Abstract
Spontaneous diastolic depolarization in the sinoatrial (SA) node enables it to serve as pacemaker of the heart. The variable cell morphology within the SA node predicts that ion channel expression would be heterogeneous and different from that in the atrium. To evaluate ion channel heterogeneity within the SA node, we used fluorescent in situ hybridization to examine ion channel expression in the ferret SA node region and atrial appendage. SA nodal cells were distinguished from surrounding cardiac myocytes by expression of the slow (SA node) and cardiac (surrounding tissue) forms of troponin I. Nerve cells in the sections were identified by detection of GAP-43 and cytoskeletal middle neurofilament. Transcript expression was characterized for the 4 hyperpolarization-activated cation channels, 6 voltage-gated Na+ channels, 3 voltage-gated Ca2+ channels, 24 voltage-gated K+ channel α-subunits, and 3 ancillary subunits. To ensure that transcript expression was representative of protein expression, immunofluorescence was used to verify localization patterns of voltage-dependent K+ channels. Colocalizations were performed to observe any preferential patterns. Some overlapping and nonoverlapping binding patterns were observed. Measurement of different cation channel transcripts showed heterogeneous expression with many different patterns of expression, attesting to the complexity of electrical activity in the SA node. This study provides insight into the possible role ion channel heterogeneity plays in SA node pacemaker activity.
Keywords: gene expression, sodium channels, potassium channels, voltage-gated calcium channels, L-type calcium channels, T-type calcium channels, cyclic nucleotide gated cation channels
primary pacemaker cells in the central region of the sinoatrial (SA) node undergo spontaneous diastolic depolarization, which ultimately triggers the action potential (AP) upstroke. The excitatory AP wave front subsequently spreads through adjacent areas of the perinodal region to the crista terminalis and atrium. Repolarization of the pacemaker cells allows the cycle to repeat. This inherent cyclic behavior enables the SA node to serve as the normal pacemaker of the heart (7, 12, 14, 26, 57).
In the central nodal and adjacent perinodal cells, the ionic basis of spontaneous diastolic depolarization and different phases of the AP have been attributed to many currents (7, 8, 14, 27, 36, 37, 42, 47, 61, 68, 77). Studies conducted on different mammalian species identified a hyperpolarization-activated nonselective cation current (If), two calcium currents [ICa,L (L-type) and ICa,T (T-type)], a sodium current (INa), and at least four voltage-dependent potassium currents [ultrarapid (IKur), rapid (IKr), and slow (IKs) delayed rectifier currents and transient outward current (Ito)]. There also appears to be a relatively small (compared with ventricular myocytes) inwardly rectifying K+ current (IK1) (17, 24, 33, 35–37, 47, 52, 54, 68, 77). However, SA node anatomy is complex and varies considerably among species (3, 5–7, 16, 18, 29, 30, 41, 42, 73), suggesting that patterns of ion channel expression may also be complex and variable among species.
The basic properties of cardiac pacemaker activity have been characterized by electrophysiological studies of multicellular SA node preparations. These studies showed clear gradations in the AP voltage range and time course recorded from primary pacemaker cells versus cells in adjacent perinodal regions (5, 7, 32, 70). Subsequent voltage-clamp studies of single SA node cells identified and quantified individual currents. Inherent limitations of these studies include the very small number of cells selected for analysis and the uncertainty of their origin (central vs. perinodal regions). Nonetheless, by establishing a framework for identification of molecular correlates underlying generation and modulation of cardiac pacemaker activity, these studies represented a significant advance.
Ferrets (Mustela putorius furo) were selected as an animal model to begin to describe more fully potential contributions of the different ion channels to generation and modulation of nodal pacemaking activity. We first describe the anatomy of ferret SA node and show that it more closely resembles human SA node than other more commonly used models (5–7, 18, 23, 29, 40, 73, 76). Using fluorescent in situ hybridization (FISH) to detect transcripts of the slow and cardiac forms of troponin I (TnIS and TnIC, respectively), we identified the location of the central region, and presumably the origin of pacemaker activity. Using these markers, we describe an extensive FISH analysis of the distribution and abundance of 33 cation channels and 3 ancillary subunit transcripts within the SA node. We performed immunofluorescence (IF) on a select group of highly expressed K+ channels to determine whether channel protein expression patterns positively correlated with transcript expression. These studies make an important contribution to our understanding of the physiology of the normal pacemaker activity in the ferret and the pathophysiology of abnormal pacemaker function in humans.
MATERIALS AND METHODS
Procedures involving ferrets were approved by the Institutional Animal Care and Use Committee of the University at Buffalo, the State University of New York, and of Duke University Medical Center, in accordance with applicable federal regulations and the ethics policies of the American Physiological Society.
SA node sections.
Hearts of male ferrets were excised and mounted on a Langendorff perfusion apparatus. After initial perfusion with normal physiological saline (to wash out blood), a second perfusion of normal saline plus 4% paraformaldehyde was conducted for 45 min. After this second perfusion, the entire nodal region (between superior and inferior vena cava) and adjacent right atrial appendage (RAA) were dissected free and incubated in 3% paraformaldehyde for 60 min. The tissue sample was incubated overnight in 40% sucrose in PBS at 4°C, followed by incubation in OCT medium (10.24% polyvinyl alcohol, 4.26% polyethylene glycol) for 5 h. The tissue was then embedded in fresh OCT medium by slow freezing in a CO2-isobutanol bath. Tissue blocks were sectioned with a Frigocut 2800E cryostat (Leica Microsystems, Wetzlar, Germany).
Fluorescent in situ hybridization.
FISH was conducted as previously described (9–11). Tissue sections were postfixed on gelatin-coated slides, incubated in prehybridization buffer, and hybridized with biotin- or digoxigenin-labeled oligonucleotides (Supplemental Table S1) at 42°C for 16–24 h.1 Labeled probes were detected with streptavidin-phycoerythrin or anti-digoxigenin antibody conjugated with fluorescein isothiocyanate (FITC). Probes for TnIC and TnIS antisense probe were used as controls. Experiments with antisense probes were run in parallel with sense probes as negative controls. Fluorescent signals were detected and quantified by confocal microscopy (LSM 410; Carl Zeiss MicroImaging, Thornwood, NY) as described previously (9, 10). All FISH experiments were repeated on at least four hearts; the number for each transcript is listed in Table 1.
Table 1.
Transcript abundance in sinoatrial node and right atrial appendage
| Transcript | SAN | RAA | n |
|---|---|---|---|
| TnIS-S* | 0.8 ± 0.0 | 0.3 ± 0.0 | 28 |
| TnIS | 86.2 ± 0.1 | 15.6 ± 0.3 | 48 |
| TnIC | 19.8 ± 0.2 | 90.4 ± 0.2 | 42 |
| Kv1.1 | 88.1 ± 0.1 | 27.5 ± 0.1 | 28 |
| Kv1.2 | 49.4 ± 0.2 | 15.0 ± 0.1 | 9 |
| Kv1.3 | 31.0 ± 0.1 | 24.5 ± 0.0 | 9 |
| Kv1.4 | 87.2 ± 0.2 | 68.8 ± 0.3 | 9 |
| Kv1.5 | 90.9 ± 0.3 | 100.0 ± 0.2 | 9 |
| Kv1.6 | 20.8 ± 0.3 | 10.0 ± 0.1 | 6 |
| Kv2.1 | 49.8 ± 0.1 | 49.7 ± 0.3 | 6 |
| Kv2.2 | 21.2 ± 0.5 | 23.1 ± 0.2 | 6 |
| Kv3.1 | 12.3 ± 0.1 | 6.0 ± 0.1 | 6 |
| Kv3.2 | 10.0 ± 0.4 | 4.6 ± 0.1 | 6 |
| Kv3.3 | 9.5 ± 0.5 | 7.2 ± 0.2 | 6 |
| Kv3.4 | 5.4 ± 0.6 | 4.2 ± 0.1 | 6 |
| Kv4.1 | 15.8 ± 0.1 | 6.9 ± 0.1 | 6 |
| Kv4.2 | 50.0 ± 0.6 | 21.3 ± 0.7 | 6 |
| Kv4.3 | 34.1 ± 0.8 | 13.2 ± 0.3 | 6 |
| Kv5.1 | 15.6 ± 0.2 | 78.0 ± 0.5 | 10 |
| Kv6.1 | 12.9 ± 0.3 | 4.6 ± 0.1 | 6 |
| Kv7.1 | 55.9 ± 0.1 | 11.3 ± 0.2 | 6 |
| Kv10.1 | 25.4 ± 0.2 | 8.9 ± 0.1 | 6 |
| mink | 28.1 ± 0.2 | 11.2 ± 0.2 | 6 |
| Kv11.1 | 36.8 ± 1 | 10.6 ± 0.3 | 6 |
| Kv11.2 | 19.6 ± 0.6 | 16.8 ± 0.1 | 6 |
| Kv11.3 | 21.8 ± 0.2 | 7.1 ± 0.1 | 6 |
| HCN1 | 49.2 ± 0.3 | 10.6 ± 0.5 | 4 |
| HCN2 | 28.0 ± 0.7 | 6.9 ± 0.3 | 4 |
| HCN3 | 9.4 ± 0.2 | 2.0 ± 0.2 | 4 |
| HCN4 | 49.1 ± 0.7 | 4.4 ± 0.6 | 4 |
| Cav1.2 | 40.6 ± 0.5 | 8.2 ± 0.7 | 4 |
| Cav3.1 | 83.9 ± 1.4 | 22.2 ± 1.1 | 4 |
| Cav3.2 | 70.2 ± 1.8 | 26.6 ± 0.9 | 4 |
| Nav1.1 | 27.5 ± 1.1 | 38.1 ± 1.1 | 4 |
| Nav1.3 | 21.2 ± 1.4 | 27.7 ± 0.9 | 4 |
| Nav1.4 | 7.7 ± 0.8 | 53.0 ± 1.8 | 4 |
| Nav1.5 | 54.9 ± 0.2 | 48.9 ± 1.2 | 4 |
| Nav1.8 | 29.5 ± 0.1 | 25.8 ± 0.9 | 4 |
| Nav1.9 | 12.4 ± 0.6 | 29.7 ± 1.2 | 4 |
Values [in arbitrary relative fluorescence units, normalized to 100 (the value obtained from Kv1.5 in right atrial appendage)] are means ± SE; n = no. of sections scanned. Sections were probed by fluorescence in situ hybridization (FISH) and scanned as described in materials and methods. TnIS, TnIC, slow and cardiac troponin I, respectively; TnIS-S*, sense probe (negative) control; SAN, sinoatrial node, RAA, right atrial appendage. To highlight variability between animals, only 1 section per heart per transcript is included in this table.
