EtOH was added to the bath solution in incremental concentrations of 3, 10, 30, and 50 mM, and each concentration was maintained for 5 to 10 min followed by a washout period of 10 to 20 min.
Data Analysis.
The stored data signals were processed by using either the Clampfit 10 program (Molecular Devices) or Mini Analysis Program (Synaptosoft Inc., Decatur, GA). Numerical data are presented as mean ± S.E.M., and n represents the number of cells tested per condition. Action potential amplitude, frequency, rise time (10–90%), half-width, and AHP decay time were analyzed with the Mini Analysis Program (Synaptosoft Inc.). Paired or unpaired t tests and one-way or two-way ANOVAs followed by Tukey post hoc tests, when appropriate, were used to test statistical inferences related to grouped data. Calculated p values of less than or equal to 0.05 were accepted as evidence of statistically significant differences.
Ih was evoked by 1.2-s hyperpolarizing steps to −130 or −140 mV from −50 mV. Ih amplitude was measured as the difference between the current level at the end of a 1.2-s hyperpolarizing step command and at the beginning after the capacitive transient had subsided. The EtOH concentration response curves were fitted by the Hill equation: Rexp = Rmax/{1+[EC50/(A)]n}, where Rexp is the expected response, Rmax is the maximal response, EC50 is the concentration of EtOH that induced the half-maximal response, (A) is the concentration of the EtOH, and n is the Hill coefficient. AHP decay time constants were obtained by fitting a two-exponential function, I(t) = If exp(−t/τfast) + Is exp(−t/τslow). If and Is are the amplitudes of fast and slow components in the AHP, respectively. τfast and τslow are decay time constants. Ih activation time constants were fitted with a single exponential function of the equation: I(t) = Ih exp(−t/τ) + ISS, where I(t) is the amplitude of the current at time t, ISS is the steady-state current during a single voltage step, Ih is instantaneous current subtracted from ISS, and τ is the time constant of activation.
Results
Morphology of SO Interneurons.
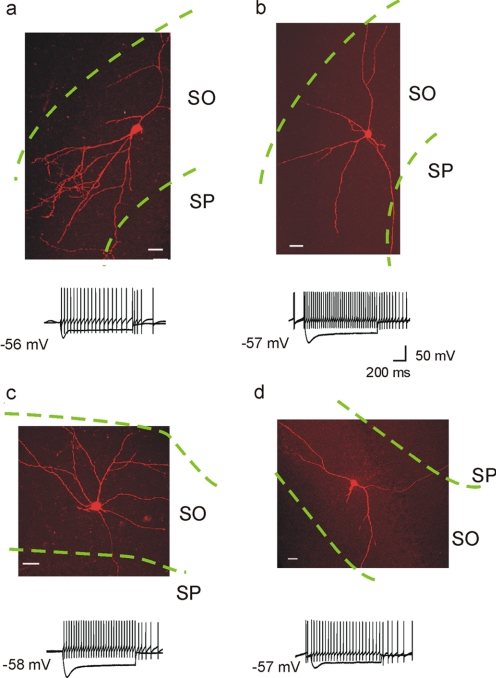
In the present study, all of the recordings were obtained from neurons with somata located within the SO of the CA1 region of the hippocampus. The recorded interneurons were processed for fluorescence staining and morphologically characterized after electrophysiological recording. The morphology of SO neurons was diverse, and most of the interneurons possessed multiple dendritic processes restricted primarily to the SO (Fig. 1). This morphology of interneurons, with somata and dendrites generally confined to the SO, is consistent with previous descriptions (Lacaille et al., 1987; McBain, 1994; Zhang and McBain, 1995; Freund and Buzsáki, 1996; Maccaferri and McBain, 1996; Klausberger, 2009).
Fig. 1.
Morphological and physiological properties of SO interneurons. The neurons were filled with Alexa Fluor 568 hydrazide during whole-cell recording. The recorded neurons were visualized and examined with confocal microscopy. The confocal images were scanned (30- to 40-μM thickness) at a Z-step of 0.5 μM, and then collapsed. Representative examples of interneurons were identified based on their morphological and physiological properties. Current-clamp recordings from each of the visualized interneurons show voltage responses to depolarizing (600 ms, +100 pA) and hyperpolarizing (600 ms, −300 pA) current injections. SP, stratum pyramidale. The region between the two green dotted lines indicates the SO region. Scale bars, 20 μm.
Membrane Properties and Spontaneous Activity in SO Interneurons.
Current-clamp recordings were obtained from a total of 67 and 65 interneurons in slices from adolescent and adult rats, respectively. All of the interneurons were “horizontally” oriented interneurons with soma and dendritic trees confined to the SO. To distinguish interneurons from pyramidal neurons by electrophysiology, we assessed responses to depolarizing current injections. The membrane properties of each group of cells, including resting membrane potential, input resistance, cell capacitance, and sAP amplitude and frequency are summarized in Table 1. Under current-clamp conditions in normal ACSF, 35 of the interneurons from adolescent rats (35/67, 52.2%) exhibited sAPs with firing rates of 4.2 ± 0.6 Hz, amplitudes of 87.6 ± 2.9 mV, and prominent AHPs. The other 32 interneurons from adolescents did not display sAP firing. In slices from adult rats, 35 (35/65, 53.8%) interneurons generated sAP firing with AHPs and had firing rates of 4.1 ± 0.8 Hz and amplitudes of 83.3 ± 4.2mV. The remaining 30 neurons from adult animals did not spontaneously fire APs and were not studied further. It is noteworthy that a higher proportion of interneurons in SO-generated sAPs, compared to interneurons from SLM (Yan et al., 2009).
