Abstract
Most life-long drug addiction begins during adolescence. Important structural and functional changes in brain occur during adolescence and developmental differences in forebrain dopamine systems could mediate a biologic vulnerability to drug addiction during adolescence. Studies investigating age differences in psychostimulant responses have yielded mixed results, possibly because of different mechanisms for increasing extracellular dopamine. Recent research from our laboratory suggests that adolescent dopamine systems may be most affected by selective dopamine uptake inhibitors. We investigated age-related behavioral responses to acute administration of several dopamine uptake inhibitors [methylphenidate, 1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine (GBR12909), and nomifensine] and releasing agents [amphetamine and methylenedioxymethamphetamine (MDMA)] in adolescent and adult male rats. Methylphenidate and amphetamine effects on stimulated dopamine efflux were determined using fast-scan cyclic voltammetry in vivo. Dopamine uptake inhibitors but not dopamine releasing agents induced more locomotion and/or stereotypy in adolescent relative to adult rats. MDMA effects were greater in adults at early time points after dosing. Methylphenidate but not amphetamine induced much greater dopamine efflux in periadolescent relative to adult rats. Periadolescent male rats are particularly sensitive to psychostimulants that are DAT inhibitors but are not internalized and do not release dopamine. Immaturity of DAT and/or DAT associated signaling systems in adolescence specifically enhances behavioral and dopaminergic responses in adolescence.
Introduction
Lifelong drug addiction usually begins with drug use during adolescence or young adulthood (Spear, 2000; Schramm-Sapyta et al., 2009). Longitudinal and retrospective studies consistently demonstrate that early exposure to drugs and alcohol is one of the strongest predictors of adult substance abuse (Spear, 2000; Chambers et al., 2003). The onset of drug addiction during adolescence is correlated with an increased severity of addiction including higher rates of morbidity and mortality (for reviews, see Spear, 2000; Schramm-Sapyta et al., 2009). Finally, the progression from initial drug use to the expression of addictive behaviors occurs more rapidly during adolescence than in adulthood. Although such studies demonstrate the importance of adolescence in human drug use, the biological basis for these vulnerabilities is not fully understood.
Adolescence is a time of both sexual maturation and attainment of adult nervous system function. Neurobiologic changes during this phase of development contribute to age-related differences in drug sensitivity (Andersen, 2003; McCutcheon and Marinelli, 2009). Dopamine systems, which mediate the rewarding effects of addictive drugs, undergo significant development and reorganization during adolescence, and may explain, in part, why this period is so important for the development of drug addiction (Spear, 2000; Andersen, 2003; Chambers et al., 2003).
Psychostimulants increase extracellular dopamine by mobilizing different storage pools of transmitter (McMillen, 1983) and so might be predicted to exhibit age-related behavioral effects to the extent that there are age-related differences in the dopamine pools. However, the pharmacological literature exploring behavioral sensitivity to psychomotor stimulants across adolescence is mixed. Several groups have reported that rats in the periadolescent period [postnatal day (PN) 30–40] are hyperactive at baseline but have smaller increases in locomotion and stereotyped behaviors than younger or older cohorts after a single dose of amphetamine or cocaine (Bolanos et al., 1998). In contrast, our laboratory has reported that cocaine induces more acute locomotor behavior and stereotypy in periadolescent than adult male rats (Caster et al., 2005; Parylak et al., 2008) and that adolescent rats will consume more of a sweetened cocaine solution (Walker et al., 2009). Periadolescent rats exhibit an “intrabinge” sensitization (Caster et al., 2005) and greater sensitization than adults 24 h after a single high dose of cocaine (Caster et al., 2007). Fast-scan cyclic voltammetry showed that 15 mg/kg cocaine enhanced dopamine efflux in dorsal striatum nearly 3-fold more in adolescent than in adult males, suggesting that greater cocaine-stimulated dopamine efflux might mediate the greater behavioral responses of periadolescents (Walker and Kuhn, 2008).
The present study compared the behavioral and neurochemical effects of methylphenidate, nomifensine, GBR12909, methylenedioxymethamphetamine (MDMA), and amphetamine. Psychostimulants can be classified as either dopamine transporter (DAT) inhibitors or amphetamine-like dopamine releasers (McMillen, 1983). This classification is based, in part, on the observation that the ability of amphetamine-like drugs to stimulate behavior is antagonized by the dopamine synthesis inhibitor α-methyl-p-tyrosine, whereas the action of DAT inhibitors is not affected (Carlsson et al., 1966; Weissman et al., 1966). In contrast, behavioral effects of DAT inhibitors, but not amphetamine-like drugs, are antagonized by reserpine, a drug that depletes catecholamine storage vesicles (Weissman et al., 1966). The ability of the DAT inhibitors but not dopamine releasers to increase extracellular dopamine in dialysis experiments is blocked by tetrodotoxin, showing that impulse flow or neuronal activity is necessary for the DAT inhibitors to exert their effects (Westerink et al., 1987; Carboni et al., 1989; Nomikos et al., 1990). The current study seek to determine whether the enhanced behavioral and neurochemical responses induced by cocaine in adolescents are induced by other stimulant drugs from both classes of psychostimulants. To accomplish this goal, we have contrasted the behavioral and neurochemical responses to representative drugs from each class in adolescents and adults. Differences in developmental effects of each drug class could identify age-related differences in DAT function and dopamine neurotransmission.