Immunofluorescence.
IF was conducted as previously described (9–11) with commercially available antibodies from Novus Biologicals (Littleton, CO) or Santa Cruz Biotechnology (Santa Cruz, CA). Primary antibodies were detected with secondary antibodies conjugated to FITC or rhodamine. Images were scanned on a Zeiss LSM 410 inverted confocal microscope. All IF experiments were repeated on three to five ferret hearts.
Image analysis.
FISH and IF images were digitized with LSM 3.9v (Zeiss) software, analyzed with Metamorph (Molecular Devices, Sunnyvale, CA) or IPLab Spectrum (Scanalytics, Rockville, MD), and assembled with Adobe Photoshop CS2. Empirical quantification of relative fluorescence levels was conducted as described in results.
RESULTS
Anatomy of ferret SA node.
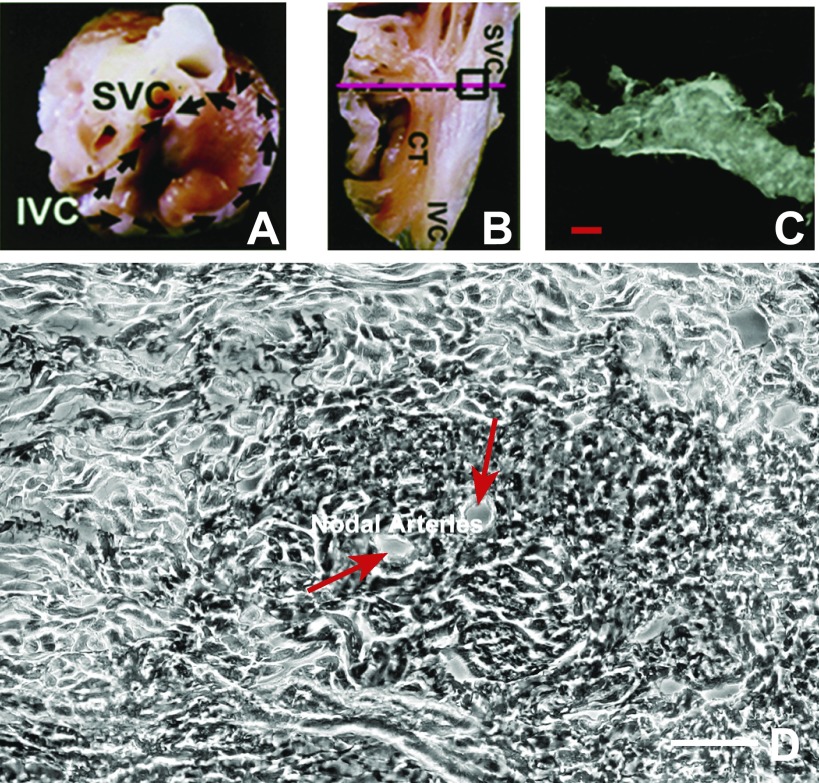
The ferret SA node is at the junction of the superior vena cava and right atrium near the sulcus terminalis region (Fig. 1, A and B). Cross sections of the right atrium revealed an ovoid-shaped SA node positioned epicardially (Fig. 1C). Two central nodal arteries are surrounded by a ring of dark cells; large pale cells form a concentric ring around the dark cells (Fig. 1D). Thus many features of ferret SA node have striking similarities to classic descriptions of human SA node (29, 74, 75).
Fig. 1.
Identification and examination of the ferret sinoatrial (SA) node. A: photograph of the ferret heart showing the aorta, superior vena cava (SVC), inferior vena cava (IVC), and right atrial (RA) appendages. Black arrows show the location of incisions that exposed the SA nodal region. B: endocardium of the medial RA appendage. The SA node is located near the crista terminalis (CT) and is marked by the box. The pink line shows the path of sections, which were perpendicular to the crista terminalis. C: light microscopic (LM) view of the section. Scale bar, 125 μm. D: enlarged image of the SA node and the proximity of the central part of the SA node to the nodal arteries, marked with red arrows. Scale bar, 50 μm. Growth-associated protein 43 (GAP-43) localization in this section is shown in Fig. 2F.
To characterize the anatomy and functional regions of ferret SA node, we next performed FISH to detect 1) cardiac and slow-twitch forms of troponin I mRNA (TnIC and TnIS, respectively; Fig. 2, A–D), 2) cytoskeletal middle neurofilament protein (NF; Fig. 2E), and 3) growth-associated protein 43 (GAP-43; Fig. 2F). TnIC has been shown to be the dominant troponin I isoform in rat atrial myocytes, while TnIS is present in the conduction system (19). Cytoskeletal middle NF is expressed in the SA node (18, 71, 79). GAP-43 is a marker for neurons (1, 13, 25, 78, 82, 83).
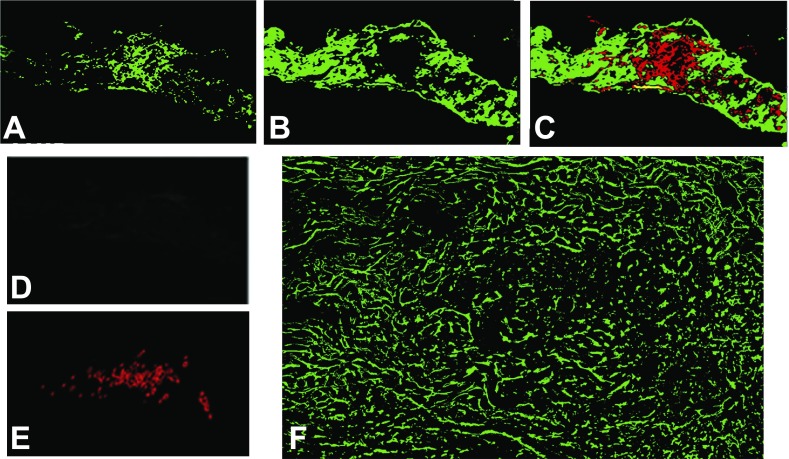
Fig. 2.
Localization of SA node and neuronal markers. The data in A–E were acquired by fluorescence in situ hybridization (FISH); F was an immunofluorescence (IF) localization. Each cross section through SA node was made at 5-μm thickness. A and B: troponin I mRNA [cardiac (TnIC) and slow twitch (TnIS)] FISH signals obtained by using TnIS antisense probe to mark the SA nodal region and TnIC antisense probe to identify atrial myocytes in this region. C, an overlay of TnIS (red) and TnIC (green) from A and B, respectively, shows that these transcripts are not coexpressed. D: FISH negative control using a TnIS sense probe; the exposure time on the photograph was identical to that in A. All FISH experiments were done simultaneously with sense and antisense probes. All sections included in this report had sense probe negative controls similar to that shown in D. E: distribution of cytoskeletal middle neurofilament protein. F: distribution of the neuronal marker GAP-43 in the SA node.
Signals generated by antisense probes to TnIS (Fig. 2A) and TnIC (Fig. 2B) show that both transcripts are expressed in the SA node. TnIS expression was prominent in the central region and nearly absent in the peripheral regions. In contrast, TnIC expression was prominently localized in the peripheral transitional region of the SA node, where the unique or differential SA nodal cells transition into the right atrial myocytes. Overlay of these two transcripts shows the nonoverlapping distribution of isoform expression (Fig. 2C). Figure 2D is an example of a TnIS sense oligonucleotide negative control, which shows the very low background fluorescence typical of our results with other probes. Finally, NF localizes to the central region of the SA node, providing further evidence of the location of the central SA node in our preparations (Fig. 2E). While both NF and TnIS were present in the central position of the SA node, the distribution of NF signal was more confined than that of TnIS. These data show that TnIS and TnIC transcript expression are useful markers for distinguishing central versus outer SA nodal regions in the ferret.