Table 1.
Membrane properties of CA1 SO interneurons from adolescent and adult rats
Values are mean ± S.E.
| Parameters | Adolescent Rats |
Adult Rats |
||
|---|---|---|---|---|
| n | n | |||
| Interneurons with sAPs | ||||
| Resting membrane potential (mV) | −59.0 ± 0.6 | 35 | −58.0 ± 0.5 | 35 |
| Input resistance (MΩ) | 169.7 ± 7.2 | 35 | 154.6 ± 8.4 | 35 |
| sAP frequency (Hz) | 4.2 ± 0.6 | 35 | 4.1 ± 0.8 | 35 |
| sAP amplitude (mV) | 87.6 ± 2.9 | 35 | 83.0 ± 4.2 | 35 |
| Interneurons without sAPs | ||||
| Resting membrane potential (mV) | −58.9 ± 0.8 | 32 | −59.5 ± 1.0 | 30 |
| Input resistance (MΩ) | 157.0 ± 11.28 | 32 | 148.9 ± 10.6 | 30 |
Effects of EtOH on sAP Firing.
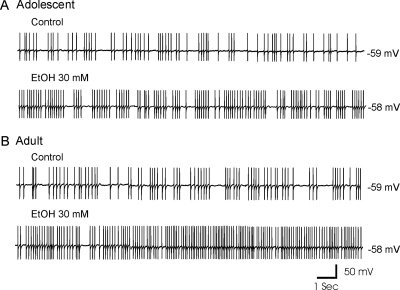
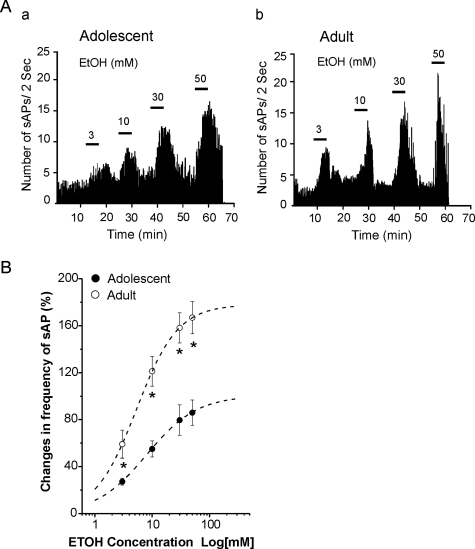
EtOH increased the sAP firing rate of SO interneurons, as we have reported previously in our study of SLM interneurons (Yan et al., 2009). However, in contrast to the effect of EtOH in SLM interneurons, in SO interneurons the promotion of sAP firing by EtOH was more potent in slices from adult rats than in those from adolescents. Figure 2 shows a typical example of sAPs recorded in interneurons from an adolescent and an adult rat. During application of 30 mM EtOH, sAP frequency increased from 4.5 to 8.9 Hz in the adolescent rat (Fig. 2A), whereas in the interneuron from the adult rat sAP frequency increased from 4.6 to 13.6 Hz (Fig. 2B). EtOH did not significantly alter resting membrane potential in interneurons from rats from either age group, and it did not significantly affect AP firing that was evoked by a depolarizing current injection (100 pA for 600-ms duration) or the amplitude of APs (data not shown) in interneurons that were not spontaneously firing. The effects of EtOH on sAP firing were concentration-dependent in both age groups (Fig. 3) and were reversed after the EtOH was washed out of the recording chamber for 15 to 30 min. A representative example of the responsiveness of an interneuron from an adolescent animal to EtOH (3–50 mM) is shown in Fig. 3Aa. EtOH at concentrations of 3, 10, 30, and 50 mM increased sAP frequency in slices from adolescents by 27.2 ± 2.9, 55.1 ± 6.8, 79.6 ± 12.9, and 86.0 ± 10.8% (n = 10), respectively (Fig. 3B). Among cells from adolescent rats, all EtOH concentrations resulted in significant increases of sAP frequency relative to control conditions [F(3, 36) = 7.57; p = 4.76E-4]. A representative example of the responsiveness of an interneuron from an adult animal to EtOH (3–50 mM) is shown in Fig. 3Ab. EtOH at concentrations of 3, 10, 30, and 50 mM increased firing frequency by 59.1 ± 11.9, 121.2 ± 12.7, 158.1 ± 12.8, and 166.8 ± 13.7% (n = 9), respectively (Fig. 3B). EtOH increased sAP frequency more powerfully in interneurons from adult rats, compared with those from adolescent rats [F(1,71) = 58.99; p = 6.53E-11; Fig. 3B]. The curves in Fig. 3C were fitted to averaged percentage increase in sAP frequency at each EtOH concentration by using the Hill equation (see Materials and Methods). Best-fit curves revealed that the EC50s for EtOH were 11.5 ± 1.7 mM for cells from adolescent rats and 6.2 ± 1.0 mM for cells from adults. The Rmaxs were 96.2 ± 12.3 and 180.0 ± 15.8% for cells from adolescent rats and adult rats, respectively. There were significant differences for EC50 [t(17) = 2.48; p = 0.02] and Rmax [t(17) = 3.99; p = 9.43E-4] between the two age groups. The concentration-response curve for interneurons from adults was shifted significantly to the left compared with the curve for interneurons from the adolescent group (Fig. 3B). There was no significant difference in the Hill coefficient between the two age groups [adolescent, 0.93 ± 0.16; adult, 1.13 ± 0.18; unpaired t test, t(17) = −0.79, p = 0.44].