Fast-scan cyclic voltammetry in anesthetized rats was used to measure electrically stimulated dopamine efflux in dorsal striatum. There is good correlation between the effects of cocaine on electrically stimulated dopamine efflux in anesthetized rats and spontaneous release and uptake events in awake rats (Greco and Garris, 2003; España et al., 2008). Michaelis-Menten parameters for uptake are not different in anesthetized and awake rats (Garris et al., 2003). A single compound was chosen from each category, DAT inhibitors and dopamine releasers. We chose methylphenidate because it effectively increased locomotor behavior and behavioral rating in all ages. We chose amphetamine (1 mg/kg) because our initial observations suggested a trend for PN28 rats to be more activated than adults in the earliest intervals. Neurochemical experiments were performed only in the youngest and oldest age groups because behavioral stimulation was most disparate in these groups.
Materials and Methods
Subjects
Male Sprague-Dawley rats were acquired from Charles River Laboratories (Raleigh, NC) and housed in self-ventilated cages by age. Animals were housed in a vivarium with a 12-h light/dark cycle and given ad libitum access to food and water. Rats PN28, 42, and 65 (±1 day) were used to correspond to early adolescence, midadolescence, and adulthood, respectively (Spear, 2000). These animals were shipped and received on PN21, 35, and 58 and given 1 week to acclimate in our facility. All experiments were approved by the Duke University Institutional Animal Care and Use Committee.
Drugs
Methylphenidate, nomifensine, GBR12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], urethane, and amphetamine were purchased from Sigma-Aldrich (St. Louis, MO), and solutions were made fresh in saline and injected intraperitoneally at 1 ml/kg. MDMA was obtained from RTI International (Research Triangle Park, NC), courtesy of the National Institute on Drug Abuse.
Locomotor Activity
Motor activity was determined in eight open-field photocell devices (Kinder Scientific, Inc., Poway, CA). The devices consisted of a Plexiglas arena (40 cm for each dimension) with corn cob bedding on the floor. Computer software supplied by the manufacturer recorded interruptions of photobeams spaced 2.54 cm (1 inch) apart and reported distance traveled. Assignment to test chambers was counterbalanced across testing days with respect to age. Habituation test sessions began when rats were placed in the open arena without injection. After this 1-h session, all rats were injected with one of the drugs, and data recording was started immediately.
Observational Behavioral Measurements
The topography of behavior was assessed simultaneously with locomotor activity by recording the occurrence of inactivity, rearing, grooming, locomotion, sniffing, continuous sniffing, and behavioral rating during three observation periods consisting of 15 s each, every 5 min, beginning 5 min after dosing. This approach ensured that the automated locomotor behavior measurements were not confounded by a greater stereotypy response in a particular age group and not another. Observations after injection of 1 mg/kg amphetamine were done at 10-min intervals for 1 h. Stereotypy included head weaving or bobbing, patterned locomotion, paw treading, and dyskinesia. A single observer, blinded to the drug treatment, watched all the rats in individual experiments. For each of the three 15-s observation periods, a summed behavioral rating score according to a noncontinuous 6-point behavioral rating scale that has been described previously (Walker et al., 2001). This scale provides a relative measure of behavioral activity with higher numbers denoting more intense behavioral activity than lower numbers. These three scores were then averaged to obtain a score for that minute. The scoring system was as follows: 1, inactive; 2, grooming or locomotion or sniffing or rearing; 3, sniffing with locomotion and/or rearing, or continuous sniffing; 4, continuous sniffing with continuous motion; 5, frequent stereotyped movements with locomotion; and 6, almost continuous stereotyped movements, restricted to one place in the cage.
In Vivo Electrochemistry
In Vivo Methods.
Rats were anesthetized with urethane (1.5 g/kg i.p.) and positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Body temperature was maintained at 37°C with a Deltaphase Isothermal Pad (Braintree Scientific, Braintree, MA). A bipolar stimulating electrode (Plastics One Inc., Roanoke, VA) was positioned in the medial forebrain bundle, and biphasic stimulation parameters were 300 μA, 2 ms each phase. The stereotactic coordinates (in millimeters) anteroposterior (AP) and mediolateral (ML) from bregma and dorsoventral (DV) from dura follow: the stimulating electrode was placed at −4.6 AP, +1.4 ML, and −7.5 to −9.0 DV. The carbon-fiber microelectrode was directed at the center of the caudate (+1.2 AP, 2.0 ML, and −4.5 to −5.6 DV). To compensate for the smaller size of the PN28 rats, the ML placement of the stimulating electrode was +1.35.
The locations of the stimulating and working electrodes were optimized to give maximal dopamine responses. Extracellular dopamine concentrations resulting from 60 pulse stimulation trains at frequencies from 10, 20, 30, 40, 50, to 60 Hz were recorded. Immediately after the final baseline data collection, the rat was administered 10 mg/kg methylphenidate or 1 mg/kg amphetamine intraperitoneally. These doses were used in behavioral experiments. The time course of drug effects on extracellular dopamine was monitored at 20 Hz because the effect of uptake inhibition is frequency-dependent and most robust at this frequency. Twenty-Hertz stimulations commenced immediately after drug injection (approximately 1 min) and were repeated at 2.5, 5, 7.5, 10, 15, and 30 min after drug. Drug responses to stimulations at the other frequencies were recorded between 20 and 40 min after drug administration.
Electrochemistry.