In several species, the SA node is innervated well by the autonomic nerves, which play an important role in SA node function (29, 30, 43, 73, 75). Because the presence of neuronally expressed ion channels could confound our analysis, we sought to identify autonomic nerve fibers in the ferret SA node. A well-characterized marker of autonomic nerves is GAP-43, a protein associated with axonal growth and regeneration, which has been identified in cultured sympathetic neurons, cholinergic neurons, and sympathetic and parasympathetic autonomic ganglia of normal adult rats (1, 13, 25, 78, 82, 83). We observed GAP-43 expression as filamentous tracts within both the central and surrounding SA nodal regions (Fig. 2F). There was less GAP-43 surrounding the SA nodal arteries. Furthermore, our data showed marked differences between the widespread filamentous pattern of antibody to GAP-43 and antibody to NF.
Distribution and expression levels of cation ion channel transcripts.
To understand the contributions of different cation channels to cardiac pacemaker activity, we examined the distribution of channel transcripts, using FISH. This analysis included the four hyperpolarization-activated cation channels (HCN1–HCN4), six voltage-gated Na+ channels (Nav1.1, Nav1.3–Nav1.5, Nav1.8, and Nav1.9), three voltage-gated Ca2+ channels (Cav1.2, Cav3.1, and Cav3.2), and 20 voltage-gated K+ channel α-subunits from multiple families (Kv1.1–Kv1.6, Kv2.1 and Kv2.2, Kv3.1–Kv3.4, Kv4.1–Kv4.3, Kv7.1, Kv10.1, Kv11.1–Kv11.3). We also probed for three K+ channel ancillary subunits (minK, Kv5.1, Kv6.1).
We detected transcript for all 33 ion channels and 3 subunits in the ferret SA node. It was perhaps surprising that the many of the same transcripts were detected in both the SA node and the RAA despite the marked differences in their electrophysiological properties. It was clear that many channels showed regional localization across the SA nodal sections. However, caution should be exercised in comparing the relative abundance of different transcripts, given the well-known factors that confound this type of comparison. For example, the efficiency of hybridization and labeling may vary between probes. With these caveats in mind, we quantified the fluorescence emitted by each transcript probe relative to an internal standard in the central nodal region and the RAA (Table 1).
The strongest fluorescent signals in the SA node were from Kv1.1, Kv1.4, Kv1.5, and the T-type calcium channel transcripts Cav3.1 and Cav3.2; all had signals greater than 70 relative fluorescence units (RFU). Several channels were apparently expressed at somewhat lower levels, with RFU values between 34 and 60. These included Kv1.2, Kv2.1, Kv4.2, Kv4.3, Kv7.1, HCN1, HCN4, Kv11.1, Cav1.2, and Nav1.5. Many signals were very weak (<10 RFU), suggesting low levels of expression. However, despite the low level of expression of these channels, we cannot rule out the possibility that these transcripts give rise to channels with important physiological function.
There were also dramatic differences in SA node expression compared with that of the RAA. While we never observed TnIS and TnIC expression overlap, the ratio of SA node to RAA expression was 5.5-fold for the former and 0.22-fold for the latter. Some channels had nearly the same degree of preference for SA node: Kv1.1, Kv1.2, Kv7.1, and Cav1.2. All four HCN transcripts were at least 4-fold greater in the SA node; strikingly, HCN4 was 11-fold more abundant in the SA node. Fewer channels showed strong preference for the RAA; the notable exception was Nav1.4, whose transcript expression was 6.9-fold higher in the RAA.
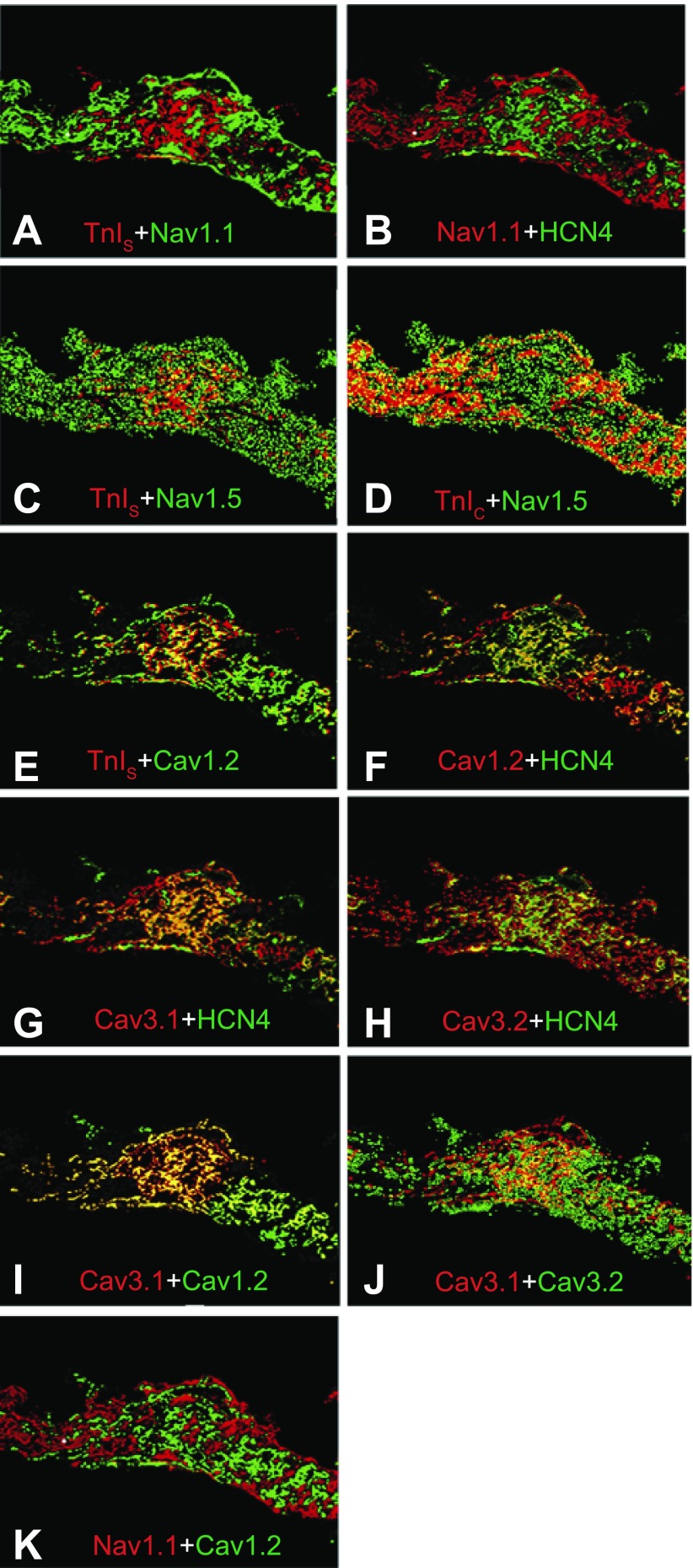
To evaluate the distribution of each transcript studied, we initially focused on a hallmark of pacemaker activity in rabbit, mouse, and human SA node, hyperpolarization-activated cation channels HCN1-HCN4 (Fig. 3) (22, 28, 41, 45, 67, 69, 71, 80, 84). Comparison of transcripts from all four hyperpolarization-activated cation channels showed that expression of HCN1 and HCN4 were highest, although there were significant levels of HCN2 and HCN3 (Fig. 3, C–F). As expected, overlays of HCN transcript expression showed significant overlap (Fig. 4, A–D), the most extensive of which was between HCN1 and HCN4. Broad overlap between TnIS and HCN1 transcripts (Fig. 4A) demonstrates that HCN1 is expressed almost exclusively in the central nodal region in the ferret. Colocalization of HCN2–4 with HCN1 suggests that any of these four channels might participate in generation of ferret SA node pacemaker activity.
