Abstract
Nitric oxide relaxes myometrium in a cGMP-independent manner. Although cGMP activates its cognate kinase, this is not required for the inhibitory effect of nitric oxide. Thus, nitric oxide-mediated cGMP elevation does not enjoy the same set of substrates as it does in other smooth muscles. To further understand the regulation of relaxation of uterine muscle by cGMP, we have studied the actions of peptide-mediated cGMP action in guinea pig myometrium. We used both functional and biochemical studies of the action of the particulate guanylyl cyclase activator uroguanylin and its receptor, particulate guanylyl cyclase type C, to address the relationship between cGMP elevation acting in the membrane signaling domain to that of the nonmembrane region of the cell. Uroguanylin relaxed oxytocin-induced contractions in a dose-dependent fashion only in pregnant myometrium. Both relaxation and cGMP accumulation after uroguanylin stimulation were blocked by the putative particulate guanylyl cyclase type C inhibitors 2-chloro-ATP and isatin (1H-indole-2,3-dione), but not by the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-A]quinoxalin-1-one (ODQ). Uroguanylin stimulated cGMP accumulation only in the pregnant myometrium. Caveolin-1 expression increased in pregnancy toward term. In the caveolin-1-containing membrane domain, uroguanylin, but not the nitric-oxide donor, led to the elevation of cGMP that was insensitive to ODQ. Particulate guanylyl cyclase C was expressed and prouroguanylin was detected in pregnant myometrium. We conclude that a uroguanylin–particulate cyclase-cGMP relaxation pathway is present and cGMP is compartmented in myometrium. The agonist-mediated selectivity of relaxation to cGMP is of fundamental pharmacological interest in understanding signal transduction in smooth muscle.
Introduction
We and others have shown that nitric oxide (NO)-mediated relaxation of myometrial smooth muscle is independent of global elevations in cGMP (Kuenzli et al., 1996, 1998; Bradley et al., 1998; Hennan and Diamond, 1998, 2001), and moreover, that levels of myometrial cGMP-dependent protein kinase I (PKGI) are decreased during pregnancy (Word and Cornwell, 1998). Despite the apparent uncoupling of relaxation in myometrium (MYO) from cGMP accumulation, it is clear that activation of PKGI and protein phosphorylation still occur in myometrium after NO stimulation (Tichenor et al., 2001). Nonetheless, blockade of cGMP accumulation by treatment with soluble guanylyl cyclase (sGC) inhibitors does not prevent NO-mediated myometrial relaxation (Buxton et al., 2001). This apparent lack of cGMP involvement in relaxation despite cGMP activation of its cognate kinase, PKG, remains unexplained, although the hypothesis that cGMP action in myometrium is compartmented has been proposed (Buxton, 2004).
Evidence of cyclic nucleotide compartmentation was first proven at the cellular level for cAMP in cardiac myocytes by Buxton and Brunton (1983). This phenomenon has now been shown for cGMP action as well (Kuenzli et al., 1996; Castro et al., 2006; Piggott et al., 2006). In considering how cGMP could regulate smooth muscle relaxation independently of the global elevations of cGMP that can be measured after activation of sGC by NO donors, we have considered the role of particulate cGMP accumulation (Buxton, 2004). Although the non-cGMP-dependent actions of NO in myometrium will offer interesting insights into the disparate biochemistry of uterine versus other smooth muscles, our hypothesis that cGMP action is compartmented in the uterine myocyte means that cGMP elevation after particulate guanylyl cyclase (pGC) activation acts at effectors disparate from those activated by soluble guanylyl cyclase-mediated cGMP elevation. Understanding how cGMP acts after particulate guanylyl cyclase activation could offer heretofore unrecognized targets for treatment of preterm labor, a condition affecting as many as 12% of pregnancies in the United States (Behrman and Butler, 2006). Preterm delivery, which results from preterm labor 50% of the time, cannot be prevented by current treatments; there is no Food and Drug Administration-approved tocolytic to prevent uterine contractions; and no tocolytic available in the United States has been developed based on actions in the myometrium (Tan et al., 2006).
It has been shown that mRNA expression of a 16-aa peptide known as uroguanylin (uGN) is up-regulated in pregnant myometrium of the rat (Girotti and Zingg, 2003). In animal studies, specific binding sites for heat-stable enterotoxin binding sites as a measure of pGC type C (pGC-C) expression were found in extraintestinal tissues such as the kidney, airway epithelium, liver, stomach, brain, and adrenal glands (Forte et al., 1989; Krause et al., 1990). Because uGN is well characterized to activate pGC-C in the GI system and kidney (Ohbayashi et al., 1998; Forte et al., 2000), we examined the possibility that activation of pGC-C, known to signal through PKGII to promote ion secretion across epithelial cells of the renal and GI system (Forte et al., 2000; Vaandrager, 2002), might act in myometrium. Presence of the receptor for uGN, pGC-C, has not previously been examined in myometrium.
Here, we describe studies of the action of uGN on uterine smooth muscle relaxation in the guinea pig and provide evidence for the expression of pGC-C and a role for particulate cGMP in uGN-mediated relaxation.
Materials and Methods
Contractile Studies.