Fig. 2.
EtOH increased sAP firing rate in CA1 SO interneurons from adolescent and adult rats. A, sAP firing recorded from an interneuron from an adolescent rat before (top) and after (bottom) the addition of 30 mM EtOH. B, sAP firing recorded from an interneuron from an adult rat before (top) and after (bottom) the addition of 30 mM EtOH.
Fig. 3.
EtOH increased sAP firing rate in CA1 SO interneurons from adolescent and adult rats in a concentration-dependent manner. A, time course of sAP firing was recorded continuously at baseline and after the addition of 3, 10, 30, and 50 mM EtOH. EtOH caused a concentration-dependent increase in the frequency of sAP firing in neurons from adolescent (a) and adult (b) rats. B, EtOH concentration-response curves for percentage change in sAP frequency in neurons from adolescent (●; n = 10) and adult (○; n = 9) rats. The individual data points show the mean percentage change in sAP frequency plotted against log of the EtOH concentration, indicating that EtOH enhanced sAP firing more powerfully in neurons from adolescent rats than in those from adults at each concentration tested (two-way ANOVA, p < 0.01). Averaged EtOH concentration-response curves were fitted by using the Hill equation. *, p < 0.01.
Effects of EtOH on sAP AHP Decay Time.
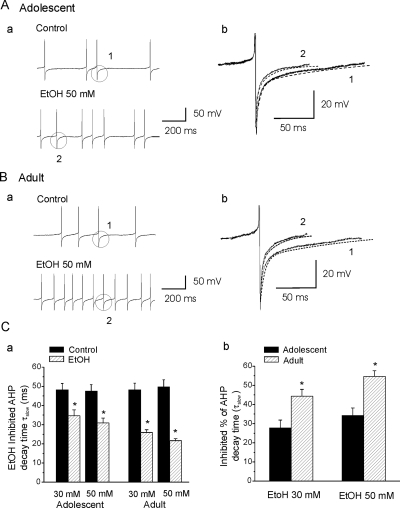
AHP plays a functional role in the firing and discharge frequency of CA1 hippocampal interneurons, and EtOH effects on the AHP time constant are involved in its ability to increase sAP firing rates (Yan et al., 2009). Therefore, we assessed EtOH-induced changes in the time course of AHP decay in SO interneurons from animals in both age groups. AHP decay time constants were obtained by fitting a two-exponential function (see Materials and Methods). EtOH (30 or 50 mM) did not change the τfast decay time components in interneurons from adolescent or adult rats. In cells from adolescent rats, the AHP τfast decay time (10–90%) was 11.4 ± 0.9 ms (n = 10), which did not change significantly after application of 30 mM EtOH [10.8 ± 0.8 ms; n = 10; paired t test, t(9) = 1.45, p = 0.18]. Bath application of EtOH (50 mM) also did not affect the AHP τfast decay time [from 10.7 ± 0.7 to 10.3 ± 0.7 ms, n = 10; paired t test, t(9) = 1.15, p = 0.28]. In addition, 30 or 50 mM EtOH did not significantly alter AHP τfast decay time in SO interneurons from adult rats [30 mM, from 11.6 ± 1.1 to 11.0 ± 0.9 ms, n = 9, paired t test, t(8) = 1.10, p = 0.66; 50 mM, from 11.4 ± 0.9 to 10.5 ± 0.7 ms, n = 9, paired t test, t(8) = 2.27, p = 0.06]. However, EtOH (30 and 50 mM) did decrease the AHP τslow decay time in SO interneurons from animals of both age groups. The results are illustrated in Fig. 4. As demonstrated in Fig. 4Aa, bath application of 50 mM EtOH accelerated the frequency of sAPs recorded from an interneuron from an adolescent rat. The AHP slow decay time was 48.4 ms at baseline and 30.6 ms after bath application of 50 mM EtOH (Fig. 4Ab). In cells from adolescent rats, the average AHP τslow decay time (10–90%) at baseline was 48.2 ± 3.3 ms, and it was significantly reduced to 34.4 ± 3.0 ms by 30 mM EtOH [n = 10; paired t test, t(9) = 5.8, p = 2.44E-04], and 50 mM EtOH reduced τslow from 47.6 ± 3.3 to 30.1 ± 2.5 ms [n = 10; paired t test, t(9) = 6.4, p = 1.26E-04] (Fig. 4Ca). Figure 4B shows that bath application of 50 mM EtOH also accelerated the frequency of sAPs isolated from an interneuron from an adult rat. Typical voltage traces are shown in Fig. 4B. In this interneuron, the AHP decay time was 48.1 ms at baseline and 21.2 ms after bath application of 50 mM EtOH. In cells from adults, the average AHP τslow decay time was significantly reduced to 29.9 ± 1.4 from 48.2 ± 3.5 ms after application of 30 mM EtOH [n = 9; paired t test, t(8) = 6.12, p = 1.49E-04] and from 49.7 ± 3.8 to 21.1 ± 1.0 ms after bath-applied 50 mM EtOH [n = 9; paired t test, t(8) = 8.11, p = 3.97E-05] (Fig. 4Ca). We compared the EtOH-induced decrease in the percentage of AHP τslow decay time in interneurons from adolescent and adult rats, and the results are plotted in Fig. 4Cb. In adolescent rats, AHP τslow decay time were decreased 27.9 ± 3.9% (n = 10) and 34.3 ± 3.9% (n = 10) by EtOH at 30 and 50 mM, respectively. EtOH also decreased AHP τslow decay time 44.3 ± 3.5% (n = 9; 30 mM) and 54.7 ± 3.0% (n = 9; 50 mM) in interneurons from adult rats. EtOH decreased AHP τslow decay time more powerfully in cells from adult rats compared with those from adolescents [unpaired t test, t(17) = −2.90, p = 0.009 at 30 mM; t(17) = −3.89, p = 0.0012 at 50 mM], indicating that the EtOH sensitivity of AHP decay time constants may be a mechanism underlying the developmental sensitivity of interneurons to EtOH.