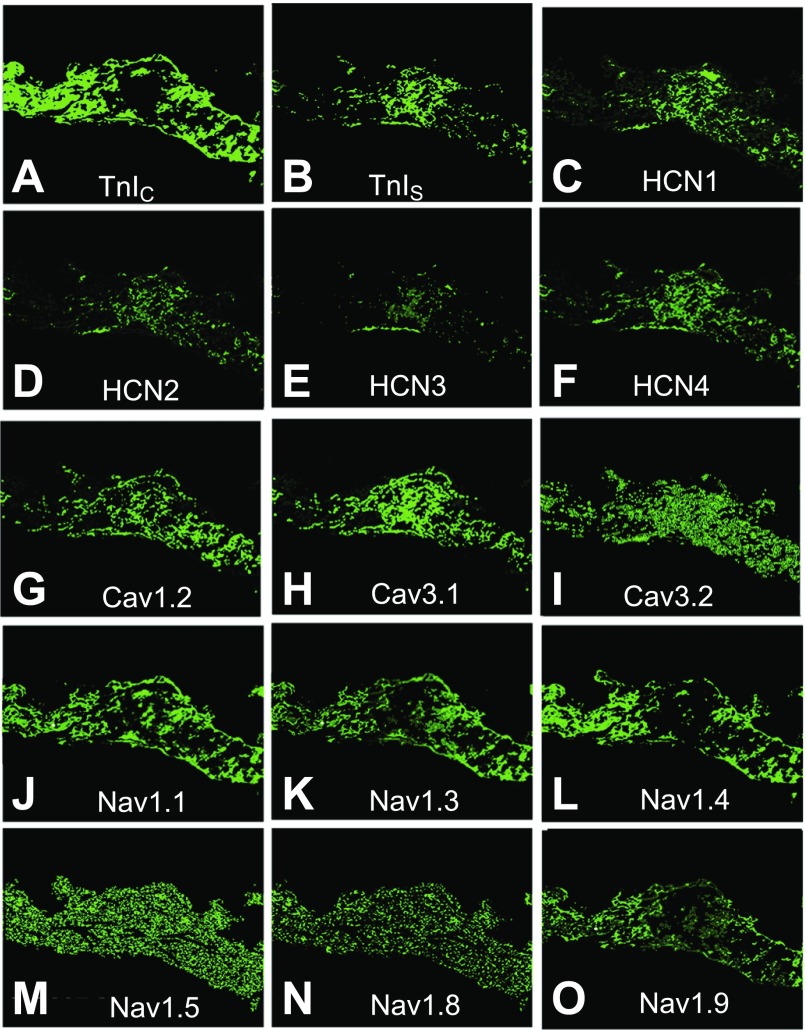
Fig. 3.
Analysis of ion channel mRNA expression by FISH in cross sections of ferret SA node. TnIC (A; marker for atrial myocytes); TnIS (B; SA nodal cell marker); hyperpolarization-activated cation channels HCN1 (C), HCN2 (D), HCN3 (E), and HCN4 (F); voltage-gated Ca2+ channels Cav1.2 (G), Cav3.1 (H), and Cav3.2 (I); and voltage-gated Na+ channels Nav1.1 (J), Nav1.3 (K), Nav1.4 (L), Nav1.5 (M), Nav1.8 (N), and Nav1.9 (O).
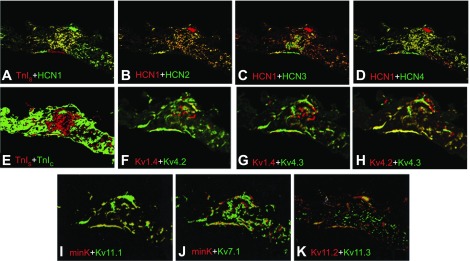
Fig. 4.
Colocalization of hyperpolarization-activated cation channels and K+ channels. Images are overlays from other figures with false color added to allow comparison of expression patterns. Individual proteins or transcripts are red (first listed) or green. All images are from FISH, except F–H, which are IF. Coexpression appears as yellow. TnIS with HCN1 (A), HCN1 with HCN2 (B), HCN1 with HCN3 (C), HCN1 with HCN4 (D), TnIS with TnIC (E), Kv1.4 with Kv4.2 (F), Kv1.4 with Kv4.3 (G), Kv4.2 with Kv4.3 (H), minK with Kv11.1 (I), minK with Kv7.1 (J), and Kv11.2 with Kv11.3 (K) are shown. The images in A–E were created by merging Fig. 3, A–F, and those in G–K by merging Fig. 6, R–V.
Voltage-gated Na+ channels are important for pacemaking in rabbit, rat, and mouse SA node (33, 38, 46, 52); therefore, we evaluated their expression in ferret SA node. The Na+ channels fell into two general expression patterns. Some, including Nav1.5, 1.8, and 1.9, were uniformly and widely expressed; Nav1.1, Nav1.3, and Nav1.4 were more localized (Fig. 3, J–O). Absence of transcript in the central nodal region was most marked in the case of Nav1.4 and to a lesser degree in Nav1.3 and Nav1.9. Magnitude of expression was greatest for Nav1.5 and lowest for Nav1.4.
Using TnIS, TnIC, and HCN4 to identify the central and surrounding regions of the SA node, we examined the distribution of Nav1.1 and Nav1.5 (Fig. 5, A–D). The highly localized Nav1.1 showed little overlap with TnIS and HCN4. In contrast, the diffusely expressed Nav1.5 overlapped expression with both TnIS and TnIC, suggesting that it is distributed throughout the SA nodal region. From our data, it was unclear whether Nav1.1 and 1.5 were expressed in different cell types or whether their mRNA was localized differently within the same cells.
Fig. 5.
Colocalization of voltage-gated Na+ channels and voltage-gated Ca2+ channels. Images presented from Fig. 3 with false color added as in Fig. 4. All images are from FISH. TnIS with Nav1.1 (A), Nav1.1 with HCN4 (B), TnIS with Nav1.5 (C), TnIC with Nav1.5 (D), TnIS with Cav1.2 (E), Cav1.2 with HCN4 (F), Cav3.1 with HCN4 (G), Cav3.2 with HCN4 (H), Cav3.1 with Cav1.2 (I), Cav3.1 with Cav3.2 (J), and Nav1.1 with Cav1.2 (K) are shown.
L-type and T-type calcium channels are expressed in the SA nodes of other species (47, 48, 69, 71); therefore we tested for the presence of transcripts of one L-type (Cav1.2) and two T-type (Cav3.1 and Cav3.2) voltage-gated calcium channels in ferrets. All three were expressed at significant levels. The T-type channels appeared to be expressed more abundantly than Cav1.2 (Fig. 3, G–I). Expression of Cav3.1 was most dense in the central region, while Cav3.2 expression was diffuse throughout the sections. Cav1.2 expression was “patchy” and widespread; there was considerable overlap with TnIS and HCN4 in the central region (Fig. 5, E and F). Cav3.1 expression also overlapped with HCN4, but overlap between Cav3.2 and HCN4 was minimal (Fig. 5, G and H); these results were reflected in the extensive overlap of Cav1.2 and Cav3.1 transcripts and minimal overlap between Cav3.1 and 3.2 transcripts (Fig. 5, I and J).
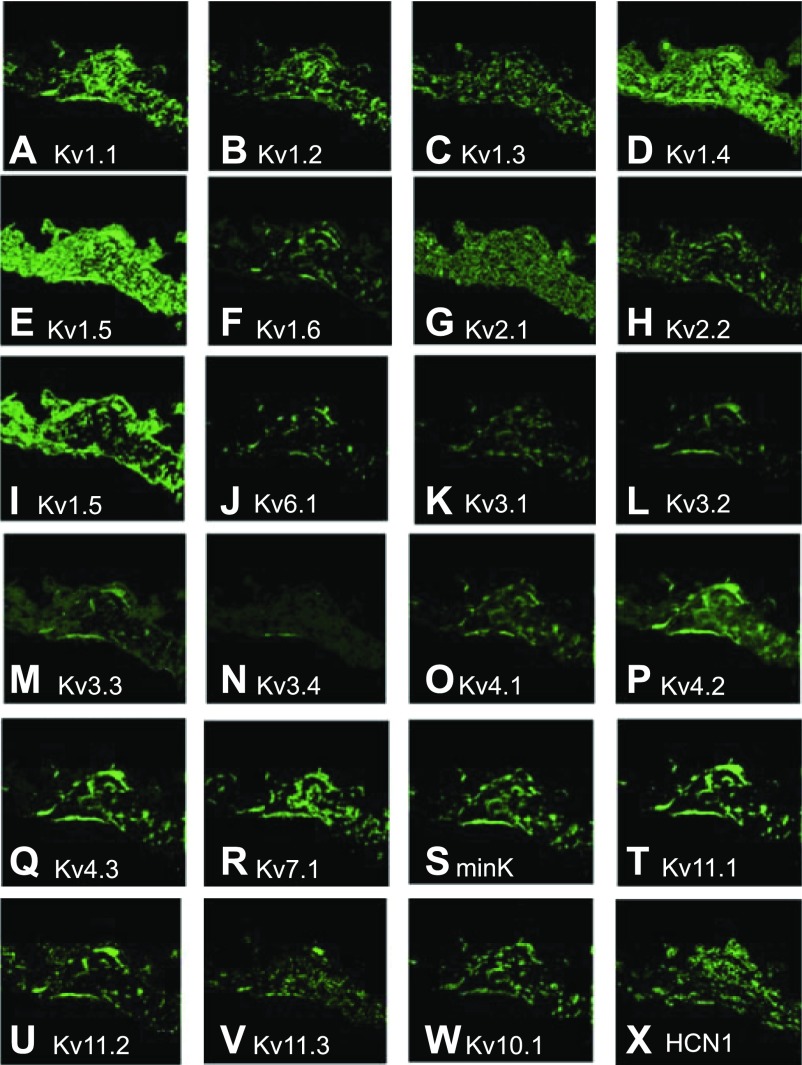
Several K+ currents have been detected in the SA nodes of various species (17, 24, 33, 35–37, 47, 52, 54, 68, 77); however, the large number of voltage-gated K+ channels and ancillary subunits often makes definitive identification of their molecular basis challenging (51). In the case of Kv1 channels, all transcripts were detected throughout the SA node (Fig. 6, A–F). Kv1.1, 1.4, and 1.5 displayed the most widespread expression levels, with Kv1.2 and 1.3 at intermediate levels and Kv1.6 the lowest. Kv1.1 expression was intense in the central region (Fig. 6A), while both Kv1.4 (Fig. 6D) and Kv1.5 (Fig. 6E) displayed nearly uniform expression throughout the nodal region. The remaining transcripts (Kv1.2, 1.3, and 1.6; Fig. 6, B, C, and E) expressed poorly in all regions, although there appeared to be somewhat higher expression of Kv1.2 in the central region.
