Abstract
In vivo effects of GABAB receptor-positive modulators suggest that they have therapeutic potential for treating central nervous system disorders such as anxiety, depression, and drug abuse. Although these effects generally are thought to be mediated by positive modulation of GABAB receptors, such modulation has been examined primarily in vitro. The present study was aimed at further examining the in vivo positive modulatory properties of the GABAB receptor-positive modulators, 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl) phenol (CGP7930) and (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one (rac-BHFF). Both compounds enhanced loss of righting induced by baclofen in mice. However, CGP7930 was less effective and rac-BHFF was less potent for enhancing loss of righting induced by γ-hydroxybutyrate (GHB), which, like baclofen, has GABAB receptor agonist properties. In contrast with baclofen- and GHB-induced loss of righting, the hypothermic effects of baclofen and GHB were not enhanced by rac-BHFF but were enhanced by CGP7930 only at doses that produced hypothermia when given alone. CGP7930-induced hypothermia was not attenuated by the GABAB receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348), at doses that blocked baclofen-induced hypothermia, and was not increased by the nitric-oxide synthase inhibitor Nω-nitro-l-arginine methyl ester, at doses that increased the hypothermic effects of baclofen and GHB. The results provide evidence that CGP7930 and rac-BHFF act in vivo as positive modulators at GABAB receptors mediating loss of righting, but not at GABAB receptors mediating hypothermia. Conceivably, CGP7930, but not rac-BHFF, acts as an allosteric agonist at these latter receptors. Taken together, the results provide further evidence of pharmacologically distinct GABAB receptor subtypes, possibly allowing for a more selective therapeutic interference with the GABAB system.
Introduction
Allosteric modulators alter the activity of the endogenous ligand by binding to receptor sites that are different from the orthosteric site where the endogenous ligand acts (Christopoulos, 2002; Pin and Prézeau, 2007; Conn et al., 2009; Wang et al., 2009). There is currently much interest in allosteric modulators, because by discriminating between activated and nonactivated receptors, they may have a broader therapeutic window than ligands that indiscriminatingly alter the activity of all receptors. Allosteric modulators have been identified for various receptors, including GABAA and GABAB receptors. Because GABAB receptors are implicated in various psychiatric disorders (Kerr and Ong, 1995; Pilc and Nowak, 2005; Frankowska et al., 2007), including drug dependence (Markou et al., 2004; Addolorato et al., 2009), modulation of these receptors could provide new treatments.
Several novel compounds have been characterized as positive modulators of GABAB receptors in vitro [e.g., 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)phenol (CGP7930) (Urwyler et al., 2001; Adams and Lawrence, 2007), N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) (Urwyler et al., 2003), (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one (rac-BHFF) (Malherbe et al., 2008), and BHF177 (Maccioni et al., 2009)], and in vivo results suggest them to have anxiolytic- and antidepressant-like properties (Cryan et al., 2004; Frankowska et al., 2007; Jacobson and Cryan, 2008). In addition, positive modulators of GABAB receptors reduce self-administration of alcohol (Orrù et al., 2005; Liang et al., 2006; Maccioni et al., 2008, 2009), cocaine (Filip et al., 2007), and nicotine (Mombereau et al., 2007; Paterson et al., 2008). Although all of these effects generally are thought to be mediated by positive modulation of GABAB receptors, to date such modulation has been examined almost exclusively in vitro. Examination of positive modulating properties in vivo may help to further understand the mechanism by which these compounds exert their potential therapeutic effects.
CGP7939 and rac-BHFF have been reported to increase loss of righting in mice induced by a subthreshold dose of the GABAB receptor agonist baclofen (Carai et al., 2004; Malherbe et al., 2008). These findings, together with the observation that CGP7930 and rac-BHFF did not produce loss of righting when given alone, were taken as evidence that CGP7930 and rac-BHFF have positive modulating properties at GABAB receptors in vivo. To characterize these in vivo effects in more detail, the present study established dose-response curves for GABAB receptor agonists and used shifts of these curves to quantify the relative potency and effectiveness of the positive modulators.
GABAB receptors can be activated by baclofen, but also by other drugs, such as γ-hydroxybutyrate (GHB) (Mathivet et al., 1997). However, the GABAB receptor mechanisms underlying the effects of baclofen and GHB do not seem to be identical. First, the GABAB receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348) is often less potent in antagonizing the effects of GHB than the effects of baclofen (Koek et al., 2004, 2007b, 2009; Carter et al., 2006). Second, N-methyl-d-aspartate antagonists enhance the behavioral effects of GHB but not baclofen (Koek et al., 2007a; Koek and France, 2008). Preferential activity of GHB at GABAB heteroreceptors on glutamatergic neurons and preferential activity of baclofen at GABAB autoreceptors on GABAergic neurons could conceivably account for some of these differences (Carter et al., 2009). Recent in vitro evidence suggests that CGP7930 and its analog 2,6-di-tert-butyl-4-(3-hydroxy-2-spiropentylpropyl)-phenol (BSPP) selectively potentiate activity at GABAB autoreceptors, but not at heteroreceptors (Chen et al., 2006; Parker et al., 2008). This suggests the possibility, examined here, that CGP7930, and perhaps rac-BHFF, preferentially enhance in vivo effects of baclofen compared with those of GHB.
GABAB receptor activation not only produces loss of righting, but also other in vivo effects, such as hypothermia (Kaupmann et al., 2003). Hypothermia, which occurs at lower doses than loss of righting, probably is mediated by a population of GABAB receptors in a particular brain region (i.e., hypothalamus) that differs from the population of GABAB receptors involved in loss of righting. To examine whether these GABAB receptor populations differ in their susceptibility to enhancement by positive modulators, the present study characterized the effects of CGP7930 and rac-BHFF on baclofen- and GHB-induced hypothermia, which have not been studied before, and compared these effects with those on loss of righting, which to date have been studied at a single agonist dose.