Virgin female guinea pigs or timed pregnant animals at different stages of gestation were sacrificed under isofluorane anesthesia according to an Institutional Animal Care and Use Committee-approved protocol. Virgin animals were estrogen-primed with 1 mg of estradiol 17β in corn oil (0.5 ml) injected subcutaneously each day for 2 days, and the animals were sacrificed on day 3. Estrogen treatment has the effect of bringing animals into estrus so that control comparisons are uniform. Uterine horns were removed and placed in HEPES-buffered Krebs' solution without Ca2+ containing 118 mM NaCl, 4.75 mM KCl, 1.2 mM KH2PO4, 0.25 mM NaHCO3, 1.2 mM MgSO4, 20 mM dextrose, and 25 mM Na HEPES, pH 7.4. Tissues were opened longitudinally in a dissecting dish. For pregnant samples, fetuses were removed along with their placenta, and regions of uterus between placentas in the upper third of the horn were dissected for contractile experiments. Muscle was dissected away from the decidua (DEC), and the myometrium was cut to 1 × 4-mm strips and mounted between a fixed point and a force transducer (Kent Scientific, Torrington, CT) in 5 ml of tissue baths filled with Kreb's buffer (118 mM NaCl, 4.75 mM KCl, 1.8 mM CaCl2, 1.20 mM KH2PO4, 25 mM NaHCO3, 1.2 mM MgSO4, and 20 mM glucose, pH 7.4). Transducer voltages were amplified and converted to digital signals by an analog-to-digital board mounted within a computer system running the DaisyLab 10 data acquisition system (I/O Tech, Norton, MA). Strips were maintained at 37°C, aerated with 95% O2/5% CO2, and loaded with initial tensions of 1.2-g force as described previously (Kuenzli et al., 1996). During the course of a 1-h equilibration period, tissues were routinely challenged first with high potassium (60 mM KCl) and subsequently with 100 nM oxytocin (OT) followed by washout. Tissues were allowed to equilibrate for 1 h before experiments.
uGN (Sigma-Aldrich, St. Louis, MO) was added to organ baths (1:1000) from a concentrated stock solution made daily and protected from light for which ascorbate (10−4 M) was the diluent. The effects on both spontaneous and OT-stimulated tissues were studied over 15 min of addition followed by washout and were quantified as area under the force curve for 15 min before treatment and 15 min after treatment. Additions of ascorbate (blank) or peptide were blinded from the experimenter to serve as control. The putative pGC-C inhibitor 2-chloro-ATP (2Cl-ATP) was disolved in Kreb's buffer and added to the tissue bath from a 1:1000 working stock. 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) was dissolved in dimethyl sulfoxide and diluted 1:10,000 from a working stock. Isatin (1H-indole-2,3-dione; Frolova et al., 1988) was dissolved in hot ethanol (70°C) and diluted 1:100,000 from a working stock. Dimethyl sulfoxide at a final concentration of 0.01% or ethanol at 0.001% did not have any effect on contraction.
Uroguanylin Expression by RT-PCR.
Total RNA was prepared by using the TRIzol method (Invitrogen, Carlsbad, CA) from 50 mg of (wet weight) of dissected myometrium. Resulting RNA was resuspended in 30 μl of diethyl pyrocarbonate-treated H2O. Any DNA contamination remaining was removed by treatment at 37°C with 10 U of RNase-free DNase I (Promega, Madison, WI). The enzyme was then inactivated by adding 25 mM EDTA with heating at 55°C for 10 min. cDNA was synthesized from 1 μg of total RNA using 500 ng oligo-dT15 (Promega), 2 mM deoxynucleoside-5′-triphosphates, 10 mM dithiothreitol, and 200 units Superscript II reverse transcriptase (Invitrogen).
Primers for PCR of uroguanylin were designed based on the human uroguanylin gene sequence (GUCA2B) from which the uroguanylin peptide is cleaved. Primers designed to detect β-actin cDNA served as a cDNA control, and human kidney DNA (Ambion, Austin, TX) served as an expression-positive sample control. Thermal cycling was performed for 40 cycles at 95°C for 1 min, 60°C for 45 s, 72°C for 1 min. For analysis, 5 μl of each resulting product was separated by electrophoresis for 1 h at −100 mAmps on a 1% agarose gel. Gels were bathed in 2 mg/ml ethidium bromide for 10 min and imaged with UV light in a gel-documentation apparatus from Alpha Innotech (San Leandro, CA). Differences in RT-PCR patterns obtained from replicate (8–12) samples were then quantified by using dot densitometry of gel bands to look at relative intensities and molecular weights based on a standard bp ladder. PCR products were verified by sequence analysis (University of Nevada Reno Genome Center).
Western Blot Analysis.
Tissues collected for RT-PCR were divided to provide samples for immunoblot analysis of the presence of uGN in endometrium versus myometrium and in full-thickness uterine samples through term pregnancy. Tissues were pulverized in a liquid N2-precooled (−196°C) stainless-steel mortar and pestle and reconstituted into buffer containing 5 mM KH2PO4, 5 mM K2HPO4, 5 mM EGTA, and the protease inhibitor 4-(2-aminoethyl) benzenesulphonyl fluoride hydrochloride (AEBSF; 1 mM), pH 7.4. Homogenates (glass–glass, on ice) were centrifuged (48,000g) for 40 min, and aliquots of the supernatant were diluted with a 4× solution of sample buffer (240 mM Tris base, 4 mM dithiothreitol, 270 mM SDS, 40% glycerol, and 0.12% BP blue, pH 6.8) and boiled for 10 min. Samples were loaded onto 8 to 15% polyacrylamide gels (gradient and fixed cross-linking as needed), and proteins were separated by electrophoresis for 1 h at a constant 150 mV. Gel samples were transferred to polyvinylidene fluoride sequencing membrane for 2 h at 4°C by using a wet transfer apparatus (6 V). Blots were blocked overnight in 1 mM Tris buffer containing 0.5% gelatin and 0.05% Tween 20, pH 7.4 in a sealed container at room temperature and washed three times using the same buffer. Blots were probed in the above buffer containing 0.1% gelatin for 1.5 h with primary antibody recognizing caveolin 1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), pGC-C (Abcam Inc., Cambridge, MA), or pro-uGN (Calbiochem, San Diego, CA) as needed. Respective secondary antibodies conjugated to either infrared 680 or infrared 800 fluorescent dye (1:100,000; Invitrogen or Rockland Immunochemicals, Gilbertsville, PA) were used for detection. Antibody incubations were carried out in 1:1 Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) and phosphate-buffered saline (PBS) with 0.1% Tween 20 (v/v) at 4°C. Bands were visualized by using an infrared imaging system (LI-COR Biosciences V2.04) using both the 700- and 800-nm channels. Relative protein levels were quantified by densitometry and normalized to GAPDH (1:1500 mouse IgG; Santa Cruz Biotechnology, Inc.) for each individual sample.
Measurement of cGMP.