Fig. 4.
The effects of EtOH on AHPs after sAPs in SO interneurons from adolescent and adult rats. A, the sAPs were obtained before (control) and after application of 50 mM EtOH (a) in an interneuron from an adolescent rat; and traces of single AHPs in the absence (1) and presence (2) of 50 mM EtOH illustrate that EtOH decreased AHP decay time τslow (1 and 2) (b). The dashed lines represent fits to a two-exponential function (see Materials and Methods). B, the same experimental protocol as A, using interneuron responses from an adult rat. C, summarized data show EtOH inhibited AHP decay time (a); and mean percentage change in AHP decay time τslow after the application of EtOH (30 and 50 mM) to slices from adolescent (n = 10) and adult (n = 10) rats (b). EtOH reduced AHP decay time and did so with greater efficacy in neurons from adults than in those from adolescents. *, p < 0.01, unpaired t tests.
EtOH Enhanced sAP Firing via Ih.
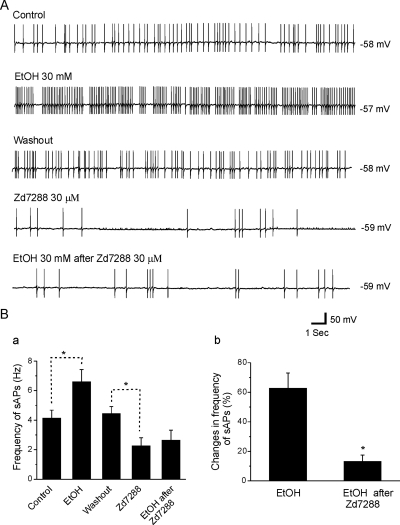
Previous studies have shown that Ih contributes to resting membrane potentials and spontaneous pace-making activity in hippocampal interneurons (Maccaferri and McBain, 1996; Lupica et al., 2001), and EtOH-induced enhancement of neuronal firing rates is mediated by Ih in SLM interneurons from hippocampal area CA1 (Yan et al., 2009). To investigate whether Ih also contributed to the EtOH-induced increases in SO interneuron firing that we have observed, we assessed the effects of Ih channel antagonists on EtOH-enhanced sAP firing in hippocampal interneurons from adolescent and adult rats. We found that the Ih antagonist ZD7288 significantly attenuated EtOH-induced increases of sAP firing rates in SO interneurons from adolescent animals. Typical responses are shown in Fig. 5A. In this interneuron, bath application of 30 mM EtOH increased sAP frequency from 4.53 to 9.08 Hz. The firing rates returned to baseline after the 15-min washout of EtOH. When 30 μM ZD7288 was added to the bath (5–7 min) the sAP firing rate was reduced to 1.83 Hz, and subsequent EtOH application did not significantly increase this firing rate. The averaged results of these experiments are shown in Fig. 5Ba. The mean sAP frequency from seven cells was significantly increased to 6.6 ± 0.8 Hz from 4.1 ± 0.5 Hz after the initial bath application of 30 mM EtOH, then the firing returned to 4.4 ± 0.5 Hz after the 15-min washout. Bath application of ZD7288 (30 μM) for 7 min irreversibly decreased sAPs firing frequency. The sAP firing was reduced to 2.0 ± 0.4 Hz by ZD7288 [n = 7; one-way ANOVA, F(4, 30) = 10.92, p = 1.39E-5]. Reapplication of EtOH did not significantly raise the sAP firing frequency, raising it only to 2.4 ± 0.5 Hz. ZD7288 also induced a small, nonsignificant hyperpolarization (−59.4 ± 0.9 to −60.9 ± 1.5 mV). We also found similar results when we replaced ZD7288 with another Ih channel blocker, CsCl. The frequency from five cells was significantly increased to 11.6 ± 1.3 Hz from 4.90 ± 0.5 Hz by 30 mM EtOH and reduced to 2.4 ± 0.2 Hz by 2 mM CsCl [n = 5; one-way ANOVA, Tukey test, F(3, 16) = 53.9, p = 1.37E-8]. After reapplication of 30 mM EtOH in the presence of 2 mM CsCl, the frequency of sAP firing was 2.50 ± 0.4 Hz, which was not significantly different from baseline in CsCl. Figure 5Bb illustrates the effect of EtOH on sAP firing frequency in the absence and presence of ZD7288. EtOH had a very weak effect on sAP firing in the presence of ZD7288 (16.5 ± 7.7%) compared with its effect in the absence of ZD7288 [74.1 ± 8.2%, n = 7; unpaired t test, t(12) = 4.74, p = 4.82E-4]. This indicates that EtOH-induced increases in sAP firing rates in SO interneurons may be caused by its suppression of Ih.