Surprisingly, the present study found that CGP7930 produced hypothermia when given alone. To study the involvement of GABAB receptors in these effects, their antagonism by CGP35348 was examined, in comparison with antagonism of baclofen- and GHB-induced hypothermia. In rats, the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) enhances baclofen-induced hypothermia (Rawls et al., 2004, 2006). Our preliminary observations (unpublished) showed this enhancement to also occur in mice. Thus, the present study further examined the mechanisms underlying CGP7930-, baclofen-, and GHB-induced hypothermia by testing whether l-NAME enhanced the hypothermic effects of CGP7930 in a manner similar to that observed with baclofen and GHB.
Materials and Methods
Animals.
A total of 160 adult male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), weighing 26 to 37 g at the beginning of the experiments, were housed in groups of four in an environmentally controlled room (temperature, 24°C; relative humidity, 45%) under a 14/10-h light/dark cycle (light on at 7:00 AM) with food (rodent sterilizable diet; Harlan Teklad, Madison, WI) and water continuously available. The animals were maintained and the experiments were conducted in accordance with the Institutional Animal Care and Use Committee at the University of Texas Health Science Center, San Antonio, TX and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996).
Apparatus.
Body temperature was measured with a digital thermometer (model BAT7001H) and a thermistor probe (model RET-3), both manufactured by Physitemp Instruments, Inc. (Clifton, NJ).
Procedure.
Righting was assessed repeatedly in the same animals before and at different intervals (15–120 min) after drug administration. Loss of the righting reflex was scored as 1, otherwise the score was 0. Righting was considered absent when a mouse, after having been placed on its back, did not right itself within 15 s (i.e., the plantar surface of none of the feet made full contact with the floor).
Immediately before drug administration, baseline temperature was measured by inserting the lubricated probe 2 cm into the rectum. Thereafter, body temperature was recorded repeatedly in the same animals at different intervals (ranging from 15 to 120 min) after drug administration.
Drug tests were conducted in groups of mice (body temperature: n = 4–6; righting: n = 8) selected nonsystematically from the population of mice (body temperature: 80; righting: 80) available for the present studies. Individual mice were tested with drugs on average 10 times (range = 5 to 15), and no mouse received the same drug test twice. In an effort to control for the effects of repeated testing, drug doses were tested in a nonsystematic order with at least 1 week between tests.
Data Analysis.
The effects of drugs given alone on loss of righting were examined by calculating the percentage of animals showing loss of righting for each dose and each postadministration interval. To further examine drugs given alone and drugs given together, for each dose (or dose combination) the loss of righting scores obtained from 0 to 120 min postadministration was summed for each animal (maximum total score = 6). Total scores were averaged across animals, and mean values ± S.E.M. were plotted as a function of dose. Dose-response data were analyzed by log-linear regression (Tallarida, 2000) of individual values by using Prism (GraphPad Software, Inc., San Diego, CA), with the following equation: effect = slope × log(dose) + intercept. Deviations from linearity were examined by the replicates test. F ratio tests in Prism were used to compare dose-response curves with respect to their slopes and intercepts. For example, a nonsignificant F ratio for slopes and a significant F ratio for intercepts show that dose-response curves are parallel but occupy different positions on the dose axis. Effects of 320 mg/kg CGP35348 on loss of righting produced by CGP7930 or rac-BHFF together with baclofen or GHB were analyzed by Student's t test.
Effects of drugs on body temperature when given alone were analyzed by two-way analysis of variance with dose as between-subjects factor and time as within-subjects factor, followed by Dunnett's test (NCSS 2007 program; NCSS Statistical Software, Kaysville, UT). To examine the effects of drugs when given together, for each dose combination the area under the body temperature time curve (AUC) from 0 to 120 min postadministration was calculated for each animal by using Prism version 5.02 for Windows (GraphPad Software, Inc.), which used the trapezoid rule to calculate the area below the baseline value obtained immediately before drug administration. AUC values were averaged across animals, and the mean values ± S.E.M. were plotted as a function of dose. Dose-response data were analyzed by log-linear regression as described for loss of righting. In addition, pretreatment interval effects were analyzed by two-way analysis of variance with interval and dose as between-subjects factors. Antagonist effects were analyzed by calculating for each dose of the antagonist the agonist dose needed to produce 50% of the maximal response (ED50) and the ratio of this ED50 with the agonist ED50 after vehicle. Dose ratios were plotted as a function of antagonist dose, and the resulting Schild plot (Arunlakshana and Schild, 1959) was analyzed with linear regression.
Drugs.
Baclofen and l-NAME HCl were purchased from Sigma-Aldrich (St. Louis, MO). GHB was provided by the National Institute on Drug Abuse (Bethesda, MD). CGP7930 and rac-BHFF were synthesized by K. Cheng at the National Institute on Drug Abuse (Bethesda, MD), and CGP35348 was synthesized by J. Agyin at the University of Texas Health Science Center (San Antonio, TX). All compounds were dissolved in sterile water, except CGP7930, which was suspended in sterile water with 0.6% methylcellulose, and rac-BHFF, which was suspended in a 4:1:15 mixture containing Cremophor EL, 1,2-propanediol, and distilled water (Malherbe et al., 2008). All compounds were injected intraperitoneally, except CGP7930, which was also administered orally, in a volume of 5 to 20 ml/kg. When more than one drug was administered, they were administered at the same time (except where noted). Doses are expressed as the form of the compound listed above.