Tissues were mounted for contractile studies; once treated under various conditions they were frozen within 5 s by using a liquid nitrogen spray gun (Brymill, Ellington, CT). For total cGMP content, without allowing samples to thaw, tissues were homogenized with a Kontes-Duall glass homogenizer (Thermo Fisher Scientific) in 1 ml of 7% trichloroacetic acid dissolved in acetone while being constantly immersed in a methanol-dry ice slurry. Precipitated protein was removed by centrifugation, trichloroacetic acid was removed by triplicate extraction with H2O-saturated diethyl ether, and samples were heated to 70°C for 10 min to evaporate residual ether. After lyophilization, samples were resuspended in PBS containing 137 mM NaCl, 2.7 mM KCl, 0.9 mM KH2PO4, 6.4 mM Na2HPO4, pH 7.4) and assayed for cGMP by enzyme-linked immunoassay using antibodies obtained from Cayman Chemical (Ann Arbor, Ml) as done previously (Buxton et al., 2001). Protein in the acid precipitate was neutralized and assayed by the method of Lowry (Butcher and Lowry, 1976).
For cGMP content in soluble versus the detergent-insoluble, glycolipid-rich fractions (DIGs as described below), homogenates of frozen powder were prepared in PBS, pH 7.4, with addition of the phosphodiesterase inhibitor zaprinast (10 μM) using a tissue grinder and clarified with low-speed centrifugation (50g for 5 min), and the resulting supernatant was spun at 48,000g for 40 min at 4.0°C. This procedure creates simple soluble and particulate fractions that can be the basis of evidence for cyclic nucleotide compartmentation in muscle (Buxton and Brunton, 1983; Buhimschi et al., 2000).
Preparation of DIGs Signaling Domains.
Homogenates of frozen powder were prepared in DIGs buffer containing 150 mM NaCO3, pH 11, 0.001 mM leupeptin, 0.0005 mM AEBSF, 5 mMNaF, 10 mM EGTA, and 10 mM EDTA, pH 7.4 using a tissue grinder and clarified with low-speed centrifugation (50g for 5 min), and the resulting supernatants were prepared for separation of DIGs signaling domain and nonsignaling domain fractions. Experiments were performed in the presence of 10 μM zaprinast to prevent cGMP degradation. Protein was measured in the starting homogenate and the final fractions using the method of Lowry (Butcher and Lowry, 1976). The homogenate was placed in a plastic test tube (600 mg of protein per tube) immersed in an ice slurry and sonnicated by using a microprobe (160 W) at 2-s pulses for 10 s ×2 at 70% duty cycle. The resulting lysate was mixed with 50% Optiprep (Sigma-Aldrich) and placed in four to eight ultracentrifuge tubes. Two milliliters of 36% Optiprep was layered on top followed by 2 ml of 6% Optiprep. In an SW41t rotor, gradients were centrifuged at 115,000g for 24 h. Fractionation was achieved by aspirating 1.5-ml samples from the top down by using a blunt-end cannula. The 6 to 36% boundary layer fraction (DIGs fraction) was diluted 5-fold in MBS buffer containing 25 mM MES, pH 6.5, 150 mM NaCl, 0.001 mM leupeptin, 0.0005 mM AEBSF, 5 mM NaF, 10 mM EGTA, and 10 mM EDTA, ± zaprinast (10 μM), centrifuged at 22,000g for 20 min to pellet insoluble proteins, and entered into subsequent experiments based on protein.
Guanylyl Cyclase Activity Assay.
GC activity was determined by monitoring the conversion of αP32-GTP to P32-cGMP in vitro (Kimura and Murad, 1974). In brief, assay mixtures contained 50 mM Tris-HCl, pH 7.6, 0.02% bovine serum albumin, 4 mM MgCl2, 1 mM GTP (5 × 105 cpm/tube), 7.5 mM creatine phosphate, 135 U/mg creatine phosphokinase, and 10 μM zaprinast. Reactions were initiated by adding pGC-C from DIGs/caveolar preparations to assay mix containing uGN and other additions as required. Incubations were performed at 32°C for 15 min during which stimulated activity was linear and terminated with 50 mM ice-cold sodium acetate, pH 4.0, with heating to 90°C for 3 min. cGMP was separated from reactants by using Dowex-50 ion exchange, and cGMP production was measured with a scintillation counter.
Results
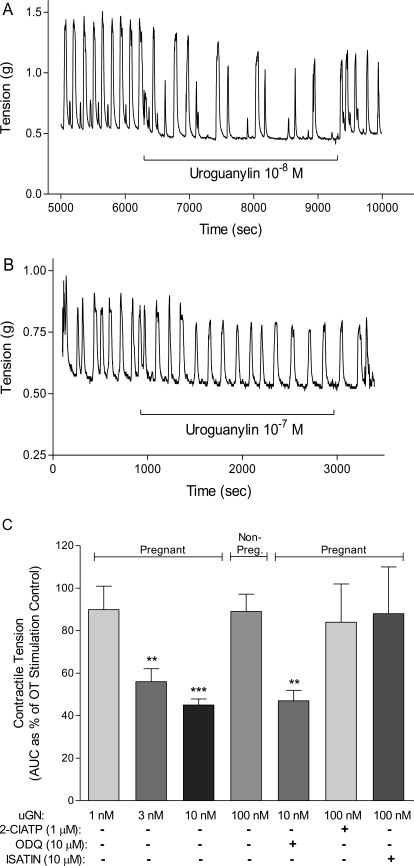
Addition of 100 nM uGN to guinea pig myometrial tissues from estrogen-primed nonpregnant (NP) guinea pigs failed to reduce OT (100 nM)-induced contractions (Fig. 1, B and C). Despite the appearance of a small regularization of the contraction seen (Fig. 1B), no significant effect was measurable when tested in duplicate tissue strips from six animals (Fig. 1C). However, when 10 nM uGN was added to myometrial strips from pregnant guinea pigs (34 days), there was a marked reduction in both the frequency of contractions and peak tension (Fig. 1A). The effect of uGN quantified as tension over time (area under the curve, 15 min) was dose-dependent with significant inhibition of OT-induced contractions at 3 nM uGN (Fig. 1C).