Fig. 6.
Analysis of voltage-gated K+ channel subunit mRNA expression by FISH in cross sections of ferret SA node. In all panels green fluorescence in each section indicates the presence of specific mRNA expression of different K+ and associated channel antisense probes. Note the differential and distinct pattern of expression for each channel type. Channel types: Kv1.1–6 (A–F), Kv2.1 (G) and Kv2.2 (H), Kv5.1 (I), Kv6.1 (J), Kv3.1–4 (K–N), Kv4.1–3 (O–Q), Kv7.1 (R), minK (S), Kv11.1–3 (T–V), Kv10.1 (W), HCN1 (X).
Kv2 transcripts were distributed throughout the SA node region (Fig. 6, G and H); however, Kv2.1 distribution was uniform while Kv2.2 expression was spotty. Expression patterns of Kv5.1 and Kv6.1 (Fig. 6, I and J), two ancillary subunits previously shown to modify Kv2.1 function (34, 56), were different from those of Kv2.1 and Kv2.2. This disparity was striking for Kv5.1, which was much more extensively expressed outside the central region. Kv3 transcripts (Fig. 6, K–N) were the most poorly expressed group; low levels of each transcript were present in all nodal regions. Patterns of expression for Kv3.1 and 3.2 appeared to be more intense than the weak and diffuse expression for Kv3.3 and 3.4.
Among Kv4 transcripts, Kv4.2 and 4.3 were most highly expressed, while Kv4.1 expression was lower (Fig. 6, O–Q). Kv4 transcripts were detected in all areas of the SA node, but expression patterns were nonuniform and displayed localized spots and/or “tracks.” This effect was minimal for Kv4.1 but prominent for 4.2 and 4.3. In addition to Kv4.2 and Kv4.3, Kv1.4 is also responsible for producing a transient outward current (Ito) with rapid inactivation in ventricular and atrial myocytes of several species, including ferret (4, 10, 53). However, recovery from inactivation is fast in Kv4.2 and Kv4.3 and slow in Kv1.4 channels. In ferret left ventricular myocytes, the Kv4-based Ito has little overlap with the Kv1.4-based current (10, 58, 85). Immunofluorescent overlays of the expression of these three channel proteins show that Kv1.4 expression does not overlap with that of either Kv4.2 or Kv4.3 (Fig. 4, F and G), suggesting the presence of two Ito phenotypes with fast and slow kinetics as in the ventricle. Kv4.2 and Kv4.3 show extensive overlap in expression (Fig. 4H), suggesting the possibility of heteromultimer formation, as has been described in mouse ventricles (21), which would be most abundant in the nodal periphery.
Kv7.1 and Kv11.1 are the channels responsible for the slow and rapid forms of the delayed rectifier (IK) current, respectively (2, 50, 62, 63, 85). Kv7.1 (Fig. 6R), minK (ancillary subunit KCNE1; Fig. 6S) and Kv11.1 (HERG1; Fig. 6T) all displayed similar patterns with tracks of moderately high expression throughout the nodal regions. Kv11.2 (HERG2; Fig. 6U), 11.3 (HERG3; Fig. 6V), and 10.1 (EAG; Fig. 6W) could also be detected, with all displaying similar track patterns, although of variable intensity and width.
The expression and distribution of ion channel ancillary subunits could make an important contribution to the electrophysiological properties and amplitude of SA node currents. Comparison of minK expression with that of Kv11.1 and 7.1 (Fig. 4, I and J) shows considerable overlap between Kv11.1 and minK but much less overlap with Kv7.1, suggesting that the contribution of the slow delayed rectifier current (IKs) may be small in the ferret SA node. We also examined the colocalization (Fig. 4K) of the neural HERG-related channels Kv11.2 and Kv11.3 (66). They show some colocalization; however, they do not appear to be expressed in the same cells throughout most of our SA node section, suggesting that they may be present in distinct neural populations.
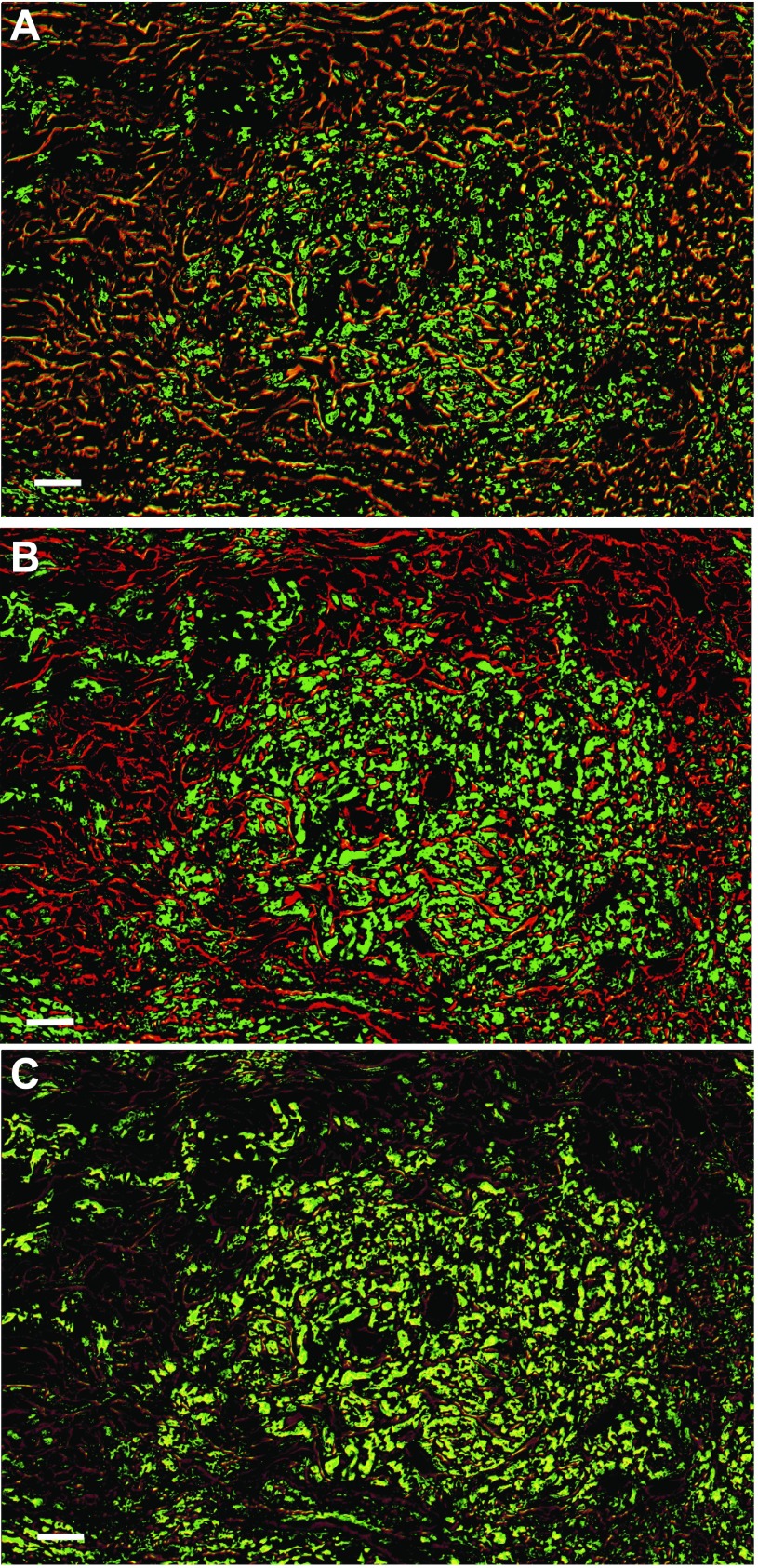
Since different cationic currents contribute to the different phases of the SA node action potential, it is important to examine the overlap of different families. For example, we have demonstrated that the degree of overlap between Cav3.1 and Cav1.2 was more extensive than that of Cav3.1 and Cav3.2, as reflected in the orange-yellow color in the central region of the SA node. Clearly the same area of cells in the central node express TnIS and HCN4 (Fig. 5, E and F) in a pattern that is shared by the two T-type channels, Cav3.1 and Cav3.2, with HCN4 (Fig. 5, G and H). Figure 7, A and B, show that both Cav1.2 and 3.1 were expressed in the central node and that there is extensive expression of GAP-43 surrounding the central portion of the node, suggesting extensive innervation. Of the two channels, only Cav1.2 localized with GAP-43. Figure 7C shows that the two channels were extensively coexpressed within the nodal cells.