Voltammetry procedures were similar to our previously published methods (Walker and Kuhn, 2008). Fast-scan cyclic voltammetry was conducted with an EI-400 potentiostat (Ensman Instrumentation, Bloomington, IN). The potential at carbon fiber electrodes was held at −400 mV, ramped to 1 V, and then back to −400 mV at 300 V/s. Cyclic voltammograms were recorded at 10 Hz. Carbon-fiber microcylinder electrodes, prepared from 7-μm-diameter T-300 fibers with approximately 50 to 100 μm of exposed carbon fiber (Amoco, Greenville, SC), were used in the in vivo experiments along with a silver/silver chloride reference wire.
Changes in extracellular dopamine were determined by monitoring the current over a 100-mV window at the peak oxidation potential for dopamine. The electroactive substance was identified as dopamine by comparing background subtracted cyclic voltammograms from the in vivo stimulations with those collected at the same electrode in vitro after the experiment. Oxidation currents in vivo were converted to dopamine concentrations by calibrating the electrodes with dopamine standard solutions in a flow injection system after experimental use.
Data Analysis
Group averages are expressed as the mean ± S.E.M., and n is the number of rats. Effects of age and time after injection on locomotor behavior and behavioral rating were determined using two-way ANOVA with repeated measures on time. Drug-induced changes in dopamine efflux were expressed relative to the baseline in each rat because of age differences in baseline [DA]max. Group averages of percentage of dopamine changes (within animal) were calculated and displayed. The effects of age (PN28 versus PN65 rats) on percentage of changes in drug-induced dopamine efflux were analyzed by two-way ANOVA with repeated measures on time. When significant main effects were found, post hoc analysis with Newman-Keuls multiple-comparison test was to determine differences between groups. Statistical analysis was conducted with NCSS 2000 software (NCSS, Kaysville, UT). Differences were considered to be significant when p < 0.05. Outliers were determined using a statistical outlier test (Grubbs; GraphPad Software Inc., San Diego, CA).
Results
Motor Behavior.
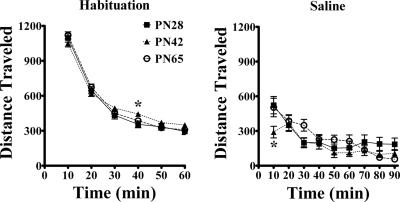
Age differences in spontaneous motor behavior during exploration of a novel open-field environment and after saline injection intraperitoneally were investigated. Each naive rat was placed in the test chambers for 1 h to habituate before injection of drug. Habituation data from all rats were compiled and are displayed in Fig. 1, left, resulting in a data set with a large N (PN28 = 109, PN42 = 110, and PN65 = 111). Horizontal activity was highest when rats were introduced to the chamber and activity habituated over the 1-h session. No age differences were seen (p = 0.56). Activity varied with time (F5,1629 = 633; p < 0.001) and age and time significantly interacted (F10,1629 = 2.52; p = 0.005). The interaction was caused in large part because PN42 rats were less active early and more active late, relative to PN28 and PN65 rats. Post hoc analysis showed that the only significant difference at individual time points was that activity was greater in PN42 than PN28 at 40 min of habituation.
Fig. 1.
Horizontal activity during habituation to a novel open-field device and after saline injection intraperitoneally. Initial habituation data from all male rats used in subsequent drug experiments were combined in the left graph: PN28 (n = 109), PN42 (n = 110), and PN65 (n = 111). There was no main effect of age but a significant age by time interaction. *, PN42 > PN28 at 40 min. A subset of these rats was injected with saline intraperitoneally after habituation, and the results are shown in the right graph: PN28 (n = 17), PN42 (n = 18), and PN65 (n = 15). No overall age difference was observed after saline injection, although the interaction of age and time was significant. *, PN42 < PN28 and PN65 at 10 min. Group means ± S.E. are shown in this and all figures. Error bars are smaller than the symbols in some cases.
After the 1-h habituation period, saline or drug was injected intraperitoneally. Figure 1, right, shows the effect of saline injection on distance traveled. As during habituation, age had no overall effect on locomotion (p = 0.21). Activity varied over time (F8,376 = 21; p < 0.001) and time and age interacted (F16,376 = 1.93; p = 0.02). Activity in PN42 rats was significantly lower than the other two groups at 10 min after saline injection (p < 0.05), and PN28 rats trended higher in the final three intervals, although post hoc analysis did not indicate significant differences for individual intervals.
Methylphenidate.
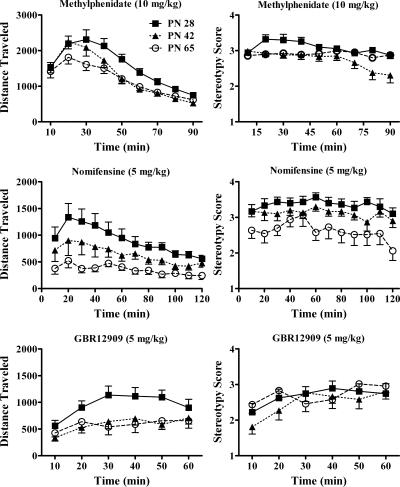
The age-dependent effects of DAT inhibitors methylphenidate, nomifensine, and GBR12909 on horizontal activity and experimenter-observed behaviors were determined. Figure 2 shows time courses of effects for each drug, and Fig. 4 shows session totals for locomotion and session means for behavioral rating. The locomotor stimulating effect of 10 mg/kg methylphenidate (n = 20 for PN28 and PN65 and 19 for PN42) was greatest in the youngest rats. ANOVA indicated an effect of age (F2,56 = 5.25; p = 0.008), and post hoc analysis indicated that PN28 rats exhibited more locomotion than PN42 and PN65 rats. Effects of interval after methylphenidate injection (F8,448 = 101, p < 0.001) and an interaction of interval and age also were found (F16,448 = 2.63; p < 0.001). Activity in PN42 rats was high in early intervals relative to adults, similar to PN28, but then it fell to lower levels similar to PN65.