Fig. 1.
Uroguanylin relaxes oxytocin-stimulated contractions in a dose-dependent, pGC-C-mediated fashion in pregnant guinea pig myometrium. A, in the pregnant guinea myometrium (50–60 days gestation) 10 nM uGN relaxes the tissue with a reproducible effect on peak height and frequency of contraction. B, no such effect is seen in tissues from estrogen-primed nonpregnant animals even at 100 nM. Traces are representative examples. Effects were reproducible after washout and were seen both early and late in the recording. C, contractile tension was measured in grams from area under the curve (AUC) for 15 min of oxytocin-stimulated contractile activity in replicate pregnant guinea pig tissues (n = 6) in the presence or absence of 2Cl-ATP, ODQ, or isatin. The uGN relaxation was dose-dependent and significant at 3 nM uGN. Uroguanylin stimulation in the presence of 2Cl-ATP (1 μM) or isatin (10 μM) prevented the relaxation to 100 nM uGN. ODQ (10 μM) had no significant effect on the uGN-mediated relaxation. Data are mean ± S.E.M. from two replicate tissues from each of six animals (50–60 days gestation). **, p < 0.01; ***, p < 0.001.
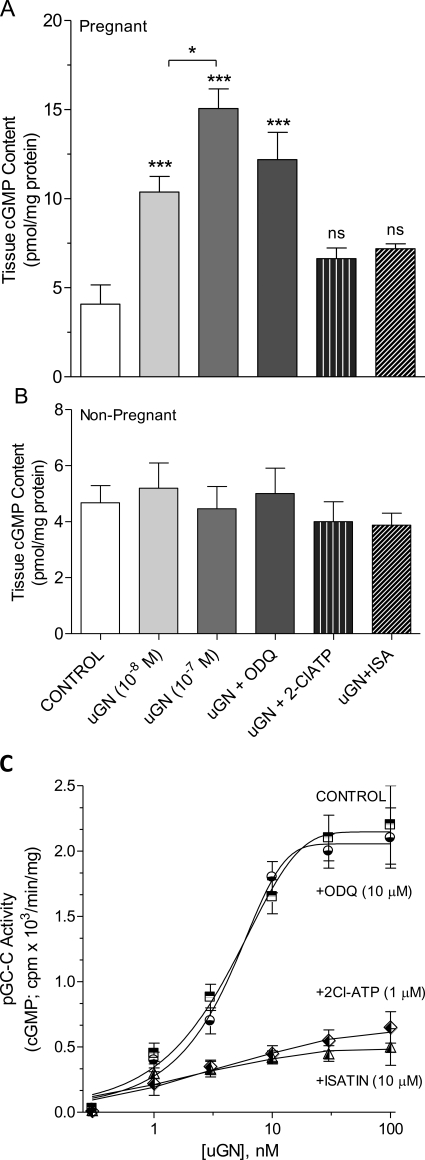
Because uGN is known to stimulate pGC-C in intestinal epithelium, we tested the possibility that uGN was acting via an increase in cGMP accumulation in the muscle. When the putative pGC-C antagonists 2Cl-ATP or isatin were added to the tissue bath, followed 3 min later by addition of 10 nM uGN, relaxation was prevented (Fig. 1C). The effect of uGN to relax the tissue was not caused by stimulation of the soluble guanylyl cyclase, because addition of ODQ had no effect. Direct measurement of cGMP accumulation in tissues studied under contractile conditions after the addition of uGN resulted in cGMP elevation only in pregnant tissues (Fig. 2). Under these conditions, addition of 2Cl-ATP (1 μM) or isatin (10 μM) attenuated cGMP accumulation, whereas ODQ (10 μM) had no effect. The effects of both 2Cl-ATP and isatin as putative pGC-C inhibitors were studied in a broken cell guanylyl cyclase assay using the myometrial homogenate (Fig. 2C). Uroguanylin produced a dose-dependent increase in activity measured as cGMP accumulation after the addition of increasing concentrations of uGN to the assay. When the process was repeated in the presence of ODQ at a concentration known to block myometrial soluble GC (10 μM), no effect was seen. However, both isatin and 2Cl-ATP added at concentrations consistent with their action as pGC-C antagonists (Sousa et al., 2010) blocked cGMP formation to uGN addition (Fig. 2C).
Fig. 2.
Uroguanylin stimulates cGMP accumulation only in pregnant guinea pig myometrium. A and B, cGMP elevation was stimulated by uGN in a dose-dependent fashion and was insensitive to the addition of 10 μM ODQ in pregnant myometrium (A), but not nonpregnant myometrium (B). Addition of 2Cl-ATP (1 μM) or isatin (10 μM) prevented cGMP accumulation to 100 nM uGN. Data are mean ± S.E.M. from two replicate tissues from six nonpregnant and four-six pregnant animals (50–60 days gestation). *, p < 0.05; ***, p < 0.001; ns, nonsignificant compared with control. C, particulate GC-C activity was measured in vitro in the myometrial homogenate from four pregnant animals (50–60 days) after the addition of uGN in the presence and absence of putative GC inhibitors. Data are mean ± S.E.M. from three experiments performed in triplicate.
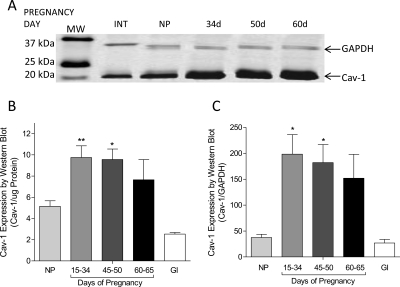
An effect of pGC-C activation leading to cGMP accumulation and tissue relaxation in pregnant, but not nonpregnant, myometrium suggests the possibility that there is an effect of pregnancy on the myometrial signaling domain. Caveolin-1 expression is elevated in pregnant guinea pig myometrium when detected by Western blot (Fig. 3A). Data gathered from early, mid, and late gestation demonstrates an elevation of caveolin expression whether quantified by protein mass (Fig. 3B) or GAPDH expression in the same samples (Fig. 3C).