Results
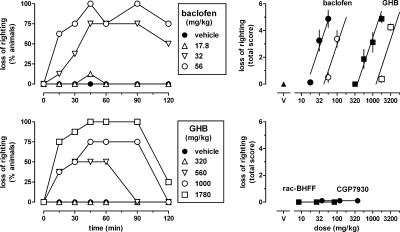
Baclofen and GHB produced loss of righting in a dose- and time-dependent manner (Fig. 1, left). Using the total of the loss of righting scores at each of the time points as a measure of drug effect, dose-response data were collected for baclofen and GHB, given alone and together with 320 mg/kg CGP35348 (Fig. 1, top right, filled and unfilled symbols, respectively). None of the dose-response data obtained in the present study deviated significantly from linearity, unless stated otherwise. The dose-response curves of GHB and baclofen had a common slope [F(1,52) = 2.89, P > 0.05] and significantly different ED50 values [baclofen: 34 (95% confidence limits: 28–41) mg/kg; GHB: 870 (750–2890) mg/kg; F(1,53) = 67.53, P < 0.0001]. Thus, baclofen was almost 30-fold more potent than GHB in producing loss of righting. CGP35348 significantly increased the ED50 values for baclofen [F(1,37) = 37.63, P < 0.0001] and GHB [F(1,45) = 37.37, P < 0.0001] in a similar manner (3- and 3.3-fold, respectively). Unlike baclofen and GHB, the positive GABAB receptor modulators CGP7930 and rac-BHFF did not produce any loss of righting (Fig. 1, bottom right).
Fig. 1.
Effects of the GABAB receptor agonist baclofen, GHB, and the GABAB receptor-positive modulators CGP7930 and rac-BHFF on righting in C57BL/6J mice (n = 8 per dose). Left, symbols represent the percentage of animals showing loss of righting at different times after intraperitoneal administration of baclofen or GHB. Right, symbols represent mean ± S.E.M righting scores, totaled for each animal across the six different times after injection. Open symbols in the upper right represent data obtained when baclofen and GHB were given together with 320 mg/kg of the GABAB receptor antagonist CGP35348.
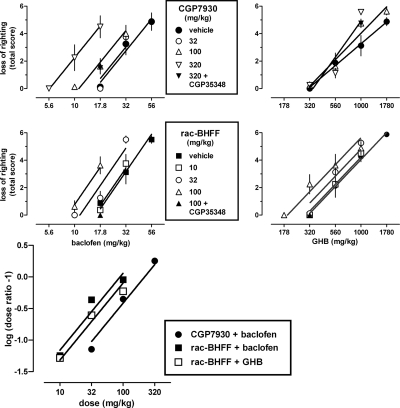
CGP7930 dose-dependently enhanced both baclofen- and GHB-induced loss of righting, but did so in a different manner (Fig. 2, top). CGP7930 shifted the dose-response curve of baclofen to the left in a parallel manner. The dose-response curves of baclofen in the presence of CGP7930 had a common slope [F(3,80) = 1.16, P > 0.20] and significantly different ED50 values [F(3,83) = 14.82, P < 0.0001]. At the highest dose of 320 mg/kg, CGP7930 decreased the ED50 of baclofen 2.8-fold, from 34 (28–41) to 12 (10–15) mg/kg. In contrast, CGP7930 shifted the dose-response curve of GHB in a nonparallel manner, as evidenced by significant different slope values for the dose-response curves of GHB in the presence of CGP7930 [F(2,81) = 3.54, P < 0.05]. As a result, the extent to which CGP7930 increased the potency of GHB depended on the effect level, with an almost 2-fold shift at maximal effect levels, but no apparent shift at intermediate and minimal effect levels. CGP7930 enhanced the effects of baclofen in a different manner than the effects of GHB; however, in both cases CGP35348 attenuated the enhanced effects. A dose of 320 mg/kg CGP35348 significantly attenuated the effects of 320 mg/kg CGP7930 combined with 17.8 mg/kg baclofen (t = 2.90, df = 14, P < 0.05) and combined with 1000 mg/kg GHB (t = 2.51, df = 13, P < 0.05) (Fig. 2, top, downward triangles).
Fig. 2.
Top and middle, effects of the GABAB receptor-positive modulators CGP7930 (top) and rac-BHFF (middle), administered intraperitoneally, on loss of righting produced by baclofen (top and middle left) and GHB (top and middle right). Symbols represent mean ± S.E.M righting scores, totaled for each animal (n = 8 mice per dose) across the six different times after injection. Data obtained in the presence of 320 mg/kg CGP35348 are indicated by ▴. Bottom, Schild-like plot of dose ratios for baclofen and GHB, calculated from the ED50 values of the dose-response curves shown in the top and middle, as a function of the dose of the positive modulator.
rac-BHFF dose-dependently enhanced baclofen- and GHB-induced loss of righting (Fig. 2, middle), as evidenced by parallel [F(3,104) = 2.25, P > 0.05], leftward shifts [F(3,107) = 7.49, P < 0.0001] of the dose-response curves. At the highest dose of 100 mg/kg, rac-BHFF decreased the ED50 of baclofen 1.9-fold, from 31 (27–35) to 16 (10–15) mg/kg and decreased the ED50 of GHB 1.6-fold, from 740 (640–850) to 460 (400–540) mg/kg. A dose of 320 mg/kg CGP35348 attenuated the enhanced effects of 100 mg/kg rac-BHFF combined with 17.8 mg/kg baclofen (t = 2.90, df = 14, P < 0.05) and 320 mg/kg GHB (t = 2.51, df = 13, P < 0.05) (Fig. 2, middle, upward triangles).
To characterize the enhancing properties of CGP7930 and rac-BHFF, ED50 values for baclofen and GHB in the presence of different doses of the modulators were used to calculate dose ratios for each modulator/agonist combination, except for the combination of CGP7930 and GHB, which did not yield parallel shifts. These ratios, shown in a Schild-like plot (Fig. 2, bottom), could be fitted with straight lines with a common slope [F(2,3) = 0.60, P > 0.20] not significantly different from 1 [i.e., 1.2 (0.92–1.5)] and significantly different intercepts [F(2,5) = 6.53, P < 0.05]. These lines were used to estimate the dose of the modulator needed to shift the agonist dose-response curve 2-fold to the left, which was 220 mg/kg for CGP7930 combined with baclofen, 89 mg/kg for rac-BHFF combined with baclofen, and 120 mg/kg for rac-BHFF combined with GHB. Thus, rac-BHFF was 2.5-fold more potent than CGP7930 in enhancing the effects of baclofen and 1.3-fold more potent in enhancing baclofen than in enhancing GHB.