Fig. 2.
Effects of inhibitors of the DAT on horizontal activity and behavioral rating in male rats at ages PN28, PN42, and PN65 in 10-min intervals are shown after administration of methylphenidate (10 mg/kg), nomifensine (5 mg/kg), or GBR12909 (5 mg/kg) (n values are indicated in the text). Behavioral observations were made simultaneously with the automated activity measurements. ANOVA indicates main effects of age for all three compounds for locomotion and interactions of age and time for methylphenidate and GBR12909. Methylphenidate and nomifensine effects on behavioral rating varied significantly with age and an interaction of age and time was found for methylphenidate.
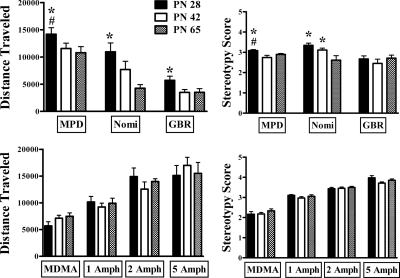
Fig. 4.
Summary of effects of all drugs on horizontal activity and behavioral rating. Data for distance traveled in Figs. 2 and 3 were summed for each age group. Behavioral rating data were averaged for the entire drug session. *, significantly different from PN65; #, p < 0.05, significantly different from PN42.
Observer-rated behavioral activation in a subset of these animals (n values: PN28 and PN65 = 16 and PN42 = 15) mirrored those for locomotion. Behavioral rating was highest in the youngest rats and ANOVA indicated a significant effect of age (F2,44 = 6.35; p = 0.004). Post hoc analysis showed that PN28 rats had significantly higher ratings than PN42 and PN65 rats (p < 0.05). Time after methylphenidate varied significantly (F8,352 = 7.83; p < 0.001) and time and age significantly interacted (F16,352 = 3.10; p < 0.001). Behavioral rating was higher in PN28 than PN65 in early intervals and waned in PN42 in late intervals.
Nomifensine.
Figures 2 and 4 display the effects of 5 mg/kg nomifensine on behavior of PN28, PN42, and PN65 male rats (n = 10 or 11/age). Nomifensine-induced locomotor behavior was inversely related to age. ANOVA indicated an overall effect of age (F2,28 = 6.57; p = 0.005). Post hoc analysis showed that PN28 rats ambulated more than PN65 rats (p < 0.05). Activity in PN42 rats was intermediate but not statistically different from other ages by post hoc analysis. Activity varied with time after injection (F11,308 = 11.6; p < 0.001), and there was no interaction with age (p = 0.13).
The effect of nomifensine on behavioral rating exhibited a similar age dependence. Age significantly affected behavioral rating (F2,28 = 5.82; p = 0.008). Newman-Keuls test indicated that nomifensine increased behavioral rating in both adolescent age groups more than in adult rats (p < 0.05). Behavioral rating varied with time after injection (F11,308 = 2.43; p = 0.007). Time did not interact with age (p = 0.81).
GBR12909.
GBR12909 (5 mg/kg) increased locomotor behavior and behavioral rating but only the effect on locomotion was age-related. GBR12909 effects on locomotion varied significantly across development (F2,37 = 3.92; p = 0.03; n = 13 or 14/age). Post hoc analysis showed that PN28 rats exhibited more locomotor behavior than PN65 rats (p < 0.05). GBR12909 effects varied with time after injection (F5,185 = 9.59; p < 0.001). The interaction of age and time was not significant (p = 0.07).
In contrast to the locomotor effects, GBR12909 did not affect behavioral rating differently across the age groups (n = 8). ANOVA reported a significant effect of time after injection (F5,104 = 6.63; p < 0.001), no age effect (p = 0.61) and no age × time interaction (p = 0.15).
Amphetamine.
Figures 3 and 4 show the effects of dopamine releasing drugs on spontaneous behavior. Multiple doses of amphetamine were tested because it is the prototypical drug in this class, and we wanted to span the dose range from low to high induction of stereotypy. A single dose of another amphetamine, MDMA, also was investigated.
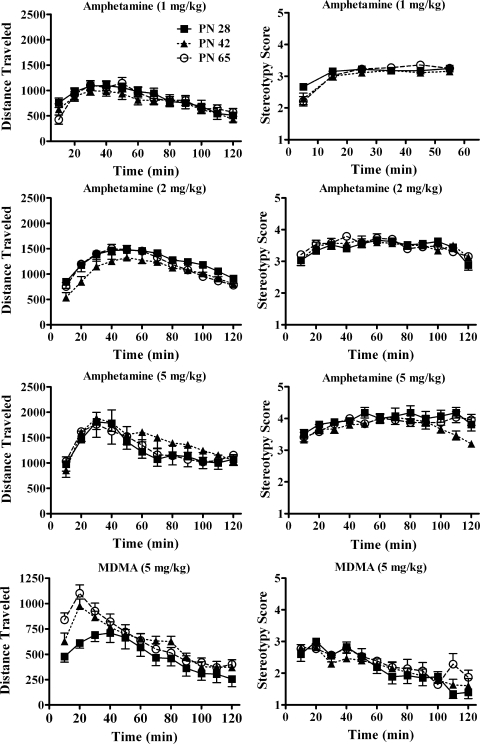
Fig. 3.