Fig. 3.
Caveolin-1 expression is increased in pregnant guinea pig. Western blot experiments (A) reveal increased expression of caveiolin-1 protein in myometrial samples from pregnant guinea pigs in early (15–34 days), mid (45–50 days), and late pregnancy (60–65 days) whether quantified as per protein loaded (B) or GAPDH expression (C). Intestine (GI) sampled from pregnant animals served as a positive comparative smooth muscle control and did not change as a result of pregnancy (not shown). Blots were imaged for the IR secondary antibody label in 680- and 800-nm channels as described, and data were recorded as relative intensity. Data are mean ± S.E.M. of determinations of pregnant tissue samples from 8 to 12 animals and nonpregnant controls (n = 3). *, p < 0.05; **, p < 0.01 compared with nonpregnant.
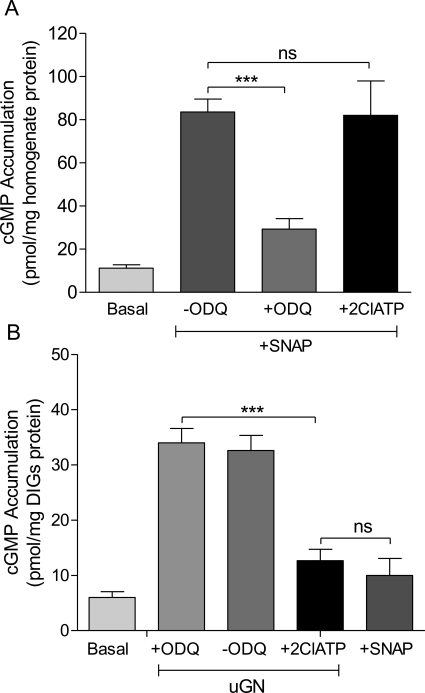
The well known dissociation of nitric oxide-mediated myometrial relaxation from cGMP accumulation is in marked contrast to the effect of uGN to relax myometrium in a cGMP-dependent fashion seen here (Figs. 1C and 2). Examination of cGMP accumulation in the myometrial homogenate after addition of the nitric-oxide donor S-nitroso-N-acetylpenicillamine (SNAP) is ODQ-sensitive and 2Cl-ATP-insensitive (Fig. 4A) consistent with the expected action of NO to stimulate soluble guanylyl cyclase. In the particulate caveolin-enriched fraction of the muscle that represents the membrane signaling domain, the effect of uGN is sensitive to 2Cl-ATP, whereas ODQ is without effect and no cGMP accumulation is seen after the addition of SNAP (Fig. 4B). Control experiments in the soluble fraction demonstrated that uGN (100 nM) did not stimulate soluble GC, whereas SNAP (10 μM) did.
Fig. 4.
cGMP accumulation in the DIGs signaling domain in pregnant myometrium is agonist-specific. A, cGMP accumulation was measured in the soluble cell fraction of the myometrial tissue homogenate as stimulated by the NO donor SNAP (100 μM) in the presence or absence of the addition of ODQ (10 μM) or 2Cl-ATP (1 μM). B, cGMP measurements in the DIGs signaling domain fraction prepared on a density gradient were not stimulated by the soluble guanylyl cyclase activator (SNAP, 100 μM) but were significantly elevated by uGN (10 nM) and blocked by 2Cl-ATP (1 μM). SNAP had no effect in the DIGs fraction. Data are mean ± S.E.M., n = 6. ***, p < 0.001; ns, nonsignificant comparison.
An effect of uGN to stimulate cGMP accumulation in the myometrium suggests the presence of both uGN and the pGC-C, neither of which have heretofore been examined in myometrium. PCR studies of pro-uGN revealed its absence in uterine smooth muscle but its presence in kidney cDNA (data not shown; primers for pro-uGN were: forward GAGTGACCTGGAGGCACAGT; and reverse CCTCCCCCTCCAACTCTATG). Expected product size was 391 bp (Maake et al., 2003). This result is consistent with a source for uGN as extra-uterine.
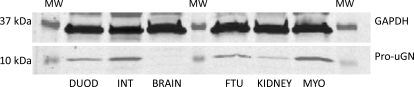
Using an antibody that detects human uGN as the prohormone, however, we detected this protein in duodenum (DUOD) and small intestine (INT) as expected. Pro-uGN was not detected in brain because this protein is not expected to cross the blood-brain barrier, but was detected in kidney and both the full thickness uterus (FTU) and isolated MYO (Fig. 5).
Fig. 5.
Pro-uGN is available to the uterus as a source of uGN for pGC-C stimulation. Western blot experiments with antibody to human pro-uGN compared with GAPDH protein expression were conducted on tissue samples from DUOD, small INT, brain, kidney, the MYO, or FTU from pregnant animals. Data are representative of the reproducible (n = 3) appearance of pro-uGN in pregnant guinea pig tissues.
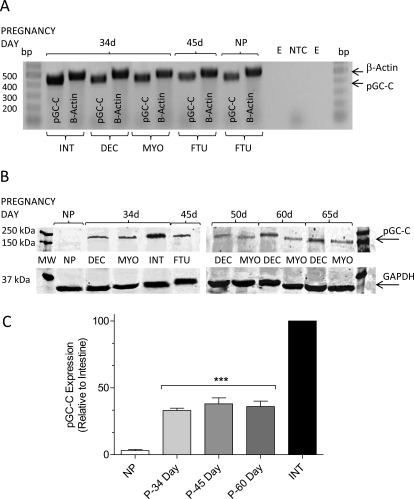
To probe for the uGN receptor, pGC-C, we used RT-PCR primers designed to amplify a 450-bp product to probe for pGC-C in guinea pig tissues. β-Actin was amplified from the same samples as an expression control (500 bp) and to assure the absence of genomic contamination (750 bp). Guinea pig INT served as a positive control and showed unequivocally that mRNA for the pGC-C is expressed in the 34-day pregnant animal in DEC, the MYO, and the FTU. The message is also seen in the NP uterine tissue (Fig. 6A).