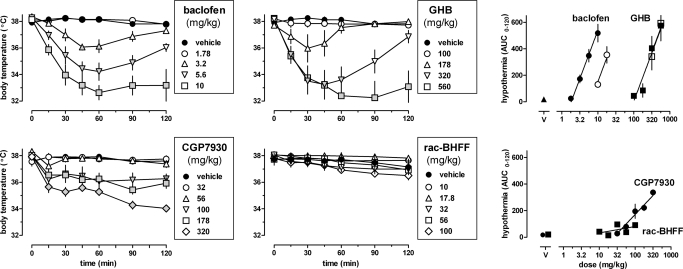
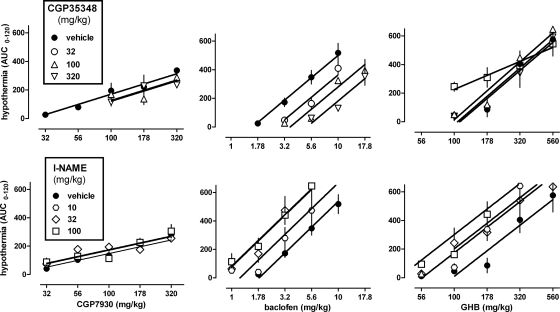
When given alone, baclofen, GHB, and CGP7930, but not rac-BHFF, decreased body temperature in a dose- and time-dependent manner (Fig. 3, left and center) [baclofen and GHB, dose: F(4,23) ≥ 9.48, P < 0.001; time: F(6,138) ≥ 19.42, P < 0.001; dose × time: F(24,138) ≥ 8.28, P < 0.001; CGP7930, dose: F(5,21) = 25.15, P < 0.001; time: F(6,126) = 30.43, P < 0.001; dose × time: F(30,126) = 7.27, P < 0.001; rac-BHFF, dose: F(5,18) = 1.15, P > 0.20; time: F(6,108) = 21.46, P < 0.001; dose × time: F(30,108) = 1.91, P < 0.01]. The lowest dose that produced statistically significant hypothermia was 3.2 mg/kg for baclofen, 178 mg/kg for GHB, and 100 mg/kg for CGP7930. Baclofen and GHB produced maximal hypothermia at 30 to 60 min after injection, and maximal effects of CGP7930 were apparent approximately 90 min after injection. The lowest body temperature observed with CGP7930 was 34 (0.3) °C, which was not significantly different [t (8) ≤ 1.96, P > 0.05] from that obtained with baclofen [32.6 (0.5)] or GHB [32.3 (1.2)]. None of the values obtained with rac-BHFF differed significantly from vehicle control. Using AUC as a measure of drug effect, dose-response data were collected for baclofen and GHB, given alone and together with 320 mg/kg CGP35348 (Fig. 3, top right, filled and unfilled symbols, respectively). The dose-response curves of GHB and baclofen had a common slope [F(1,44) = 0.41, P > 0.20] and significantly different ED50 values [baclofen: 4.7 (3.8–5.8) mg/kg; GHB: 250 (210–280) mg/kg; F(1,45) = 72.41, P < 0.0001]. Thus, baclofen was 53-fold more potent in producing hypothermia than GHB. CGP35348 significantly increased the ED50 value for baclofen 3.4-fold [F(1,29) = 45.02, P < 0.0001], but did not significantly alter the ED50 for GHB [F(1,29) = 0.01, P > 0.20]. CGP7930 produced hypothermia [ED50 = 100 (73–130)], such as baclofen and GHB, but its dose-response curve (Fig. 3, bottom right) was significantly shallower [F(1,39) = 19.78, P < 0.0001]. CGP7930 also produced hypothermia when administered orally (data not shown), with an ED50 [i.e., 140 (104–201) mg/kg] that did not differ significantly [F(1,35) = 2.64, P > 0.10] from its ED50 after intraperitoneal administration [i.e., 100 (73–130) mg/kg], and with a common slope [F(1,34) = 2.52, P > 0.10]. In contrast with CGP7930, rac-BHFF did not produce hypothermia, as evidenced by the slope of the regression line not being significantly different from zero [F(1,18) = 2.29, P > 0.10].
Fig. 3.
Effects of baclofen, GHB, CGP7930, and rac-BHFF on body temperature in C57BL/6J mice. Symbols represent mean ± S.E.M. (n = 4–6 mice per dose). Left and center, gray-filled symbols represent data that are significantly different from vehicle control (Dunnett's test, P < 0.05). Right, AUC values shown were calculated for each dose from the time response data shown (left and center). Open symbols in the top right represent data obtained when baclofen and GHB were given together with 320 mg/kg of the GABAB receptor antagonist CGP35348.
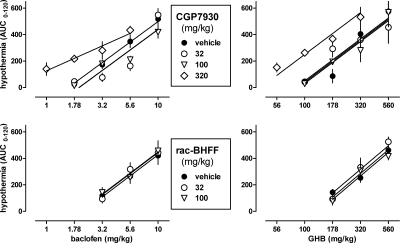
CGP7930 enhanced the hypothermic effects of baclofen and GHB (Fig. 4, top). At 320 mg/kg, CGP7930 significantly shifted the dose-response curves of baclofen [F(1,36) = 18.84, P < 0.001] and GHB [F(1,37) = 14.39, P < 0.001] to the left, in a parallel manner for GHB [F(1,36) = 2.08, P > 0.10] but not for baclofen [F(1,36) = 6.50, P < 0.05]. At lower doses of CGP7930 (i.e., 32 and 100 mg/kg), the dose-response curves of baclofen and GHB were similar to control and had a common slope [baclofen: F(2,54) = 1.17, P > 0.20; GHB: F(2,50) = 0.90, P > 0.20] and a common ED50 [baclofen: F(2,56) = 2.26, P > 0.10; GHB: F(2,52) = 0.07, P > 0.20]. None of the dose-response curves deviated from linearity, except the dose-response curve of baclofen in the presence of 32 mg/kg CGP7930 (P < 0.001). When 100 mg/kg CGP7930 was given 60 min before baclofen (3.2, 5.6 mg/kg) or GHB (178, 320 mg/kg) (data not shown), the results were not significantly different [F(1,12) ≤ 1.32, P > 0.20] from those obtained when CGP7930 was coadministered with baclofen or GHB (Fig. 4, top). In contrast with CGP7930, rac-BHFF did not alter the hypothermic effects of baclofen and GHB (Fig. 4, bottom). The dose-response curves of baclofen and GHB obtained in the presence of different doses of rac-BHFF could be fitted with common slopes [baclofen: F(2,40) = 0.07, P > 0.20; GHB: F(2,36) = 0.92, P > 0.20, respectively] and common ED50 values [baclofen: F(2,42) = 0.13, P > 0.20; GHB: F(2,38) = 0.87, P > 0.20].