Effects of dopamine releasing drugs on horizontal activity and behavioral rating in male rats at ages PN28, PN42, and PN65 (n values are indicated in the text). All methods and descriptions are the same as described in Fig. 1. Multiple doses of amphetamine (1, 2, and 5 mg/kg) were tested to thoroughly examine age differences for this dopamine-releasing compound. None of the three amphetamine doses induced a main effect of age on locomotor or stereotyped behavior. An interaction of age and time was found only for 5 mg/kg amphetamine on behavioral rating. Age did not induce a significant effect on MDMA (5 mg/kg)-stimulated horizontal activity or behavioral rating, but an interaction of age and time was found for MDMA-induced locomotor behavior as activity was lowest in the early intervals in PN28 rats.
The effect of 1 mg/kg amphetamine on ambulatory behavior did not differ by age (F2,42 = 0.30; p = 0.74; n = 14–16/group). Time after injection significantly affected ambulations (F11,462 = 15.3; p < 0.001), and the interaction with age was not significant (p = 0.22).
The effect of 1 mg/kg amphetamine on behavioral rating was also not age-dependent (p = 0.33; n = 15 or 16/group). Time after injection was significant (F5,215 = 44; p < 0.001), but its interaction with age did not quite reach significance (F10,215 = 1.80; p = 0.062).
The effect of 2 mg/kg amphetamine on ambulatory behavior did not exhibit an overall effect of age (F2,42 = 0.67; p = 0.52; n = 9–11/group). Time after injection significantly affected ambulations (F11,462 = 12.4; p < 0.001), but the interaction with age was not significant (p = 0.086).
The effect of 2 mg/kg amphetamine on behavioral rating in the same rats was also not age-dependent (F2,27 = 0.22; p = 0.80). Time after injection was significant (F11,297 = 10.8; p < 0.001), but its interaction with age was not significant (p = 0.41).
The effect of 5 mg/kg amphetamine on ambulatory behavior did not exhibit an overall effect of age (F2,24 = 0.22; p = 0.80; n = 8–10/group). Time after injection significantly affected ambulations (F11,297 = 10.8; p < 0.001), but its interaction with age was not significant (p = 0.67).
The effect of 5 mg/kg amphetamine on behavioral rating in the same rats also was not age-dependent (F2,24 = 2.54; p = 0.10). Time after injection was significant (F11,297 = 6.31; p < 0.001). The interaction of time and age was significant (F22,264 = 1.63; p = 0.04) because in mid-to late intervals, ratings tended to be highest in PN28 and lowest in PN42 rats.
Methylenedioxymethamphetamine.
Figures 3 and 4 show the effect of 5 mg/kg MDMA on locomotor behavior (n = 15 or 17/group). Unlike the DAT inhibitors tested, locomotor effects of MDMA were not enhanced in periadolescents. In fact, the trend was the opposite. The effect of age on MDMA-stimulated locomotor behavior was not significant (F2,46 = 2.74; p = 0.075). The effect of time was significant (F11,476 = 50; p < 0.001). The time course graph (Fig. 3) shows that in the initial intervals after injection MDMA effects were less in PN28 rats relative to PN42 and PN65. ANOVA confirmed this as an interaction of age and time (F22,476 = 2.86; p < 0.001). Thus, the early surge in activity after MDMA was attenuated in periadolescents.
A subset of the animals tested for locomotor behavior also were rated for stereotypy (n = 6–8). The effects of 5 mg/kg MDMA on behavioral rating were not age dependent (p = 0.5). There was a significant effect of time after injection (F11,198 = 16.5; p < 0.001) but no interaction with age (p = 0.4).
Neurochemistry.
Fast-scan cyclic voltammetry was used to measure electrically stimulated dopamine efflux in dorsal striatum. A single compound was chosen from each category (DAT inhibitors versus dopamine releasers). Methylphenidate was chosen because it effectively increased locomotor behavior and behavioral rating in all ages and effects were age-dependent. The dose of 1 mg/kg amphetamine was chosen because there was at least a trend for PN28 rats to be more activated than adults (p < 0.10 for the interaction of age and time for both locomotion and rating). Neurochemical experiments were performed only in the youngest and oldest age groups because behavioral stimulation was most disparate in these groups.
Methylphenidate.
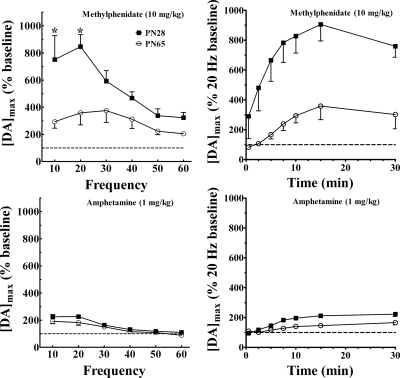
Methylphenidate effects on stimulated extracellular dopamine levels were determined across a range of frequencies and as a time course at one frequency, 20 Hz. Methylphenidate (10 mg/kg) increased stimulated extracellular dopamine concentrations relative to baseline in all rats. Figure 5 shows the increases in extracellular dopamine relative to the maximal efflux at each frequency before methylphenidate administration. Age strongly influenced the methylphenidate-induced relative increases in stimulated dopamine (Fig. 5). Methylphenidate increased dopamine levels more in adolescents (F1,8 = 12.4; p = 0.008; n = 5 for PN28 and PN65). Frequency exerted an effect on the relative stimulation of extracellular dopamine levels (F5,39 = 9; p < 0.001). Methylphenidate increased dopamine in adolescents more than adults particularly at the lowest frequencies leading to an interaction of age and frequency (F5,39 = 3.56; p = 0.01).