Fig. 6.
Particulate guanylyl cyclase is expressed in pregnant myometrium. A, RT-PCR for pGC-C was performed on samples from pregnant and nonpregnant guinea pigs using primers designed from the human consensus sequence of pGC-C. β-Actin was amplified as an expression control (500 bp) and to rule out genomic contamination (750-bp amplicon not seen). Empty lanes (E) and the nontemplate control (NTC) confirmed the specificity of the PCR products shown for small INT, uterine DEC, isolated MYO, or FTU samples from pregnant and NP animals. B, Western blot using antibodies specific for GAPDH or the pGC revealed that pGC-C is expressed in pregnant, but not nonpregnant, animals. No increase in expression of pGC-C was seen with pregnancy between midgestation (34 days) and term. pGC-C in both INT-positive controls and myometrial samples were sequenced and identified as pGC-C. C, data for relative pGC-C expression in replicate experiments plotted as density measurements from Western blots with expression in the intestine set to 100%. Data are mean ± S.E.M. from 8 to 12 animals in experiments repeated three times. ***, p < 0.001.
Particulate GC-C protein expression was examined by Western blot (Fig. 6B). Despite the presence of the message for pGC-C seen in the nonpregnant tissue, protein was absent in the nonpregnant uterus (Fig. 6B), whereas it was readily detectable in pregnant samples where GAPDH served as a positive control. Protein expression in pregnant myometrium, although only approximately 30% that of expression seen in the intestine, was significant (Fig. 6C).
Discussion
The notion that the action of soluble messengers such as cyclic nucleotides can subserve their signaling roles in an agonist-specific fashion was first proven more than 25 years ago for cAMP and is now well known for explaining signaling in cardiac muscle (Brunton et al., 1981; Buxton and Brunton, 1983; Iancu et al., 2007, 2008a,b). Compartmentation of cGMP action has received less attention but has been suggested to explain the cGMP-independent effects of NO in smooth muscle (Buxton, 2004) and has been described in the heart (Castro et al., 2006; Fischmeister et al., 2006).
In the myometrium, a wealth of evidence has established that nitric oxide-induced relaxations are independent of cGMP accumulation (Kuenzli et al., 1996, 1998; Bradley et al., 1998; Buxton et al., 2001), thus leaving the role of global cGMP in uterine smooth muscle relaxation unexplained. However, as one envisions a mechanism of NO action in myometrium, the fact that cGMP is not involved raises the intellectually displeasing possibility that cGMP does not signal relaxation in myometrium under any conditions. It has been hypothesized that although cGMP elevation by NO-mediated activation of soluble GC does not signal relaxation, a role for peptide-mediated cGMP accumulation may (Buxton, 2004).
Particulate GC activity has been examined previously in the myometrium (Buhimschi et al., 2000; Telfer et al., 2001), but a role for pGC-C activation and signaling, possibly leading to the relaxation of the uterine smooth muscle has not been well documented. Unlike the results described in guinea pig by others (Buhimschi et al., 2000) who saw no effect of the pGC-C agonist uGN on cGMP elevation, we saw that uGN relaxes pregnant myometrium in a dose-dependent fashion and that both relaxation (Fig. 1) and cGMP accumulation measured under physiological conditions (Fig. 2) were prevented by the pGC-C inhibitors 2Cl-ATP and isatin. We cannot explain the failure of others to measure the changes we have seen. We suspect that the preparation of the DIGs fraction may have allowed the action of uGN to be seen because it may preserve critical elements of the signaling domain. The failure of the sGC inhibitor ODQ to inhibit the functional and biochemical effects of uGN stimulation together with the effect of pGC-C inhibitors suggests that particulate, but not soluble, GC subserve the relaxation to uGN and that this is a feature only of pregnant tissues (Figs. 1C and 2).
The fact that it is only pregnant uterine muscle that responds to uGN suggests that there may be an effect of pregnancy on the signaling domain that permits an effect of uGN. The importance of the signaling domain in myometrium has been shown in studies of cholesterol depletion that significantly alter both spontaneous activity and the effects of oxytocin (Buxton and Vittori, 2005; Zhang et al., 2007). Removal of cholesterol seems to remove the phasic activity in the tissue consistent with an effect on relaxation mechanisms. We reasoned that the importance of the signaling domain conferred by the cholesterol content of the membrane suggested that critical elements of relaxation signaling may reside in the caveolar fraction. We therefore examined the expression of caveolin-1 in myometrial smooth muscle by Western blot and found that it is elevated in pregnant guinea pig tissues compared with the uterine muscle from nonpregnant, estrogen-primed animals (Fig. 3).
When the effects of the NO-donor SNAP on tissue cGMP accumulation were compared with the detergent-insoluble glycolipid-rich fraction (DIGs) containing caveolin-1 (Fig. 4), 2Cl-ATP had no effect, whereas ODQ blocked the effect of the NO donor to raise cGMP levels. In the DIGs fraction, on the other hand, ODQ had no effect on cGMP accumulation stimulated by uGN that was blocked by 2Cl-ATP (Fig. 4). In control experiments, addition of uGN (100 nM) did not stimulate cGMP accumulation in the soluble fraction, whereas SNAP (10 μM) did. Moreover, SNAP did not elevate cGMP content in the DIGs fraction (Fig. 4), which is consistent with compartmentation of the effects of NO versus uGN.
Stimulation of cGMP accumulation in myometrium was unexpected based on existing notions of pGC-C expression (Giannella and Mann, 2003), although the functional effect of uGN described here suggests it. We have used both RT-PCR and Western blot analysis to address the presence of pGC-C in guinea pig myometrium (Fig. 6). Using β-actin primers as both positive control for the PCR and evidence to rule out genomic contamination, we saw expression of pGC-C by PCR consistent with the presence of mRNA expression in myometrium and the decidua in pregnant guinea pig uterus (Fig. 6A). Expression was also seen in the nonpregnant, estrogen-primed uterus and the intestine control. Sequence analysis of the 450-bp amplicon confirmed that we have documented the expression of pGC-C in guinea pig (data not shown). The presence of protein for pGC-C was confirmed by Western blot analysis of pGC-C (Fig. 6, B and C).