Fig. 4.
Effects of CGP7930 (top) and rac-BHFF (bottom) on hypothermia induced by baclofen (left) and GHB (right). Symbols represent mean ± S.E.M. AUC values (n = 4–6 mice per dose).
CGP7930-induced hypothermia was not significantly attenuated by the GABAB receptor antagonist CGP35348; the dose-response curves shown in Fig. 5, top left had a common slope [F(2,37) = 0.24, P > 0.20] and a common ED50 [F(2,39) = 1.62, P > 0.20]. In contrast, CGP35348 shifted the dose-response curve for baclofen-induced hypothermia to the right [F(3,59) = 29.33, P < 0.001], in a parallel manner [F(3,56) = 0.62, P > 0.20] (Fig. 5, top center). These antagonist effects of CGP35348 were quantified by means of a Schild regression plot (not shown). The plot, with a slope [i.e., −0.54 (−0.77, −0.31)] significantly different from −1 (P < 0.05), yielded an apparent pA2 value, as an empirical potency estimate, of 3.55 (3.37–3.80) and did not differ significantly [F(2,2) = 1.05, P > 0.20] from the plot based on data obtained only at 60 min after the injection of the antagonist (data not shown). Doses of CGP35348 that antagonized the effects of baclofen failed to antagonize the effects of GHB (Fig. 5, top right). At the highest dose tested (i.e., 1000 mg/kg), CGP35348 significantly (P < 0.05) shifted the dose-response curve of GHB to the left in a nonparallel manner (P = 0.05). This dose of CGP35348 significantly decreased body temperature when given alone [dose: F(4,25) = 2.51, P = 0.067; time: F(6,150) = 7.93, P < 0.001; dose × time: F(24,150) = 2.72, P < 0.001] to a minimum value of 36.7 (S.E.M. = 0.35) °C, 45 min after injection (data not shown).
Fig. 5.
Effects of CGP35348 (top) and l-NAME (bottom) on hypothermia induced by CGP7930 (left), baclofen (center), and GHB (right). Symbols represent mean ± S.E.M. AUC values (n = 4–6 mice per dose).
CGP7930-induced hypothermia was not significantly affected by l-NAME. The dose-response curves shown in Fig. 5, bottom left had a common slope [F(2,105) = 1.87, P > 0.10] and a common ED50 [F(2,107) = 1.75, P > 0.10]. In contrast, l-NAME dose-dependently enhanced baclofen- and GHB-induced hypothermia (Fig. 5, bottom center and right) at doses that did not lower body temperature when given alone [data not shown; dose: F(4,25) = 0.09, P > 0.20; time: F(6,150) = 11.42, P < 0.001; dose × time: F(24,150) = 1.67, P = 0.034]. At 10 to 100 mg/kg, l-NAME significantly shifted the dose-response curves of baclofen [F(3,79) = 11.10, P < 0.001] and GHB [F(3,71) = 7.58, P < 0.001] to the left, in a parallel manner [baclofen: F(3,76) = 0.44, P > 0.20; GHB: F(3,68) = 0.85, P > 0.20]. l-NAME maximally shifted the baclofen dose-response curve 2.4-fold to the left at 32 and 100 mg/kg and maximally shifted the GHB dose-response curve 2.5-fold to the left at 100 mg/kg. Thus, l-NAME seemed to be more potent in enhancing the hypothermic effects of baclofen than those of GHB.
Discussion
The main finding of the present study is that the positive GABAB receptor modulators CGP7930 and rac-BHFF enhanced baclofen- and GHB-induced loss of righting, but not hypothermia. These results suggest that CGP7930 and rac-BHFF act in vivo as positive modulators at GABAB receptors involved in loss of righting, but not at GABAB receptors mediating hypothermic effects of GABAB receptor agonists. Thus, different GABAB receptor populations may differ in their susceptibility to positive modulatory effects, possibly allowing for a more selective therapeutic interference with the GABAB system.
Baclofen and GHB induced loss of righting in mice, consistent with previous observations (Carter et al., 2005), and antagonism by CGP35348 confirmed a role for GABAB receptors in these effects. CGP7930 and rac-BHFF enhanced baclofen- and GHB-induced loss of righting without producing loss of righting when given alone, in agreement with previous findings (Carai et al., 2004; Malherbe et al., 2008). Antagonism of the enhancement by CGP35348 indicated the involvement of GABAB receptors. In contrast with previous studies using a subthreshold dose of baclofen (Carai et al., 2004; Malherbe et al., 2008) and GHB (Carai et al., 2004), dose-response curves for baclofen and GHB were established. CGP7930 shifted the baclofen dose-response curve to the left in a parallel manner, but increased the slope of GHB dose-response curve. Conceivably, this may be related to different GABAB receptors mediating the effect of baclofen and GHB (i.e., GABAB autoreceptors and heteroreceptors, respectively; e.g., (Carter et al., 2009) and CGP7930 selectively potentiating autoreceptor activity (Chen et al., 2006). BHFF was 2.5-fold more potent than CGP7930 in enhancing baclofen and was less potent in enhancing GHB than baclofen. Taken together, these results suggest the possibility that GABAB receptor-positive modulators preferentially enhance in vivo effects of baclofen compared with those of GHB. If confirmed, this would be further evidence that the GABAB receptor mechanisms involved in the effects of baclofen and GHB are not identical.