Fig. 5.
Effects of ZD7288 on sAP firing frequency in hippocampal SO interneurons from adolescent rats. A, sAP firing was recorded before (control) and after bath application of EtOH (30 mM) and washout of the EtOH solution. Then ZD7288 (30 μM) was applied, followed by 30 mM EtOH. ZD7288 irreversibly decreased sAP firing frequency and blocked the effects of EtOH on sAP frequency. B, summarized results show the effects of EtOH alone, ZD7288 alone, and the application of EtOH after ZD7288 on the frequency of sAP firing (a; n = 7, one-way ANOVA; *, p < 0.01) and the percentage change in sAP frequency after EtOH application before and after ZD7288 (b; n = 7, unpaired t test; *, p < 0.01).
Effects of EtOH on Ih.
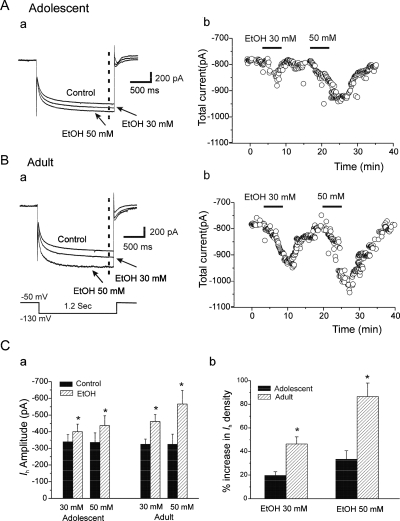
The mediation of EtOH-induced increases in sAP firing by Ih prompted us to explore whether it could be of developmental significance in SO interneurons. We therefore assessed the effects of ethanol on Ih currents recorded in interneurons from animals in both age groups. We evoked Ih by using 1.2-s hyperpolarizing voltage steps to −130 mV from the −50-mV holding potential. The average Ih amplitude was −339.5 ± 43.2 pA (n = 10) in interneurons from adolescent animals and −325.4 ± 30.5 pA (n = 14) in interneurons from adults. These Ih amplitudes valves are larger than those we have recorded previously from SLM interneurons (Yan et al., 2009). Bath application of EtOH (30 and 50 mM) reversibly increased Ih amplitude in cells from animals in both age groups (Fig. 6, A and B). Typical current traces of Ih in the absence and presence of EtOH (30 and 50 mM) of interneurons recorded from adolescent and adult are shown in Fig. 6, A and Ba. The total currents corresponding to the dashed lines in Fig. 6, Aa and Ba were calculated and plotted before and during EtOH application (Fig. 6, A and Bb). EtOH increased Ih amplitude in interneurons from both age groups (Fig. 6Ca). In interneurons from adolescent animals, EtOH (30 mM) significantly enhanced Ih amplitude from −342.5 ± 41.0 to −400.5 ± 45.2 pA [n = 10, paired t test, t(9) = 7.89, p = 2.48E-05] and 50 mM EtOH further increased Ih amplitudes to −437.2 ± 58.7 pA from −345.9 ± 54.3 pA [n = 10; paired t test, t(13) = 9.6, p = 5.23E-06]. In interneurons from adult rats, EtOH also increased Ih amplitudes from −325.3 ± 30.3 and −324.6 ± 40.8 pA to −461.6 ± 41.7 pA [30 mM EtOH, n = 14, paired t test, t(13) = −6.60, p = 1.71E-05] and −566.5 ± 80.8 pA [50 mM EtOH 50, n = 14, paired t test, t(13) = 5.93, p = 4.93E-05], respectively (Fig. 6Ca).
Fig. 6.
Effects of EtOH on Ih recorded in SO interneurons from adolescent and adult rats. A, a, representative traces of current obtained before (control) and after application of 30 and 50 mM EtOH in an interneuron from an adolescent rat. The current traces were evoked by a 1.2-s hyperpolarizing voltage step from a holding potential of −50 to −130mV. b, time course of the effects of EtOH (30 and 50 mM) on currents from the same interneuron. Total current (see a) was measured at the vertical dashed line. B, the same experimental protocol as A in an interneuron from an adult rat. C, a, summarized results show that EtOH (30 and 50 mM) increased Ih amplitudes in interneurons from adolescent (n = 10) and adult (n = 14) rats. *, p < 0.01, paired t test. b, the bar graph shows the effects of EtOH (30 and 50 mM) on Ih density in SO interneurons from adolescent (solid bars, n = 10) and adult (hatched bars, n = 14) rats. Ih density was obtained by dividing the Ih amplitude in each cell by that cell's capacitance. EtOH enhanced Ih density more powerfully in neurons from adult animals than from adolescent animals. *, p < 0.01, unpaired t test.