Fig. 5.
Age differences in the time course and frequency dependence of methylphenidate and amphetamine effects on dopamine overflow in caudate of anesthetized rats. Drug effects are expressed as a percentage of the change in DAmax (drug/predrug baseline). For methylphenidate, [DA]max was averaged across groups of PN28 (n = 5) and PN65 (n = 5) rats and for 1 mg/kg amphetamine, PN28 (n = 6) and PN65 (n = 7) rats. The drug effects were first determined at 1 min after injection and then at 2.5, 5, 7.5, 10, and 30 min. The dashed lines serve as a visual reference for the baseline values. *, p < 0.05, significantly different from the corresponding age group.
Age differences in methylphenidate effects on relative dopamine increases were prominent throughout the first 30 min after administration at 20-Hz stimulations (Fig. 5). ANOVA of age by time indicated significant effects of age (F1,8 = 12.9; p = 0.007), time (F6,48 = 23.4; p < 0.001), and the interaction of the two (F6,48 = 3.60; p = 0.005). Age differences in dopamine concentrations were greatest between 7.5 to 10 min after intraperitoneal injection.
Amphetamine.
Amphetamine effects on stimulated extracellular dopamine levels were determined using the methods described for methylphenidate in PN28 and PN65 male rats. Amphetamine effects were analyzed relative to predrug baseline levels (percentage of baseline) at each frequency tested. Amphetamine (1 mg/kg) increased stimulated extracellular dopamine in a frequency-dependent manner (F5,55 = 44.6; p < 0.001). Across this range of frequencies, age did not exert a statistical effect (p = 0.11), and there was no interaction with frequency (p = 0.50).
The relative increase in extracellular dopamine by amphetamine was greater in the adolescent than adult rats as indicated by main effect of age (F1,11 = 8.23; p = 0.015; n = 6 for PN28 and n = 7 for PN65). Amphetamine effects varied with time after administration (F8,82 = 24.7; p < 0.001), and age and time significantly interacted (F8,82 = 6.36; p < 0.001). This interaction reflects the results showing that peak amphetamine effects occurred between 10 to 30 min for adolescents and between 30 to 60 min for adults.
Amphetamine enhanced extracellular dopamine more in adolescent rats at 20 Hz, and the peak increases were earlier in adolescents than adults. Relative to methylphenidate effects however, age differences caused by amphetamine were more modest.
Discussion
The present study demonstrates that selective DAT inhibitors induce more spontaneous motor behavior in adolescent than adult rats. In contrast, dopamine releasing drugs did not. The early increase in locomotor behavior induced by MDMA was greater in adult than adolescent rats. The age differences spanned a large range of maximal locomotor stimulation, suggesting that the phenomenon is robust and consistent. Effects on stimulated dopamine efflux reflected the behavioral differences: methylphenidate stimulated dopamine efflux more in adolescents, but amphetamine did not. Thus, greater relative stimulation of dopamine by selective DAT inhibitors in periadolescents partly explains the distinct age differences.
Reported effects of amphetamine vary across laboratories. Vasilev et al. (2003) reported that amphetamine (1.5 mg/kg) induced less locomotion and stereotyped behavior in PN28 to 30 than 90-day-old adult hooded males. Bolanos et al. (1998) also found lower locomotor effects of 0.5 and 1.5 mg/kg amphetamine in PN35 rats than in PN80 male rats. Although these results differed from the present results no age differences in the effects of 1, 2 or 5 mg/kg amphetamine, they support our conclusion that developmental effects of amphetamine differ from those of dopamine uptake inhibitors. Wooters et al. (2006) showed that the acute locomotor effects of methylphenidate in periadolescent males were approximately double those in the adults. These reports generally agree with the present findings.
Age differences in pharmacokinetics could contribute to age differences in the behavioral effects of psychostimulants. Determining the kinetics of each of these drugs across development was beyond the scope of the present study. Unfortunately, the literature provides few answers for the issue. In general, blood levels of these psychostimulants may be slightly lower in adolescents than adults (Spear, 2007). We have previously examined brain cocaine levels in adolescent and adult males using a repeated dose model and found no significant differences across age (Caster et al., 2005). Age-related differences in acute cocaine metabolism have not been identified, which would explain greater the behavioral responses of adolescents. A review article mentions that brain amphetamine levels are lower in adolescent (PN25) than adult rats (Spear and Brake, 1983). This age-related pharmacokinetic difference did not correlate with the age-related behavioral differences reported in that article. If amphetamine concentrations are in fact lower in the adolescent brain, this would confound the present results. However, the weight of the evidence from this and our other studies with cocaine showing that four DAT inhibitors are more effective in adolescents and two dopamine releasers are not suggests that it is unlikely that age differences in pharmacokinetics is a sufficient explanation.