Fig. 7.
Cav1.2 and Cav3.1 are coexpressed in the SA node. FISH on Cav1.2, Cav3.1, and anti-GAP-43 antibody was performed as described in materials and methods. Bars, 25 μm. A: GAP-43 (red) overlaid with Cav1.2 (green) shows considerable expression of Cav1.2 both in the SA node and in the surrounding neurons. B: GAP-43 with Cav3.1 shows that the Ca2+ channel is excluded from the neurons but is expressed in the central nodal area. C: Cav1.2 with Cav3.1 shows that the channels are expressed in the same cells in the central SA nodal tissue.
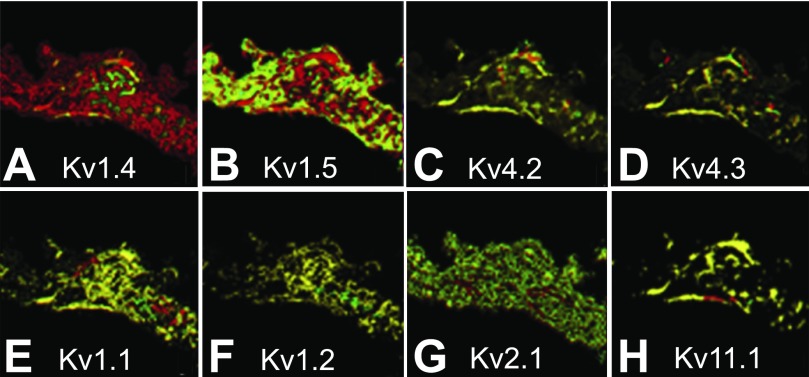
A weakness of the FISH approach for detection of channels is that it is an indirect measure of protein expression. As we have previously shown with Kv1.4 (10, 51), the level of mRNA does not necessarily correlate with protein, although this effect does not seem to be common. A second potential issue is that mRNAs detected by FISH may not overlap with protein location within a cell, potentially distorting channel distribution in large distended cells. To ascertain whether this might be the case for channels in the SA node, we performed immunolocalization of several K+ channels (Fig. 8). As is true in ferret ventricles (10), Kv1.4 transcript expression is much greater than protein expression in the ferret SA node (Fig. 8A). In addition, Kv1.5 transcript expression was also greater than protein expression in the SA node (Fig. 8B). For the other transcripts tested, there are close correlations between transcript and protein (Fig. 8, C–H).
Fig. 8.
Comparison of mRNA and protein expression of K+ channel distribution in ferret SA node. FISH was performed with antisense oligonucleotide probes labeled with rhodamine (red), IF was performed as described in materials and methods with antibodies labeled with Alexa 488 (green). The overlaid images shown are Kv1.4 (A), Kv1.5 (B), Kv4.2 (C), Kv4.3 (D), Kv1.1 (E), Kv1.2 (F), Kv2.1 (G), and Kv11.1 (H). Note the significant difference in expression level of Kv1.4 and Kv1.5 mRNA and protein expression.
DISCUSSION
The goals of this study were to describe the anatomy of the ferret SA node and to characterize expression of ion channel transcripts within the node and surrounding regions. We choose ferrets for this study because the size of their SA node made them amenable to microscopic studies and future electrophysiological characterization. As we have shown, a second advantage of the ferret is that the anatomy of its SA node more closely resembles that of the human than the popular rabbit and mouse models. Finally, the availability of a sequence in the domestic ferret may ultimately lead to the development of transgenic ferrets, which could enable us to investigate the role played by each channel in the ferret SA node.
There are striking similarities in SA node architecture between human and ferret (7, 12, 16, 20, 23, 27, 29, 74–76). Both show the presence of central nodal arteries surrounded by concentrically arranged small dark and large pale cells. In contrast, the rabbit SA node has no central nodal artery and no distribution of dark and lightly stained cells as seen in ferret and human (73). The physiological consequences of the ferret architecture are potentially important to understanding human physiology and pathophysiology. For example, changes in intravascular pressure in the SA nodal artery should affect heart rate in the ferret but not the rabbit (31). The morphology could be indicative of different cell types expressing a different array of ion channels. The combination of central nodal arteries and distribution of dark and light cells like those of the human SA node led us to suggest that the location and expression levels of ion channel transcripts are similar in humans and ferrets (15, 73, 74).
More importantly, our data are unique in that they are based on extensive analysis of contiguous nodal areas, and hence offer more detail on specific localization and distribution patterns of channel transcripts. In particular, our data show that distribution patterns of different cation channel transcripts were quite variable. While more limited in scope than our FISH analysis, the IF analysis of select Kv channel proteins confirms that there is not a strict one-on-one correspondence between mRNA levels and protein expression for some channels. Finally, our results represent a significant advancement in evaluating both the distribution and the potential function of ion channel transcripts in SA node, as well as pointing out some pitfalls that may be encountered in such analyses.
In attempting to understand SA node function, identification of molecular transcripts in thin sections of tissue does not allow extrapolation of the findings to understanding a complex three-dimensional tissue like the SA node. This limitation is a result of the combined presence of 1) multiple cell types, 2) a high degree of autonomic innervations, and 3) the distribution patterns and amounts of ion channel protein in the sarcolemma. These difficulties highlight the need for reliable markers to differentiate the true SA nodal cells from those of the surrounding atrium. For this purpose, we made extensive use of TnIS and TnIC as positive and negative markers, respectively, for SA nodal cells.
For many years, it has been understood that TnIS, found in adult skeletal muscle, is present in fetal hearts. The adult form of cardiac troponin, TnIC, replaces it late in gestation (59, 60, 64, 72). Gorza et al. (19) were the first to suggest that TnIS remained in the cardiac conduction system and SA node of rats after birth. On the basis of this observation, we used TnIS expression as a marker for cells isolated from ferret SA node and atrioventricular (AV) node (11). High levels of TnIS mRNA have also been shown in mouse SA node by quantitative PCR (49).
The presence of TnIS in the SA node is not surprising. It has been noted that several skeletal muscle contractile proteins are expressed in the cardiac conduction system (65). Furthermore, the presence of TnIS lends support to the view that, in many ways, SA node cells resemble undifferentiated atrial working cardiomyocytes (16). Here we have shown that TnIS mRNA is concentrated in the central node and highly expressed and that its separation from cells expressing TnIC is nearly absolute (Fig. 2C), making it an excellent marker for SA node ion channel gene expression.
An additional complicating factor is the extensive innervation of the SA node. To understand the roles of specific ion channels in pacemaking and their modulation by autonomic tone it is particularly important to determine which channels are expressed in the neurons of the SA node. To examine the distribution of the autonomic nerve fibers, we localized GAP-43 and neurofilament M (NF). GAP-43 is a marker for axonal growth and regeneration. It has been identified in cultured sympathetic neurons, cholinergic neurons of the postnatal rat brain, and the sympathetic and parasympathetic autonomic ganglia of normal adult rats (1, 13, 25, 78, 82, 83). Neurofilaments have been identified in sympathetic and parasympathetic neurons in the peripheral nervous system of rats (71, 79). We found that the distribution of these two proteins differed substantially. GAP-43 was present in filamentous structures within and surrounding areas of the SA node (Fig. 2F and Fig. 7). These filaments are suggestive of the axonal tracts that inhabit the node. In contrast, NF staining was most intense in the area surrounding the SA nodal arteries. The latter finding is most consistent with a concentrated distribution of autonomic fibers in the central portion of the SA node, where the release of acetylcholine and norepinephrine would have their greatest effect on pacemaking rate. Further evidence of the presence of neurons comes from expression of Kv10.1 (EAG) and Kv11.2 and 11.3 (ERG2 and ERG3). These channels are apparently expressed only in neurons (13, 67), and while experience with them as markers is much less extensive than that with GAP-43 and NF, we think it is likely that these channels mark the presence of neurons in the SA node.
Channel expression in the SA nodes of rabbit, mouse, and human has been reported (11, 15, 49, 71). Because of differences in methodologies and species, comparisons should be made with caution; however, some broad observations can be made. In general, our results are similar with respect to the abundance of Kv1.5, Kv2.1, Kv4.2, and other K+ channels. Our Ca2+ channel results also are similar. Nav1.5 is by far the most abundant sodium channel in mouse SA node, with less Nav1.4 and very little Nav1.1, 1.3, and 1.7. We find that in ferret SA node Nav1.5 is also the most abundant but that Nav1.4 localizes to areas peripheral to the central node, while there are substantial amounts of Nav1.1 and 1.3 in the central part of the SA node. The presence of Nav1.5 in the central node of the ferret differs from that of the mouse and rabbit (38, 46, 71). Finally, we concur with Marionneau et al. (49) that HCN1 and HCN4 are the most abundant among HCN species. However, while HCN4 is much more highly expressed than HCN1 in mice, we find that expression of the two isoforms is roughly equal in the ferret SA node. Localization of channel transcripts has also been performed in rabbit SA node (71). While many of the same transcripts were identified, their patterns were different. For example, Cav1.2 is nearly absent from the rabbit SA node, as is Nav1.5. Kv4.2 is expressed much more highly than Kv1.5, and HCN4 expression is 10-fold higher than HCN1. Again, these disparities could be due to differences in SA node anatomy between rabbits and ferrets.