The particulate type-C cyclase was readily detected in the intestinal control and uterine tissue from pregnant animals but was not found in the nonpregnant tissue in estrogen-primed guinea pigs (Fig. 6B). Although the qualitative detection of pGC-C by Western blot confirmed its expression in uterus, no evidence for changes in expression during pregnancy was found when GAPDH was used as an expression control. The abundance of pGC-C was apparently less, at least qualitatively, than that seen in the intestine at 34 days gestation.
For there to be expression of pGC-C and an effect of uGN on uterine relaxation in vivo, it stands to reason that uGN or a uGN-like agonist must be available under physiological conditions during pregnancy. Although this possibility has not been examined before for regulation of uterine function, it is known that pro-UGN circulates in the plasma (Fan et al., 1996; Carrithers et al., 2002) and is converted to uGN by protease activity (Hamra et al., 1996). We examined the presence of pro-uGN by Western blot in tissues from guinea pig intestine and brain and kidney and found that intestinal tissues and kidney showed the presence of pro-uGN. The protein was absent in brain, consistent with its exclusion by the blood-brain barrier, but present in both the full-thickness uterus and the smooth muscle (Fig. 5).
Our data show for the first time that a paracrine axis exists that can regulate particulate cGMP accumulation in the caveolin-rich signaling domain in myometrium and contribute to relaxation of the muscle. The unmistakable conclusion we draw from these studies is that cGMP action is compartmented in the smooth muscle and that not all cGMP interacts with all cGMP-dependent effectors, because, unlike NO-mediated relaxations, uGN-induced relaxation is cGMP-dependent.
The failure of soluble guanylyl cyclase-mediated cGMP accumulation to relax guinea pig myometrium raises unresolved questions about the role of cGMP in myometrium. Discovery of the ability of the guanylyl cyclase type C agonist uroguanylin to relax uterine smooth muscle in a cGMP-dependent manner confirms the hypothesis that cGMP can still act to regulate contraction, but that cGMP action is agonist-specific in myometrium.
This work was supported by the National Institutes of Health National Institute of Child Health and Human Development [Grant HD053028] and a Prematurity Research Initiative Grant from the March of Dimes.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.171934.
- NO
- nitric oxide
- PKG
- cGMP-dependent protein kinase
- sGC
- soluble guanylyl cyclase
- pGC
- particulate guanylyl cyclase
- pGC-C
- pGC type C
- uGN
- uroguanylin
- DIGs
- detergent-insoluble glycolipid-rich membrane fraction
- SNAP
- S-nitroso-N-acetylpenicillamine
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- isatin
- 1H-indole-2,3-dione
- 2Cl-ATP
- 2-chloro-ATP
- bp
- base pairs
- RT-PCT
- reverse transcription-polymerase chain reaction
- FTU
- full thickness uterus
- MYO
- myometrium
- DEC
- decidua
- INT
- intestine
- DUOD
- duodenum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GI
- gastrointestinal
- OT
- oxytocin
- AEBSF
- 4-(2-aminoethyl) benzenesulphonyl fluoride hydrochloride
- PBS
- phosphate-buffered saline
- MES
- 4-morpholineethanesulfonic acid
- NP
- nonpregnant.
References
- Behrman RE, Butler AS. eds (2006) Preterm Birth: Causes, Consequences, and Prevention, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- Bradley KK, Buxton IL, Barber JE, McGaw T, Bradley ME. (1998) Nitric oxide relaxes human myometrium by a cGMP-independent mechanism. Am J Physiol Cell Physiol 275:C1668–C1673 [DOI] [PubMed] [Google Scholar]
- Brunton LL, Hayes JS, Mayer SE. (1981) Compartments of cyclic AMP and protein kinase in heart: data supporting their existence and speculation on their subcellular basis, in Protein Phosphorylation: Cold Spring Harbor Conferences on Cell Proliferation 8 (Rosen OM, Krebs EG. eds) pp 227 to 235, Cold Spring Harbor Laboratory Press, Woodbury, NY [Google Scholar]
- Buhimschi IA, San Martin-Clark O, Aguan K, Thompson LP, Weiner CP. (2000) Differential alterations in responsiveness in particulate and soluble guanylate cyclases in pregnant guinea pig myometrium. Am J Obstet Gynecol 183:1512–1519 [DOI] [PubMed] [Google Scholar]
- Butcher EC, Lowry OH. (1976) Measurement of nanogram quantities of protein by hydrolysis followed by reaction with orthophthalaldehyde or determination of glutamate. Anal Biochem 76:502–523 [DOI] [PubMed] [Google Scholar]
- Buxton IL. (2004) Regulation of uterine function: a biochemical conundrum in the regulation of smooth muscle relaxation. Mol Pharmacol 65:1051–1059 [DOI] [PubMed] [Google Scholar]
- Buxton IL, Brunton LL. (1983) Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 258:10233–10239 [PubMed] [Google Scholar]
- Buxton IL, Vittori JC. (2005) Cholesterol depletion enhances both spontaneous and agonist-evoked uterine smooth muscle contractions in a reversible manner. Proc West Pharmacol Soc 48:126–128 [PubMed] [Google Scholar]
- Buxton IL, Kaiser RA, Malmquist NA, Tichenor S. (2001) NO-induced relaxation of labouring and non-labouring human myometrium is not mediated by cyclic GMP. Br J Pharmacol 134:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers SL, Pager C, Shah R, Greenberg R. (2002) Uroguanylin activates the extracellular-regulated kinase (ERK), c-jun NH2-terminal kinase (JNK), and p38 mitogen activated protein kinase in colon carcinoma cells via cGMP. FASEB J 16:A1162 [Google Scholar]