The present study confirms and extends other dopamine work. Stamford (1989) showed that nomifensine (10 mg/kg i.p.) increased electrically stimulated dopamine efflux more in the striatum of young (30-day-old) rats relative to adults, using very similar voltammetry methods to those in the current study. In addition, our laboratory showed previously that cocaine increased extracellular dopamine more in PN28 than PN65 rats (Walker and Kuhn, 2008). Thus, three studies using electrically stimulated dopamine efflux have reported that effects of cocaine, nomifensine and now methylphenidate are enhanced in dorsal striatum of periadolescents relative to adults. Other reports of age-related effects of these compounds on dopamine include microdialysis studies in nucleus accumbens. A low dose of cocaine increased extracellular dopamine more rapidly in nucleus accumbens of PN35 rats (Badanich et al., 2006). Frantz et al. (2007) did not find age differences in the nucleus accumbens shell at baseline or after 20 mg/kg cocaine i.p. This agrees with our previous report finding greater electrically stimulated dopamine efflux in dorsal striatum not nucleus accumbens core (Walker and Kuhn, 2008). Cao et al. (2007) found lower basal dopamine in ventral but not dorsal caudate putamen of PN29 rats relative to adults but did not report whether cocaine induced a significant effect. One important caveat with the current results is that our technique might not have captured spontaneous increases in basal dopamine that might have been induced by the low dose used here.
Differences in the mechanism of DAT inhibition might explain the present behavioral results. Amphetamine-like compounds are fundamentally different from cocaine and other DAT inhibitors because they are a substrate for DAT and are transported into the cell. Slightly different binding sites on DAT or interactions with DAT between these classes of stimulants may explain their differing functional effects. Some studies show that the binding sites of dopamine, cocaine, and amphetamine overlap (Beuming et al., 2008), whereas others demonstrate differences between amphetamine and other psychostimulants in their binding or inhibition of DAT (Dersch et al., 1994; Wayment et al., 1998). Differences in DAT inhibition have functional implications. For example, high-cocaine-responding rats were found to have greater dopamine uptake than low responders, and uptake in individual rats was correlated with cocaine-stimulated behavior (Briegleb et al., 2004). However, none of these relationships existed for amphetamine-stimulated behavior, suggesting that functional DAT expression on the cell surface is related to cocaine- but not amphetamine-stimulated behavioral activation (Briegleb et al., 2004). Furthermore, a novel benztropine analog that occupies DAT completely blocks behavioral and conditioned effects of cocaine but not amphetamine (Velazquez-Sanchez et al., 2009). Carboni et al. (1989) showed that the dopamine-elevating effect of amphetamine is independent of impulse flow because γ-butyrolactone blocked the dopamine increases induced by cocaine and nomifensine but not amphetamine-induced increases. Similarly, using tetrodotoxin to inhibit action potential propagation, nomifensine, cocaine, GBR12909, and methylphenidate but not amphetamine were shown to be dependent on impulse flow to increase extracellular dopamine in dialysates (Nomikos et al., 1990). In this context, the present results suggest that developmental differences in DAT structure–function are related to the cocaine but not the amphetamine binding site on DAT.
Forebrain dopamine systems continue to mature and reorganize across adolescence (for reviews, see Andersen, 2003; Kuhn et al., 2010). Dopamine innervation of the dorsal and ventral striatum is incomplete during early adolescence, and most presynaptic markers including DAT expression and dopamine stores have not yet attained adult levels. There is also an overproduction followed by regressive “pruning” of striatal dopamine receptors during adolescence. Such maturational events in dopamine systems could probably affect the behavioral responsiveness to stimulants across adolescence independent of sex hormones. However, the parallel increase in DAT expression and DA stores that have been observed do not suggest an obvious explanation for differences in the actions of DAT inhibitors and DA-releasing drugs.
That adolescent dopaminergic transmission is more regulated by DAT than adults would contribute to the present findings. We have postulated that uptake inhibition by cocaine enhances extracellular dopamine more in the adolescent striatum because at baseline, the ratio of uptake to release is greater in adolescent striatum (Walker and Kuhn, 2008). Release capacity is lower in periadolescent than adult striatum. The lesser relative effects of amphetamine could be related to the limited stores available for release in early adolescence as dopamine content and release capacity is less in dorsal striatum of adolescents than adults (Kuhn et al., 2010). Serotonin innervation is also immature in the periadolescents (Moll et al., 2000; Galineau et al., 2004), which is significant because the two dopamine releasers used in these studies would have elevated extracellular serotonin levels more than the DAT inhibitors (Kuczenski and Segal, 1997). Increased serotonin levels should attenuate the hyperlocomotion induced by hyperdopaminergia (Gainetdinov et al., 1999), an effect that should be greater in the adults. The enhanced serotonin elicited by releasers would be expected to decrease locomotor behavior preferentially in the adults, serving to attenuate the presently observed age by psychostimulant category difference.
Putative developmental differences in DAT glycosylation represent one potential mechanism for the greater sensitivity of adolescents to DAT inhibitors. Patel et al. (1994) found that DAT from the striatum of adult rats had a higher molecular weight than DAT from rats at 0, 4, and 14 days of age, and they showed that this size difference is due to adult DAT being more glycosylated. Cocaine was more potent for inhibition of dopamine uptake into cells expressing the least glycosylated mutant because nonglycosylated DAT has greater affinity for dopamine than normal DAT (Li et al., 2004). Thus, specific DAT inhibition should induce a greater relative change in extracellular dopamine in the adolescents and presumably induce more behavioral effects.