Baclofen decreased body temperature, in agreement with previous observations (Gray et al., 1987; Jacobson and Cryan, 2005) and did so by activating GABAB receptors, evidenced by antagonism by CGP35348. Schild analysis yielded a regression plot with a slope different from −1, suggesting possible nonequilibrium (e.g., Kenakin, 1997). However, the regression plot was not different when, instead of the 2-h AUC data, only data were used that were obtained 60 min after administration of CGP35348, when agonist effects of baclofen and antagonist effects of CGP35348 are probably maximal (Koek et al., 2007b). This suggests that other factors, such as a heterogeneous receptor population, might be involved (Kenakin, 1997). An apparent pA2 value of 3.55 was calculated as an empirical estimate of the potency with which CGP35348 antagonized the hypothermic effects of baclofen. This potency estimate, which is equivalent to 70 mg/kg CGP35348, agrees with the previous finding that 100 mg/kg CGP35348 shifted the dose-response curve for the cataleptic effects of baclofen 2-fold to the right (Koek et al., 2007b). These results obtained with CGP35348, together with the finding that baclofen does not produce hypothermia in GABAB receptor knockout mice (Quéva et al., 2003), indicate that baclofen-induced hypothermia is mediated by GABAB receptors and, therefore, useful as an in vivo assay of GABAB receptor activation.
Like baclofen, GHB produces hypothermia by activating GABAB receptors, evidenced by its lack of hypothermic effects in GABAB receptor knockout mice (Kaupmann et al., 2003). Consistent with the involvement of GABAB receptors, CGP35348 antagonizes many of the effects of GHB, but is often less potent in antagonizing the effects of GHB than baclofen (Koek et al., 2004, 2007b, 2009; Carter et al., 2006). This has been taken to indicate that the GABAB receptor mechanisms underlying the effects of GHB are not identical to those of prototypical GABAB agonists such as baclofen (Koek et al., 2009). Consistent with this, in the present study CGP35348 failed to antagonize GHB-induced hypothermia at doses that antagonized baclofen-induced hypothermia. At the highest dose, CGP35348 shifted the GHB dose-response curve to the left and produced hypothermia when given alone. Thus, its hypothermic effects seemed to limit its antagonism of GHB-induced hypothermia. The finding that high doses of CGP35348 produced hypothermia, like GABAB agonists, may be related to its partial agonist properties at GABAB receptors (Urwyler et al., 2005). The present results differ from a report that CGP35348 antagonized hypothermia produced by γ-butyrolactone, a prodrug of GHB, (Carai et al., 2008) and a report that GHB-induced hypothermia was antagonized by the GABAB antagonist (2S)-(+)-5,5-dimethyl-2-morpholineacetic acid (SCH50911) (van Nieuwenhuijzen and McGregor, 2009). These differences could be related in part to the use of γ-butyrolactone, which has pharmacokinetic properties different from GHB, and SCH50911, which may have pharmacological properties different from CGP35348. Be that as it may, these studies clearly indicate an important role for GABAB receptors in GHB-induced hypothermia. The present results with CGP35348 suggest that baclofen and GHB produce hypothermia through different GABAB receptor mechanisms.
rac-BHFF did not alter baclofen- and GHB-induced hypothermia at doses that enhanced baclofen- and GHB-induced loss of righting. CGP7930 enhanced baclofen- and GHB-induced hypothermia, but only at a dose that produced hypothermia when given alone. In a recent study in mice, CGP7930 did not significantly affect body temperature except for a moderate increase of less than 1°C at the highest dose tested, i.e., 300 mg/kg p.o. (Jacobson and Cryan, 2008). In the present study, CGP7930 decreased body temperature with similar potency when administered intraperitoneally (ED50 = 100 mg/kg) and orally (ED50 = 140 mg/kg). Thus, differences between the effects of CGP7930 in the study by Jacobson and Cryan (2008) and in the present study do not seem to be related to the route of administration, but may be related to mouse strain (C57BL/6J in the present study; OF1 in the prior study), because strains can differ in their sensitivity to the hypothermic effects of baclofen (Jacobson and Cryan, 2005). In the present study, the hypothermic effects of CGP7930 were not altered by doses of CGP35348 that antagonized the hypothermic effects of baclofen. This suggests that the hypothermic effects of CGP7930 do not result from enhancement of the effects of endogenous GABA at GABAB receptors. Instead, they could result from activation of the GABAB receptor by CGP7930 through a site different from the site where GABA acts, suggested by the observation that CGP7930 can directly activate the receptor (Binet et al., 2004). Thus, although the present results do not provide evidence that CGP7930 acts in vivo as a positive modulator at GABAB receptors mediating hypothermia, they are consistent with the possibility that CGP7930 behaves as an allosteric agonist at these receptors.
The NOS inhibitor l-NAME dose-dependently enhanced the hypothermic effects of baclofen and GHB in mice without affecting body temperature when given alone. These results are in agreement with previous observations on the enhancement of baclofen-induced hypothermia by l-NAME in rats (Rawls et al., 2004, 2006) and extend these results to mice and hypothermia induced by GHB. The mechanism underlying these synergistic effects has been suggested to involve GABAB receptor-mediated suppression of NO synthesis in brain regions that regulate body temperature, with NO production diminished further by l-NAME (Rawls et al., 2006). This mechanism may be involved not only in the hypothermic synergy of baclofen and l-NAME, but also in that of GHB and l-NAME, because l-NAME enhanced the hypothermic effects of baclofen and GHB in a similar manner. In contrast, the hypothermic effects of baclofen and GHB were differentially antagonized by CGP35348. These results suggest the possibility that baclofen and GHB produce hypothermia through different GABAB receptor populations that are similarly coupled to NO production.