Furthermore, 30 and 50 mM EtOH increased Ih density, which was calculated by normalizing Ih current to the cell capacitance in each cell. The percentage increases were 19.6 ± 3.1 and 46.3 ± 6.0% (22.6 ± 1.8 pF cell capacitance; n = 10) in interneurons from adolescent animals (Fig. 6Cb). In interneurons from adult animals, Ih densities were increased 46.3. ± 6.0 and 86.5 ± 11.5% after application of EtOH at 30 and 50 mM (21.5 ± 1.2 pF cell capacitance; n = 14), respectively (Fig. 6Cb). The effects of EtOH on Ih density were greater in interneurons from adult rats than in those from adolescent rats [Fig. 6Cb; unpaired t test, t(22) = −3.40 and p = 0.0029 at 30 mM; t(22) = −3.34 and p = 0.0025 at 50 mM]. These results indicate that the effect of EtOH to enhance Ih may contribute to developmental EtOH sensitivity differences among SO interneurons.
It is noteworthy that we did not observe a significant effect of ZD7288 on resting membrane potential. A previous study has shown that ZD7288 induced hyperpolarization of SO interneurons during the blockade of Ih (Lupica et al., 2001). However, in the previous study, a ZD7288 concentration of 50 μM was applied for 10 min and induced an approximately 5.0-mV hyperpolarization on average that was statistically significant. In contrast, we used 30 μM ZD7288, which was applied for 5 to 7 min and induced an approximately 1.5-mV hyperpolarization on average that was not statistically significant. IThe previous study also recorded resting membrane potentials under current-clamp conditions and in the presence of TTX, whereas we did not use TTX because spontaneous firing was a primary dependent variable in this study. Thus, although we found a qualitatively similar effect of ZD7288 on membrane potential, it was clearly not significant. t seems likely that pharmacological and experimental differences between the two studies would account for this difference in outcome relative to resting membrane potential.
Discussion
The present findings establish that the spontaneous firing of CA1 SO interneurons is increased by EtOH concentrations that are consistent with low to moderate doses of alcohol in humans, and that this effect of EtOH is more potent in interneurons from adult animals than in those from adolescents. In addition, as we have shown in previous studies of SLM interneurons (Yan et al., 2009), this EtOH-induced increase in sAP firing rate is associated with an augmentation of the hyperpolarization-activated cation current, Ih, and a decrease in AHP decay time. Despite these similarities in the effects of EtOH on SLM and SO interneurons, from a developmental perspective there is one striking difference: whereas EtOH increased firing rates more potently in SO interneurons from adults in the present study, it increased firing rates of SLM interneurons from adolescents more potently in our previous study (Yan et al., 2009). Taken together, these data indicate that although EtOH effects on hippocampal network excitability are related to its effects on the mechanisms underlying interneuron firing, the developmental mediation of those effects is distinct among different populations of interneurons in area CA1 of the hippocampal formation.
It is intriguing that the potency of EtOH on sAP firing in SO and SLM interneurons is developmentally regulated, albeit in opposite directions. We observed some physiological differences between interneurons in SO and those in SLM (Yan et al., 2009). For example, more than half of the SO interneurons that we sampled in the present study generated sAPs, whereas only approximately a quarter of those from SLM generated sAPs (Yan et al., 2009). However, there was no difference in the percentage of spontaneously firing interneurons between adolescents and adults. In addition, interneurons from SO had more depolarized membrane potentials (see Table 1; adolescent, −59 mV; adult, −58 mV), compared with SLM interneurons (adolescent, −68 mV; adult, −68 mV; Yan et al., 2009), again with no developmental differences noted. These data indicate that the developmental differences in EtOH sensitivity between these two populations of interneurons are not related to either resting membrane potentials or the other characteristics that may give rise to spontaneous firing.
During development from adolescence to adulthood, spontaneously firing SO interneurons become more sensitive to EtOH (present findings), whereas SLM interneurons become less sensitive to EtOH (Yan et al., 2009). It is noteworthy that the potency of EtOH to induce firing in both types of interneurons is quite similar during adolescence (SO EC50, 11.5 mM; SLM EC50, 10.8 mM), whereas during adulthood they are quite different (SO EC50, 6.2 mM; SLM EC50, 20 mM). That is, during adolescence EtOH promotes the spontaneous firing of SO interneurons with similar potency to SLM interneurons (Yan et al., 2009), but as animals mature into adulthood the EtOH potency on these two populations of interneurons diverges. It is noteworthy that the efficacy of perhaps the most behaviorally relevant concentration tested (10 mM) was very markedly different in cells from adolescents compared with those from adults, and this was the case in both the present study and our previous study of SLM interneurons (Yan et al., 2009). Because the divergence of EtOH responsiveness in these two interneuron populations is represented in the adult state, whereas they are similarly responsive during adolescence, these data also suggest that SO and SLM interneurons mature differentially in some essential way that regulates EtOH sensitivity.