One unique aspect of our study was the concerted effort to differentiate between nodal myocytes and neuronal expression. Of the highly expressed channels in the SA node, we found evidence that the Kv4 channels Kv7.1, its ancillary subunit minK, and Kv11.1 were more highly expressed in neurons while Kv2.1 and Kv1 channels were more highly expressed in SA nodal cells. Nav1.1, 1.3, and 1.9 also appear to be present primarily in neurons. Among Ca2+ channels, expression of Cav1.2, Cav3.1, and Cav3.2 channels was robust, demonstrating their central role in SA node function. While both Cav1.2 and Cav3.1 showed extensive localization in the SA node, they had different patterns of expression, implying different functional roles in the SA node. In addition, Cav1.2 expression is higher in or near the septum and colocalizes with GAP-43, suggesting expression in the nervous system. While HCN1 and HCN4 showed the expected expression, the expression patterns of HCN2 and HCN3 were compatible with localization in nerves.
While we have no direct evidence that the distribution patterns of ion channels in ferret sinus node closely reflect their distribution in the human SA node, the presence of these patterns needs to be taken into account when considering the function of the SA node in other species. The anatomy and complex electrophysiological properties of the SA node are essential to activating the large mass of the right atrium. In the rabbit, the leading pacemaker sequence originates in ∼5,000 cells in the intercaval region (5). From there, the propagating wave front moves at increasing size and speed toward the crista terminalis, enabling it to arrive there as a broad wave. The shape of the AP undergoes a gradual transition as it moves through the SA node, bringing the maximum diastolic potential and phase 0 amplitude closer to the values found in the crista terminalis and atrium (5, 7, 32, 70).
Physiological implications.
In many pathophysiological conditions, the primary pacemaker site in the SA node can undergo “pacemaker shift,” allowing a different group of cells within the SA node to assume the pacemaking function (5, 55). We show that the ion channel transcript levels and identities differ between the central region of the SA node and the adjacent perinodal region. These observations suggest that pacemaker activity arising in areas outside of the primary pacemaker site should have ionic currents contributing to the AP different from those in the primary pacemaker site. Hence, currents that contribute to the AP in a pacemaker site outside of the primary pacemaker may respond differently to changes in autonomic tone and Class I and III antiarrhythmic drugs.
The importance of different ion channels in pacemaking activity can be discerned from studies in patients with sinus bradycardia resulting from genetic mutations or in mice resulting from knockout of a gene encoding a specific ion channel (38, 39, 44, 48, 69, 81). Alternatively, the negative chronotropic effects of K+, Na+, and Ca2+ channel blockers can also slow the heart rate. In addition, the negative chronotropic effects of TTX on the spontaneous heart rate of the isolated mouse heart led to the identification of TTX-sensitive Na+ channels (brain type) in the mouse SA node (38); we have documented their presence in the ferret SA node. Finally, if our findings are substantiated in the human heart, we believe that the widespread distribution of Na+ channels is functionally important. In patients with diseased SA node, Class I antiarrhythmic drugs could further impair the propagation of impulses out of the SA node to the crista terminalis.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-52874 (H. C. Strauss) and RO1-HL-058913 (D. L. Campbell) and in part by equipment grant BIR-9318118 to J. D. Crapo and J. McNamara.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1.Augood SJ, Arbuthnott GW, Emson PC. Identified cholinergic neurones in the adult rat brain are enriched in GAP-43 mRNA: a double in situ hybridisation study. J Chem Neuroanat 9: 17–26, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate for form the IKs cardiac potassium current. Nature 384: 78–80, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Benvenuti LA, Aiello VD, Higuchi MD. Different cell types within the sinoatrial node. Circulation 100: 1011–1012, 1999. [PubMed] [Google Scholar]
- 4.Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev 84: 803–833, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bleeker WK, Mackaay AJC, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res 46: 11–22, 1980. [DOI] [PubMed] [Google Scholar]
- 6.Boyett MR, Dobrzynski H, Lancaster MK, Jones SA, Honjo H, Kodama I. Sophisticated architecture is required for the sinoatrial node to perform its normal pacemaker function. J Cardiovasc Electrophysiol 14: 104–106, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res 47: 658–687, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Boyett MR, Honjo H, Yamamoto M, Nikmaram MR, Niwa R, Kodama I. Regional differences in effects of 4-aminopyridine within the sinoatrial node. Am J Physiol Heart Circ Physiol 275: H1158–H1168, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Brahmajothi MV, Campbell DL. Heterogeneous basal expression of nitric oxide synthase and superoxide dismutase isoforms in mammalian heart: implications for mechanisms governing indirect and direct nitric oxide-related effects. Circ Res 85: 575–587, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Brahmajothi MV, Campbell DL, Rasmusson RL, Morales MJ, Trimmer JS, Nerbonne JM, Strauss HC. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J Gen Physiol 113: 581–600, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmajothi MV, Morales MJ, Liu S, Rasmusson RL, Campbell DL, Strauss HC. In situ hybridization reveals extensive diversity of K+ channel mRNA in isolated ferret cardiac myocytes. Circ Res 78: 1083–1089, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Brooks CM, Lu HH. The Sinoatrial Pacemaker of the Heart. Springfield, IL: Thomas, 1972. [Google Scholar]
- 13.Bruckenstein DA, Lein PJ, Higgins D, Fremeau RT., Jr Distinct spatial localization of specific mRNAs in cultured sympathetic neurons. Neuron 5: 809–819, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DL, Rasmusson RL, Strauss HC. Ionic current mechanisms generating vertebrate primary pacemaker activity at the single cell level: an integrative view. Annu Rev Physiol 54: 279–302, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, DiFrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, Dobrzynski H. Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation 119: 1562–1575, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Christoffels VM, Smits GJ, Kispert A, Moorman AF. Development of the pacemaker tissues of the heart. Circ Res 106: 240–254, 2010. [DOI] [PubMed] [Google Scholar]
- 17.DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol 46: 163–183, 1985. [DOI] [PubMed] [Google Scholar]
- 18.Dobrzynski H, Li J, Tellez J, Greener ID, Nikolski VP, Wright SE, Parson SH, Jones SA, Lancaster MK, Yamamoto M, Honjo H, Takagishi Y, Kodama I, Efimov IR, Billeter R, Boyett MR. Computer three-dimensional reconstruction of the sinoatrial node. Circulation 111: 846–854, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Gorza L, Ausoni S, Merciai N, Hastings KEM, Schiaffino S. Regional differences in troponin I isoform switching during rat heart development. Dev Biol 156: 253–264, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Gorza L, Vettore S, Vitadello M. Molecular and cellular diversity of heart conduction system myocytes. Trends Cardiovasc Med 4: 153–159, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res 90: 586–593, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Harzheim D, Pfeiffer KH, Fabritz L, Kremmer E, Buch T, Waisman A, Kirchhof P, Kaupp UB, Seifert R. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J 27: 692–703, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho SY, Seo JW, Brown NA, Cook AC, Fagg NL, Anderson RH. Morphology of the sinus node in human and mouse hearts with isomerism of the atrial appendages. Br Heart J 74: 437–442, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho WK, Brown HF, Noble D. High selectivity of the if channel to Na+ and K+ in rabbit isolated sinoatrial node cells. Pflügers Arch 426: 68–74, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Hoang TX, Nieto JH, Havton LA. Regenerating supernumerary axons are cholinergic and emerge from both autonomic and motor neurons in the rat spinal cord. Neuroscience 136: 417–423, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman BF, Cranefield PF. Electrophysiology of the Heart. New York: Futura, 1960. [Google Scholar]
- 27.Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev 73: 197–227, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Ishii TM, Takano M, Xie LH, Noma A, Ohmori H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem 274: 12835–12839, 1999. [DOI] [PubMed] [Google Scholar]
- 29.James TN. Anatomy of the human sinus node. Anat Rec 141: 109–139, 1961. [DOI] [PubMed] [Google Scholar]
- 30.James TN. Anatomy of the sinus node of the dog. Anat Rec 143: 251–265, 1962. [DOI] [PubMed] [Google Scholar]
- 31.James TN, Nadeau RA. Effects of sympathomimetic amines studied by direct perfusion of the sinus node. Am J Physiol 204: 591–594, 1963. [DOI] [PubMed] [Google Scholar]
- 32.Kerr CR, Prystowsky EN, Browning DJ, Strauss HC. Characterization of refractoriness in the sinus node of the rabbit. Circ Res 47: 742–756, 1980. [DOI] [PubMed] [Google Scholar]