CGP7930-induced hypothermia was not affected by l-NAME, suggesting CGP7930 acts through GABAB receptors not coupled to NO production. Although CGP7930 seems to be a selective GABAB receptor modulator (Urwyler et al., 2001), the possible involvement of other non-GABAB receptors in its hypothermic effects can at present not be ruled out. Be that as it may, the finding that rac-BHFF did not produce hypothermic effects suggests rac-BHFF to be a more selective in vivo GABAB receptor modulator than CGP7930.
Taken together, the present results show that CGP7930 and rac-BHFF enhance baclofen- and GHB-induced loss of righting, but not hypothermia. Effects of GABAB agonists on motor coordination and body temperature probably are mediated by different GABAB receptor populations, in brain regions such as motor cortex and cerebellum and in the hypothalamus, respectively. There is evidence that the specific GABAB receptor populations that mediate ataxia and hypothermia are under differential genetic control (Jacobson and Cryan, 2005). Based on the results of the present experiments, it is tempting to speculate that the pharmacological properties of these receptor populations differ as well. Differential enhancement of GABAB receptor populations by positive modulators has been shown in vitro: presynaptic GABAB autoreceptors seem to be sensitive to CGP7930 and the CGP7930 analog BSPP, whereas presynaptic GABAB heteroreceptors are not (Chen et al., 2006; Parker et al., 2008). Conceivably, such differential enhancement could also be involved in the in vivo effects of CGP7930 and rac-BHFF reported here.
In summary, the positive GABAB receptor modulators CGP7930 and rac-BHFF enhanced baclofen- and GHB-induced loss of righting, but not hypothermia, suggesting that they act in vivo as positive modulators at some, but not all, GABAB receptors. If different GABAB receptor populations differ in their susceptibility to positive modulatory effects, this could allow a more selective therapeutic interference with the GABAB system.
Acknowledgments
We thank Jason Persyn and Christopher Limas for technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA15692, DA17918] and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.171116.
- CGP7930
- 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)phenol
- rac-BHFF
- (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one
- BHF177
- N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine
- CGP35348
- 3-aminopropyl(diethoxymethyl)phosphinic acid
- GS39783
- N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine
- BSPP
- 2,6-di-tert-butyl-4-(3-hydroxy-2-spiropentylpropyl)-phenol
- GHB
- γ-hydroxybutyrate
- AUC
- area under the body temperature time curve
- l-NAME
- Nω-nitro-l-arginine methyl ester
- NOS
- nitric-oxide synthase
- SCH50911
- (2S)-(+)-5,5-dimethyl-2-morpholineacetic acid.
References
- Adams CL, Lawrence AJ. (2007) CGP7930: a positive allosteric modulator of the GABAB receptor. CNS Drug Rev 13:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Cardone S, Ferrulli A, Gasbarrini G. (2009) Role of the GABA(B) receptor system in alcoholism and stress: focus on clinical studies and treatment perspectives. Alcohol 43:559–563 [DOI] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. (1959) Some quantitative uses of drug antagonists. Br J Pharmacol Chemother 14:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prézeau L. (2004) The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J Biol Chem 279:29085–29091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Froestl W, Gessa GL. (2004) In vivo effectiveness of CGP7930, a positive allosteric modulator of the GABAB receptor. Eur J Pharmacol 504:213–216 [DOI] [PubMed] [Google Scholar]
- Carai MA, Lobina C, Maccioni P, Cabras C, Colombo G, Gessa GL. (2008) γ-Aminobutyric acid B (GABAB)-receptor mediation of different in vivo effects of gamma-butyrolactone. J Pharmacol Sci 106:199–207 [DOI] [PubMed] [Google Scholar]
- Carter LP, Chen W, Coop A, Koek W, France CP. (2006) Discriminative stimulus effects of GHB and GABA(B) agonists are differentially attenuated by CGP35348. Eur J Pharmacol 538:85–93 [DOI] [PubMed] [Google Scholar]
- Carter LP, Koek W, France CP. (2009) Behavioral analyses of GHB: receptor mechanisms. Pharmacol Ther 121:100–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Matthews MM, Mehta AK, Hernandez RJ, Thomson JA, Ticku MK, Coop A, Koek W, et al. (2005) Novel γ-hydroxybutyric acid (GHB) analogs share some, but not all, of the behavioral effects of GHB and GABAB receptor agonists. J Pharmacol Exp Ther 313:1314–1323 [DOI] [PubMed] [Google Scholar]
- Chen Y, Menendez-Roche N, Sher E. (2006) Differential modulation by the GABAB receptor allosteric potentiator 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)-phenol (CGP7930) of synaptic transmission in the rat hippocampal CA1 area. J Pharmacol Exp Ther 317:1170–1177 [DOI] [PubMed] [Google Scholar]
- Christopoulos A. (2002) Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov 1:198–210 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP. (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 310:952–963 [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Przegaliński E. (2007) Effects of GABA(B) receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol 574:148–157 [DOI] [PubMed] [Google Scholar]
- Frankowska M, Filip M, Przegaliński E. (2007) Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol Rep 59:645–655 [PubMed] [Google Scholar]
- Gray JA, Goodwin GM, Heal DJ, Green AR. (1987) Hypothermia induced by baclofen, a possible index of GABAB receptor function in mice, is enhanced by antidepressant drugs and ECS. Br J Pharmacol 92:863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. (2005) Differential sensitivity to the motor and hypothermic effects of the GABA B receptor agonist baclofen in various mouse strains. Psychopharmacology (Berl) 179:688–699 [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. (2008) Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology 54:854–862 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der Putten H, Mosbacher J, et al. (2003) Specific γ-hydroxybutyrate-binding sites but loss of pharmacological effects of γ-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci 18:2722–2730 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (1997) Competitive antagonism, in Pharmacologic Analysis of Drug-Receptor Interaction, 3rd ed, pp 331–373, Lippincott-Raven, Philadelphia, PA [Google Scholar]