We have shown previously that cocaine effects on behavior and stimulated dopamine efflux are greater in adult female than adult male rats and that psychostimulant responses fall across development in males (Parylak et al., 2008). The present results suggest that this latter effect exists for a broad array of DAT inhibitors. Thus, the behavioral response to clinically used psychostimulants might be expected to change across development, depending on the mechanism of action. This phenomenon could have implications for pharmacotherapy of attention-deficit hyperactivity disorder for children, adolescents, and adults.
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grant DA019114].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.167320.
- PN
- postnatal day
- GBR12909
- 1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine
- MDMA
- methylenedioxymethamphetamine
- DAT
- dopamine transporter
- ANOVA
- analysis of variance
- DA
- dopamine.
References
- Andersen SL. (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18 [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. (2006) Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol 550:95–106 [DOI] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, et al. (2008) The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11:780–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. (1998) Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res 111:25–33 [DOI] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. (2004) Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology 29:2168–2179 [DOI] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. (2007) Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology 32:2279–2289 [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. (1989) Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 28:653–661 [DOI] [PubMed] [Google Scholar]
- Carlsson A, Fuxe K, Hamberger B, Lindqvist M. (1966) Biochemical and histochemical studies on the effects of imipramine-like drugs and (+)-amphetamine on central and peripheral catecholamine neurons. Acta Physiol Scand 67:481–497 [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. (2005) Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 183:218–225 [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. (2007) A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology (Berl) 193:247–260 [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. (2003) Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 160:1041–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersch CM, Akunne HC, Partilla JS, Char GU, de Costa BR, Rice KC, Carroll FI, Rothman RB. (1994) Studies of the biogenic amine transporters. 1. Dopamine reuptake blockers inhibit [3H]mazindol binding to the dopamine transporter by a competitive mechanism: preliminary evidence for different binding domains. Neurochem Res 19:201–208 [DOI] [PubMed] [Google Scholar]
- España RA, Roberts DC, Jones SR. (2008) Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience 155:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. (2007) Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology 32:625–637 [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283:397–401 [DOI] [PubMed] [Google Scholar]
- Galineau L, Kodas E, Guilloteau D, Vilar MP, Chalon S. (2004) Ontogeny of the dopamine and serotonin transporters in the rat brain: an autoradiographic study. Neurosci Lett 363:266–271 [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. (2003) A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience 118:819–829 [DOI] [PubMed] [Google Scholar]
- Greco PG, Garris PA. (2003) In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol 479:117–125 [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. (1997) Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem 68:2032–2037 [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. (2010) The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav 58:122–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME. (2004) The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem 279:21012–21020 [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. (2009) Age matters. Eur J Neurosci 29:997–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen BA. (1983) CNS stimulants: two distinct mechanisms of action for amphetamine-like drugs. Trends Pharmacol Sci 4:429–432 [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. (2000) Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res 119:251–257 [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. (1990) In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis. Synapse 6:106–112 [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. (2008) Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav 89:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AP, Cerruti C, Vaughan RA, Kuhar MJ. (1994) Developmentally regulated glycosylation of dopamine transporter. Brain Res Dev Brain Res 83:53–58 [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. (2009) Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 206:1–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463 [DOI] [PubMed] [Google Scholar]
- Spear LP. (2007) Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol 29:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC. (1983) Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol 16:83–109 [DOI] [PubMed] [Google Scholar]
- Stamford JA. (1989) Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. J Neurochem 52:1582–1589 [DOI] [PubMed] [Google Scholar]
- Vasilev V, Veskov R, Janać B, Rakić Lj, Stojiljković M. (2003) Age-related differences in MK-801- and amphetamine-induced locomotor and stereotypic activities of rats. Neurobiol Aging 24:715–723 [DOI] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, Ferragud A, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, Canales JJ. (2009) The dopamine uptake inhibitor 3 alpha-[bis(4′-fluorophenyl)methoxy]-tropane reduces cocaine-induced early-gene expression, locomotor activity, and conditioned reward. Neuropsychopharmacology 34:2497–2507 [DOI] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan K, Li ST, Haroon J, Spohr HA, Kuhn CM. (2001) Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology 25:118–130 [DOI] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. (2008) Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol 30:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Schramm-Sapyta NL, Caster JM, Waller ST, Brooks MP, Kuhn CM. (2009) Novelty-induced locomotion is positively associated with cocaine ingestion in adolescent rats; anxiety is correlated in adults. Pharmacol Biochem Behav 91:398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayment H, Meiergerd SM, Schenk JO. (1998) Relationships between the catechol substrate binding site and amphetamine, cocaine, and mazindol binding sites in a kinetic model of the striatal transporter of dopamine in vitro. J Neurochem 70:1941–1949 [DOI] [PubMed] [Google Scholar]
- Weissman A, Koe BK, Tenen SS. (1966) Antiamphetamine effects following inhibition of tyrosine hydroxylase. J Pharmacol Exp Ther 151:339–352 [PubMed] [Google Scholar]
- Westerink BH, Tuntler J, Damsma G, Rollema H, de Vries JB. (1987) The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn Schmiedebergs Arch Pharmacol 336:502–507 [DOI] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. (2006) Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology (Berl) 188:18–27 [DOI] [PubMed] [Google Scholar]