In the present study, we found that EtOH increased sAP firing through activation of the Ih. Previous studies have demonstrated that four Ih channel subunits (HCN1–HCN4) are expressed in axons and presynaptic terminals of GABAergic interneurons in the hippocampus (Notomi and Shigemoto, 2004). One recent study indicates that EtOH increases the intrinsic firing rates of cerebellar interneurons through enhancement of Ih, resulting in the facilitation of GABAergic transmission (Hirono et al., 2009). In addition, EtOH has been shown to augment Ih and neuronal firing in ventral tegmental area dopaminergic neurons, an effect that was attenuated by ZD7288 (Okamoto et al., 2006). These findings are consistent with our general finding that EtOH promotes SO interneuron firing by promoting Ih. It is noteworthy that another study of mouse ventral tegmental area dopaminergic neurons has shown that whereas ethanol produced only increases in neuronal firing in the absence of ZD7288, when Ih was antagonized by 30 μM ZD7288 ethanol produced a transient excitation followed by a decrease of firing rate. Moreover, that late inhibition of firing was not observed in the presence of barium, suggesting that ethanol may directly increase an inhibitory potassium conductance, this inhibitory effect of ethanol only decreases the firing rate if Ih is blocked (McDaid et al., 2008).
A growing literature indicates that the effects of EtOH on neurobehavioral function are developmentally regulated. We have shown that EtOH impairs spatial learning more potently in adolescent rats than in adults (Markwiese et al., 1998), and that early postadolescent humans are more vulnerable to acute EtOH-induced learning impairment than are slightly older individuals (Acheson et al., 1998). EtOH is also a more potent antagonist of memory-related long-term potentiation (Swartzwelder et al., 1995a; Pyapali et al., 1999) and N-methyl-d-aspartate receptor-mediated synaptic function (Swartzwelder et al., 1995b; Li et al., 2002) in adolescent animals compared with adults. Conversely, EtOH is a less potent sedative in juvenile and adolescent animals than in adults (Little et al., 1996; Silveri and Spear, 1998), and the EtOH sensitivity of evoked GABAA receptor-mediated inhibitory postsynaptic currents increases steadily during juvenile and adolescent development in the rat (Li et al., 2003). Likewise, EtOH increases the frequency of action potential-dependent spontaneous IPSCs in hippocampal pyramidal cells more potently in slices from adult rats than from adolescents, in the absence of any age-dependent effect on action potential-independent miniature IPSC frequency or amplitude (Li et al., 2006). The data on spontaneous IPSCs in particular are consistent with the present findings on the EtOH sensitivity of SO interneurons from adolescent rats compared with adult rats.
To understand the implications of the effects of EtOH on interneuron excitability it is important to consider the hippocampal networks in which those interneurons function. CA1 pyramidal cells receive mainly glutamatergic and GABAergic synaptic inputs. GABAergic interneurons primarily innervate the dendrites of CA1 pyramidal cells (Megías et al., 2001) and thereby regulate pyramidal cell integration of incoming signals and synaptic plasticity (see Klausberger, 2009). The prevailing understanding is that the main glutamatergic input to SO interneurons arises from local CA1 pyramidal cells (Blasco-Ibáñez and Freund, 1995) and SO interneurons generally project to the apical tuft of CA1 pyramidal cells and other interneurons (Katona et al., 1999; Maccaferri et al., 2000). However, there are at least five separate types of SO interneurons that project, at least in part, to CA1 pyramidal cells: oriens lacunosum-moleculare cells, bistratified cells, trilaminar cells, backprojecting cells, and oriens retrohippocampal projection cells (Klausberger, 2009). Thus, in the absence of extensive characterization of our recorded interneurons, it would be highly speculative at present to propose a model whereby the developmental regulation of interneuron sensitivity to EtOH may explain the developmental differences in hippocampal and behavioral sensitivity to EtOH that we have observed previously (Swartzwelder et al., 1995a; Little et al., 1996; Acheson et al., 1998; Markweise et al., 1998). However, now that we have shown a high degree of EtOH sensitivity in two distinct populations of CA1 interneurons, each of which manifests marked developmental regulation that is associated with the EtOH sensitivity of Ih, we believe that a major research focus on interneurons in the context of adolescent EtOH sensitivity is needed.
Hippocampal interneurons are remarkably diverse. An early study described 16 distinct morphological phenotypes, with three different modes of discharge, expressing 25 or more combinations of receptors for neurotransmitters (Parra et al., 1998). Other studies have described more than 20 different CA1 interneuron subtypes based on their firing patterns, molecular expression profiles, and innervation of different subcellular domains of principal cells (Klausberger and Somogyi, 2008). Furthermore, a review (Klausberger, 2009) has divided hippocampal CA1 interneurons into four cell groups according to their expression of parvalbumin, cholecystokinin, axonal arborization density, and long-range projections, and these four cell groups contain at least 12 distinct cell types. Against the backdrop of this complexity, it is very important to begin to understand the effects of EtOH across these various types of interneurons. Add to this the developmental differences in EtOH sensitivity that we have observed (Yan et al., 2009 and present findings), and it seems likely that studies of this type will yield important information about the mechanisms whereby EtOH affects hippocampal function differently in adolescents and adults.