- Castro LR, Verde I, Cooper DM, Fischmeister R. (2006) Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Hamra FK, Freeman RH, Eber SL, Krause WJ, Lim RW, Pace VM, Currie MG, Forte LR. (1996) Uroguanylin: cloning of preprouroguanylin cDNA, mRNA expression in the intestine and heart and isolation of uroguanylin and prouroguanylin from plasma. Biochem Biophys Res Commun 219:457–462 [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. (2006) Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res 99:816–828 [DOI] [PubMed] [Google Scholar]
- Forte LR, Krause WJ, Freeman RH. (1989) Escherichia coli enterotoxin receptors: localization in opossum kidney, intestine, and testis. Am J Physiol Renal Physiol 257:F874–F881 [DOI] [PubMed] [Google Scholar]
- Forte LR, London RM, Krause WJ, Freeman RH. (2000) Mechanisms of guanylin action via cyclic GMP in the kidney. Annu Rev Physiol 62:673–695 [DOI] [PubMed] [Google Scholar]
- Frolova NA, Kravtsov VK, Biyushkin VN, Chumakov YM, Belkova ON, Malinovskii TI. (1988) Crystal and molecular structure of isatin. J Struct Chem 29:491–493 [Google Scholar]
- Giannella RA, Mann EA. (2003) E. coli heat-stable enterotoxin and guanylyl cyclase C: new functions and unsuspected actions. Trans Am Clin Climatol Assoc 114:67–85, discussion 85–86 [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Zingg HH. (2003) Gene expression profiling of rat uterus at different stages of parturition. Endocrinology 144:2254–2265 [DOI] [PubMed] [Google Scholar]
- Hamra FK, Fan X, Krause WJ, Freeman RH, Chin DT, Smith CE, Currie MG, Forte LR. (1996) Prouroguanylin and proguanylin: purification from colon, structure, and modulation of bioactivity by proteases. Endocrinology 137:257–265 [DOI] [PubMed] [Google Scholar]
- Hennan JK, Diamond J. (1998) Evidence that spontaneous contractile activity in the rat myometrium is not inhibited by NO-mediated increases in tissue levels of cyclic GMP. Br J Pharmacol 123:959–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennan JK, Diamond J. (2001) Effect of NO donors on protein phosphorylation in intact vascular and nonvascular smooth muscles. Am J Physiol Heart Circ Physiol 280:H1565–H1580 [DOI] [PubMed] [Google Scholar]
- Iancu RV, Jones SW, Harvey RD. (2007) Compartmentation of cAMP signaling in cardiac myocytes: a computational study. Biophys J 92:3317–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu RV, Ramamurthy G, Harvey RD. (2008a) Spatial and temporal aspects of cAMP signalling in cardiac myocytes. Clin Exp Pharmacol Physiol 35:1343–1348 [DOI] [PubMed] [Google Scholar]
- Iancu RV, Ramamurthy G, Warrier S, Nikolaev VO, Lohse MJ, Jones SW, Harvey RD. (2008b) Cytoplasmic cAMP concentrations in intact cardiac myocytes. Am J Physiol Cell Physiol 295:C414–C422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Murad F. (1974) Evidence for two different forms of guanylate cyclase in rat heart. J Biol Chem 249:6910–6916 [PubMed] [Google Scholar]
- Krause WJ, Freeman RH, Fort LR. (1990) Autoradiographic demonstration of specific binding sites for E. coli enterotoxin in various epithelia of the North American opossum. Cell Tissue Res 260:387–394 [DOI] [PubMed] [Google Scholar]
- Kuenzli KA, Bradley ME, Buxton IL. (1996) Cyclic GMP-independent effects of nitric oxide on guinea-pig uterine contractility. Br J Pharmacol 119:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzli KA, Buxton IL, Bradley ME. (1998) Nitric oxide regulation of monkey myometrial contractility. Br J Pharmacol 124:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maake C, Auf der Maur F, Jovanovic K, Reinecke M, Hauri D, John H. (2003) Occurrence and localization of uroguanylin in the aging human prostate. Histochem Cell Biol 119:69–76 [DOI] [PubMed] [Google Scholar]
- Ohbayashi H, Yamaki K, Suzuki R, Takagi K. (1998) Effects of uroguanylin and guanylin against antigen-induced bronchoconstriction and airway microvascular leakage in sensitized guinea-pigs. Life Sci 62:1833–1844 [DOI] [PubMed] [Google Scholar]
- Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. (2006) Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol 128:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa CM, Havt A, Santos CF, Arnaud-Batista FJ, Cunha KM, Cerqueira JB, Fonteles MC, Nascimento NR. (2010) The relaxation induced by uroguanylin and the expression of natriuretic peptide receptors in human corpora cavernosa. J Sex Med doi: 10.1111/j.1743-6109.2009.01672.x [DOI] [PubMed] [Google Scholar]
- Tan TC, Devendra K, Tan LK, Tan HK. (2006) Tocolytic treatment for the management of preterm labour: a systematic review. Singapore Med J 47:361–366 [PubMed] [Google Scholar]
- Telfer JF, Itoh H, Thomson AJ, Norman JE, Nakao K, Campa JS, Poston L, Tribe RM, Magness RR. (2001) Activity and expression of soluble and particulate guanylate cyclases in myometrium from nonpregnant and pregnant women: down-regulation of soluble guanylate cyclase at term. J Clin Endocrinol Metab 86:5934–5943 [DOI] [PubMed] [Google Scholar]
- Tichenor S, Malmquist NA, Buxton IL. (2001) Actions of S-nitroso N-acetyl penicillamine and 3-morpholinosydonimine may involve disparate signaling pathways in myometrial smooth muscle. Proc West Pharmacol Soc 44:53–56 [PubMed] [Google Scholar]
- Vaandrager AB. (2002) Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem 230:73–83 [PubMed] [Google Scholar]
- Word RA, Cornwell TL. (1998) Regulation of cGMP-induced relaxation and cGMP-dependent protein kinase in rat myometrium during pregnancy. Am J Physiol Cell Physiol 274:C748–C756 [DOI] [PubMed] [Google Scholar]
- Zhang J, Kendrick A, Quenby S, Wray S. (2007) Contractility and calcium signaling of human myometrium are profoundly affected by cholesterol manipulation: implications for labor? Reprod Sci 14:456–466 [DOI] [PubMed] [Google Scholar]