- Kerr DI, Ong J. (1995) GABAB receptors. Pharmacol Ther 67:187–246 [DOI] [PubMed] [Google Scholar]
- Koek W, France CP. (2008) Cataleptic effects of γ-hydroxybutyrate (GHB) and baclofen in mice: mediation by GABA(B) receptors, but differential enhancement by N-methyl-d-aspartate (NMDA) receptor antagonists. Psychopharmacology (Berl) 199:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Flores LR, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, France CP. (2004) Discriminative stimulus effects of γ-hydroxybutyrate in pigeons: role of diazepam-sensitive and -insensitive GABA(A) and GABA(B) receptors. J Pharmacol Exp Ther 308:904–911 [DOI] [PubMed] [Google Scholar]
- Koek W, Khanal M, France CP. (2007a) Synergistic interactions between ‘club drugs’: γ-hydroxybutyrate and phencyclidine enhance each other's discriminative stimulus effects. Behav Pharmacol 18:807–810 [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A. (2007b) Cataleptic effects of γ-hydroxybutyrate (GHB), its precursor γ-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacology (Berl) 192:407–414 [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A, France CP. (2009) Behavioral effects of γ-hydroxybutyrate, its precursor γ-butyrolactone, and GABA(B) receptor agonists: time course and differential antagonism by the GABA(B) receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). J Pharmacol Exp Ther 330:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ. (2006) The GABA(B) receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology 50:632–639 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Carai MA, Kaupmann K, Guery S, Froestl W, Leite-Morris KA, Gessa GL, Colombo G. (2009) Reduction of alcohol's reinforcing and motivational properties by the positive allosteric modulator of the GABA(B) receptor, BHF177, in alcohol-preferring rats. Alcohol Clin Exp Res 33:1749–1756 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G. (2008) Specific reduction of alcohol's motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783–comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res 32:1558–1564 [DOI] [PubMed] [Google Scholar]
- Malherbe P, Masciadri R, Norcross RD, Knoflach F, Kratzeisen C, Zenner MT, Kolb Y, Marcuz A, Huwyler J, Nakagawa T, et al. (2008) Characterization of (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one as a positive allosteric modulator of GABAB receptors. Br J Pharmacol 154:797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. (2004) Role of γ-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann NY Acad Sci 1025:491–503 [DOI] [PubMed] [Google Scholar]
- Mathivet P, Bernasconi R, De Barry J, Marescaux C, Bittiger H. (1997) Binding characteristics of γ-hydroxybutyric acid as a weak but selective GABAB receptor agonist. Eur J Pharmacol 321:67–75 [DOI] [PubMed] [Google Scholar]
- Mombereau C, Lhuillier L, Kaupmann K, Cryan JF. (2007) GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens DeltaFosB accumulation. J Pharmacol Exp Ther 321:172–177 [DOI] [PubMed] [Google Scholar]
- Orrù A, Lai P, Lobina C, Maccioni P, Piras P, Scanu L, Froestl W, Gessa GL, Carai MA, Colombo G. (2005) Reducing effect of the positive allosteric modulators of the GABA(B) receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats. Eur J Pharmacol 525:105–111 [DOI] [PubMed] [Google Scholar]
- Parker DA, Marino V, Ong J, Puspawati NM, Prager RH. (2008) The CGP7930 analogue 2,6-di-tert-butyl-4-(3-hydroxy-2-spiropentylpropyl)-phenol (BSPP) potentiates baclofen action at GABA(B) autoreceptors. Clin Exp Pharmacol Physiol 35:1113–1115 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A. (2008) Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther 326:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilc A, Nowak G. (2005) GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors. Drugs Today (Barc) 41:755–766 [DOI] [PubMed] [Google Scholar]
- Pin JP, Prézeau L. (2007) Allosteric modulators of GABA(B) receptors: mechanism of action and therapeutic perspective. Curr Neuropharmacol 5:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quéva C, Bremner-Danielsen M, Edlund A, Ekstrand AJ, Elg S, Erickson S, Johansson T, Lehmann A, Mattsson JP. (2003) Effects of GABA agonists on body temperature regulation in GABA(B(1))−/− mice. Br J Pharmacol 140:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Gomez T, Jacobs K, Tallarida RJ. (2004) Pronounced hypothermic synergy between systemic baclofen and NOS inhibitor. Eur J Pharmacol 502:271–272 [DOI] [PubMed] [Google Scholar]
- Rawls SM, Jacobs K, Tallarida RJ. (2006) Baclofen and NOS inhibitors interact to evoke synergistic hypothermia in rats. Life Sci 78:669–672 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (2000) Dose-response analysis, in Drug Synergism and Dose-Effect Data Analysis, pp 44–50, Chapman & Hall/CRC, Boca Raton, FL [Google Scholar]
- Urwyler S, Gjoni T, Koljatić J, Dupuis DS. (2005) Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology 48:343–353 [DOI] [PubMed] [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K. (2001) Positive allosteric modulation of native and recombinant γ-aminobutyric acid(B) receptors by 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol 60:963–971 [PubMed] [Google Scholar]
- Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, Koller M, Kaupmann K. (2003) N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of γ-aminobutyric acidB receptor function. J Pharmacol Exp Ther 307:322–330 [DOI] [PubMed] [Google Scholar]
- van Nieuwenhuijzen PS, McGregor IS. (2009) Sedative and hypothermic effects of γ-hydroxybutyrate (GHB) in rats alone and in combination with other drugs: assessment using biotelemetry. Drug Alcohol Depend 103:137–147 [DOI] [PubMed] [Google Scholar]
- Wang L, Martin B, Brenneman R, Luttrell LM, Maudsley S. (2009) Allosteric modulators of G protein-coupled receptors: future therapeutics for complex physiological disorders. J Pharmacol Exp Ther 331:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]