- 33.Kodama I, Nikmaram MR, Boyett MR, Suzuki R, Honjo H, Owen JM. Regional differences in the role of the Ca2+ and Na+ currents in pacemaker activity in the sinoatrial node. Am J Physiol Heart Circ Physiol 272: H2793–H2806, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Kramer JW, Post MA, Brown AM, Kirsch GE. Modulation of potassium channel gating by coexpression of Kv2.1 with regulatory Kv5.1 or Kv6.1 α-subunits. Am J Physiol Cell Physiol 274: C1501–C1510, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Lai LP, Su MJ, Lin JL, Tsai CH, Lin FY, Chen YS, Hwang JJ, Huang SK, Tseng YZ, Lien WP. Measurement of funny current (If) channel mRNA in human atrial tissue: correlation with left atrial filling pressure and atrial fibrillation. J Cardiovasc Electrophysiol 10: 947–953, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Lei M, Honjo H, Kodama I, Boyett MR. Characterisation of the transient outward K+ current in rabbit sinoatrial node cells. Cardiovasc Res 46: 433–441, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Lei M, Honjo H, Kodama I, Boyett MR. Heterogeneous expression of the delayed-rectifier K+ currents iK,r and iK,s in rabbit sinoatrial node cells. J Physiol 535: 703–714, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei M, Jones SA, Liu J, Lancaster MK, Fung SS, Dobrzynski H, Camelliti P, Maier SK, Noble D, Boyett MR. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol 559: 835–848, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei M, Zhang H, Grace AA, Huang CL. SCN5A and sinoatrial node pacemaker function. Cardiovasc Res 74: 356–365, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Lev M, Thaemert JC. The conduction system of the mouse heart. Acta Anat (Basel) 85: 342–352, 1973. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M. Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovasc Res 73: 729–738, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Noble PJ, Xiao G, Abdelrahman M, Dobrzynski H, Boyett MR, Lei M, Noble D. Role of pacemaking current in cardiac nodes: insights from a comparative study of sinoatrial node and atrioventricular node. Prog Biophys Mol Biol 96: 294–304, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Lu HH, Lange G, Brooks CM. Factors controlling pacemaker action in cells of the sinoatrial node. Circ Res 17: 460–471, 1965. [DOI] [PubMed] [Google Scholar]
- 44.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22: 216–224, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature 393: 587–591, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, Catterall WA, Scheuer T. An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci USA 100: 3507–3512, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangoni ME, Couette B, Marger L, Bourinet E, Striessnig J, Nargeot J. Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog Biophys Mol Biol 90: 38–63, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le QK, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/α1G T-type calcium channels. Circ Res 98: 1422–1430, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J, Lei M, Escande D, Demolombe S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol 562: 223–234, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang KW, Goldstein SA, Fishman GI. A minK-HERG complex regulates the cardiac potassium current IKr. Nature 388: 289–292, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Morales MJ, Brahmajothi MV, Campbell DL, Strauss HC. Molecular methods for evaluation of K+ channel expression and distribution in the heart. In: Potassium Channels in Cardiovascular Biology, edited by Archer SL, Rusch NJ. New York: Kluwer Academic/Plenum, 2000. [Google Scholar]
- 52.Muramatsu H, Zou AR, Berkowitz GA, Nathan RD. Characterization of a TTX-sensitive Na+ current in pacemaker cells isolated from rabbit sinoatrial node. Am J Physiol Heart Circ Physiol 270: H2108–H2119, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J Mol Cell Cardiol 48: 12–25, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ono K, Ito H. Role of rapidly activating delayed rectifier K+ current in sinoatrial node pacemaker activity. Am J Physiol Heart Circ Physiol 269: H453–H462, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Opthof T. The mammalian sinoatrial node. Cardiovasc Drugs Ther 1: 573–597, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ. Obligatory heterotetramerization of three previously uncharacterized Kv channel α-subunits identified in the human genome. Proc Natl Acad Sci USA 99: 7986–7991, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paes de Carvalho AP, Carlos de Mello WC, Hoffman BF. Electrophysiological evidence for specialized fiber types in rabbit atrium. Am J Physiol 196: 483–488, 1959. [DOI] [PubMed] [Google Scholar]
- 58.Rasmusson RL, Morales MJ, Wang S, Liu S, Campbell DL, Brahmajothi MV, Strauss HC. Inactivation of voltage gated cardiac K+ channels. Circ Res 82: 739–750, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Sabry MA, Dhoot GK. Identification and pattern of expression of a developmental isoform of troponin I in chicken and rat cardiac muscle. J Muscle Res Cell Motil 10: 85–91, 1989. [DOI] [PubMed] [Google Scholar]
- 60.Saggin L, Gorza L, Ausoni S, Schiaffino S. Troponin I switching in the developing heart. J Biol Chem 264: 16299–16302, 1989. [PubMed] [Google Scholar]
- 61.Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol 571: 639–649, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KvLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature 384: 80–83, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, Barton PJ. Troponin I gene expression during human cardiac development and in end-stage heart failure. Circ Res 72: 932–938, 1993. [DOI] [PubMed] [Google Scholar]
- 65.Schiaffino S. Protean patterns of gene expression in the heart conduction system. Circ Res 80: 749–750, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Shi W, Wymore RS, Wang HS, Pan ZM, Cohen IS, McKinnon D, Dixon JE. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci 17: 9423–9432, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi WM, Wymore R, Yu HG, Wu JY, Wymore RT, Pan ZM, Robinson RB, Dixon JE, McKinnon D, Cohen IS. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res 85: E11–E16, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Shinagawa Y, Satoh H, Noma A. The sustained inward current and inward rectifier K+ current in pacemaker cells dissociated from rat sinoatrial node. J Physiol 523: 593–605, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stieber J, Hofmann F, Ludwig A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc Med 14: 23–28, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Strauss HC, Bigger JT., Jr Electrophysiological properties of the rabbit sinoatrial perinodal fibers. Circ Res 31: 490–506, 1972. [DOI] [PubMed] [Google Scholar]
- 71.Tellez JO, Dobrzynski H, Greener ID, Graham GM, Laing E, Honjo H, Hubbard SJ, Boyett MR, Billeter R. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ Res 99: 1384–1393, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Toyota N, Shimada Y. Differentiation of troponin in cardiac and skeletal muscles in chicken embryos as studied by immunofluorescence microscopy. J Cell Biol 91: 497–504, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trautwein W, Uchizono K. Electron microscopic and electrophysiologic study of the pacemaker in the sino-atrial node of the rabbit heart. Z Zellforsch Mikrosk Anat 61: 96–109, 1963. [DOI] [PubMed] [Google Scholar]
- 74.Truex RC. Myocardial cell diameters in primate hearts. Am J Anat 135: 269–280, 1972. [DOI] [PubMed] [Google Scholar]
- 75.Truex RC. The sinoatrial node and its connections with the atrial tissues. In: The Conduction System of the Heart. Structure, Function, and Clinical Implications, edited by Wellens HJJ, Lie KI, Janse MJ. Philadelphia, PA: Lea & Febiger, 1976. [Google Scholar]
- 76.Truex RC, Smythe MQ, Taylor MJ. Reconstruction of the human sinoatrial node. Anat Rec 159: 371–378, 1967. [DOI] [PubMed] [Google Scholar]
- 77.Uese K, Hagiwara N, Miyawaki T, Kasanuki H. Properties of the transient outward current in rabbit sino-atrial node cells. J Mol Cell Cardiol 31: 1975–1984, 1999. [DOI] [PubMed] [Google Scholar]
- 78.Verge VM, Tetzlaff W, Richardson PM, Bisby MA. Correlation between GAP43 and nerve growth factor receptors in rat sensory neurons. J Neurosci 10: 926–934, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vitadello M, Vettore S, Lamar E, Chien KR, Gorza L. Neurofilament M mRNA is expressed in conduction system myocytes of the developing and adult rabbit heart. J Mol Cell Cardiol 28: 1833–1844, 1996. [DOI] [PubMed] [Google Scholar]
- 80.Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66: 470–494, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilde AA, Bezzina CR. Genetics of cardiac arrhythmias. Heart 91: 1352–1358, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamada M, Terayama R, Bando Y, Kasai S, Yoshida S. Regeneration of the abdominal postganglionic sympathetic system. Neurosci Res 54: 261–268, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto M, Kondo H. Gene expression of a neuronal growth-associated protein, GAP-43, in the paraganglionic carotid body as well as in the autonomic ganglia of normal adult rats. Neurosci Lett 117: 275–279, 1990. [DOI] [PubMed] [Google Scholar]
- 84.Ye B, Nerbonne JM. Proteolytic processing of HCN2 and co-assembly with HCN4 in the generation of cardiac pacemaker channels. J Biol Chem 284: 25553–25559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeola SW, Snyders DJ. Electrophysiological and pharmacological correspondence between Kv4.2 current and rat cardiac transient outward current. Cardiovasc Res 33: 540–547, 1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.