In summary, the present study demonstrates that EtOH enhanced spontaneous firing in hippocampal CA1 SO interneurons through its promotion of Ih, and this effect is more pronounced in interneurons from adult rats than in those from adolescents. This finding is of particular interest because it illustrates that the maturation of interneuron EtOH sensitivity between adolescence and adulthood varies across interneuron types and maturational increases and decreases in EtOH sensitivity are bidirectional depending on cell type.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA01489] (to H.S.S.), Merit Review grants and Senior Research Career Scientist awards from the U.S. Department of Veterans Affairs (to H.S.S. and W.A.W.), and an Institute for Medical Research, Inc. award (to H.Y.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.168450.
- EtOH
- ethanol
- SLM
- stratum lacunosum moleculare
- SO
- stratum oriens
- sAP
- spontaneous action potential
- AHP
- afterhyperpolarization
- Ih
- hyperpolarization-activated cation current
- τslow
- slow decay time constant
- PD
- postnatal day
- IPSC
- inhibitory postsynaptic current
- ZD7288
- 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride
- TTX
- tetrodotoxin
- ANOVA
- analysis of variance
- aCSF
- artificial cerebrospinal fluid.
References
- Acheson SK, Stein RM, Swartzwelder HS. (1998) Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res 22:1437–1442 [DOI] [PubMed] [Google Scholar]
- Aponte Y, Lien CC, Reisinger E, Jonas P. (2006) Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol 574:229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. (2004) Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci 24:10679–10686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco-Ibáñez JM, Freund TF. (1995) Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur J Neurosci 7:2170–2180 [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. (1998) The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res 22:236–244 [PubMed] [Google Scholar]
- Brown H, Difrancesco D. (1980) Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol 308:331–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. (2003) Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci USA 100:6813–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. (2007) Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol 97:3806–3811 [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. (1996) Interneurons of the hippocampus. Hippocampus 6:347–470 [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI. (1997) Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol 75:479–487 [PubMed] [Google Scholar]
- Galindo R, Zamudio PA, Valenzuela CF. (2005) Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J Neurochem 94:1500–1511 [DOI] [PubMed] [Google Scholar]
- Hirono M, Yamada M, Obata K. (2009) Ethanol enhances both action potential-dependent and action potential-independent GABAergic transmission onto cerebellar Purkinje cells. Neuropharmacology 57:109–120 [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T. (2009) GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci 30:947–957 [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. (2008) Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science 321:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Williams S. (1990) Membrane properties of interneurons in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience 36:349–359 [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. (1987) Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci 7:1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. (2002) Differential effect of ethanol on NMDA EPSCs in pyramidal cells in the posterior cingulate cortex of juvenile and adult rats. J Neurophysiol 87:705–711 [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. (2003) Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res 27:2017–2022 [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. (2006) Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res 30:119–126 [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. (1996) Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res 20:1346–1351 [DOI] [PubMed] [Google Scholar]
- Lupica CR, Brodie MS. (2006) Queer currents, steady rhythms, and drunken DA neurons. Focus on “hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice.” J Neurophysiol 95:585–586 [DOI] [PubMed] [Google Scholar]
- Lupica CR, Bell JA, Hoffman AF, Watson PL. (2001) Contribution of the hyperpolarization-activated current (Ih) to membrane potential and GABA release in hippocampal interneurons. J Neurophysiol 86:261–268 [DOI] [PubMed] [Google Scholar]
- Maccaferri G. (2005) Stratum oriens horizontal interneurone diversity and hippocampal network dynamics. J Physiol 562:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. (1996) The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol 497:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. (2000) Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol 524:91–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. (1984) Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol 354:319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. (1998) Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res 22:416–421 [PubMed] [Google Scholar]
- McBain CJ. (1994) Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol 72:2853–2863 [DOI] [PubMed] [Google Scholar]
- McBain CJ, Dingledine R. (1993) Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol 462:373–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. (2008) Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: Involvement of barium-sensitive potassium currents. J Neurophysiol 100:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megías M, Emri Z, Freund TF, Gulyás AI. (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102:527–540 [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. (2004) Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol 471:241–276 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. (2006) Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol 95:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. (1996) Queer current and pacemaker: The hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58:299–327 [DOI] [PubMed] [Google Scholar]
- Parra P, Gulyás AI, Miles R. (1998) How many subtypes of inhibitory cells in the hippocampus? Neuron 20:983–993 [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. (1999) Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol 19:107–111 [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. (2004) Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci 24:6521–6530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Mathers LH. (1978) Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res 157:1–10 [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. (1998) Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res 22:670–676 [DOI] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463 [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. (1995a) Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res 19:1480–1485 [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. (1995b) Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res 19:320–323 [DOI] [PubMed] [Google Scholar]
- Yan H, Li Q, Fleming R, Madison RD, Wilson WA, Swartzwelder HS. (2009) Developmental sensitivity of hippocampal interneurons to ethanol: involvement of the hyperpolarization-activated current, Ih. J Neurophysiol 101:67–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. (1995) Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol 488:661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]