Abstract
Inbred mouse strains play a critical role in biomedical research. Genetic homogeneity within inbred strains and their general amenability to genetic manipulation have made them an ideal resource for dissecting the physiological function(s) of individual genes. However, the inbreeding that makes inbred mice so useful also results in genetic divergence between them. This genetic divergence is often unaccounted for but may be a confounding factor when comparing studies that have utilized distinct inbred strains. Here, we compared the cardiac function of C57BL/6J mice to seven other commonly used inbred mouse strains: FVB/NJ, DBA/2J, C3H/HeJ, BALB/cJ, 129X1/SvJ, C57BL/10SnJ, and 129S1/SvImJ. The assays used to compare cardiac function were the ex vivo isolated Langendorff heart preparation and in vivo real-time hemodynamic analysis using conductance micromanometry. We report significant strain-dependent differences in cardiac function between C57BL/6J and other commonly used inbred strains. C57BL/6J maintained better cardiac function than most inbred strains after ex vivo ischemia, particularly compared with 129S1/SvImJ, 129X1/SvJ, and C57BL/10SnJ strains. However, during in vivo acute hypoxia 129X1/SvJ and 129S1/SvImJ maintained relatively normal cardiac function, whereas C57BL/6J animals showed dramatic cardiac decompensation. Additionally, C3H/HeJ showed rapid and marked cardiac decompensation in response to esmolol infusion compared with effects of other strains. These findings demonstrate the complex effects of genetic divergence between inbred strains on cardiac function. These results may help inform analysis of gene ablation or transgenic studies and further demonstrate specific quantitative traits that could be useful in discovery of genetic modifiers relevant to cardiac health and disease.
Keywords: ischemia/reperfusion, β-blockade, hypoxia, quantitative trait locus
for decades biomedical research has relied heavily on the use of inbred mouse strains. The genetic homogeneity within these strains allows for well-controlled and highly reproducible studies. Over 450 distinct inbred strains have been described in the literature (3) and the Jackson Laboratory alone maintains over 180 inbred mouse strains (http://jaxmice.jax.org/list/cat481365.html). The most common strain of inbred mice used in research is the C57BL/6 mouse. This strain is the most commonly used background of genetically modified mouse strains maintained by the Jackson Laboratory (http://jaxmice.jax.org/literature/catalog/JAX_Mice_Catalog.pdf) and is currently the only inbred strain whose genome has been fully sequenced (57).
Though not as widely used, other inbred strains have found niches in biomedical research due to ease of genetic manipulation or differing propensities for developing specific pathologies. For instance, FVB/N mice are commonly used in conventional transgenesis (51), and 129 substrains have been used almost exclusively for the isolation of embryonic stem (ES) cells used for gene targeting (41, 56). Alternatively, other inbred strains have found use as disease models due to naturally occurring mutations in genes linked to human disease (5, 22, 38, 43). Even in the absence of engineered or known naturally occurring mutations, different inbred mouse strains can vary drastically in their susceptibility to clinically relevant diseases. Previous reports have taken advantage of this naturally occurring variation in susceptibility to a particular disease to identify quantitative trait loci (QTL) that modify disease pathogenesis (2, 6, 13, 48, 49).
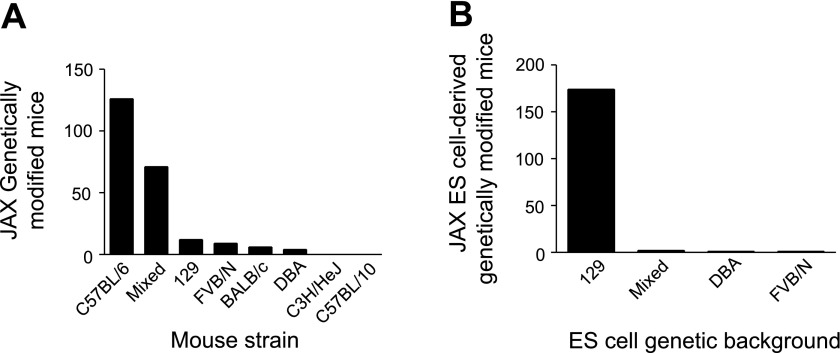
Cardiovascular disease is the single leading cause of death in the United States, affecting ∼1 in 3 Americans and causing >800,000 deaths in 2006 (27). To effectively investigate the pathological processes associated with cardiovascular disease and devise new therapeutic strategies, the cardiovascular field has embraced genetically modified inbred mouse strains as tools to help understand the fundamental physiological processes governing function in the normal and diseased heart. A survey of the Jackson Labs website shows that they maintain >225 genetically modified mouse lines used for cardiovascular research derived from inbred mouse strains. C57BL/6 is the most common genetic background (Fig. 1A). However, mutations are also maintained on other common inbred backgrounds as well. The majority of these mice were created using ES cells derived from a substrain of the 129 inbred strain, then often backcrossed and maintained on a C57BL/6 or mixed C57BL/6 × 129 background (Fig. 1B).
Fig. 1.
Summary of strain background of genetically modified mice and embryonic stem (ES) cells. A: genetic background of genetically modified mouse strains designed for cardiovascular research maintained at Jackson Labs. B: genetic background of ES cells used to create genetically modified mouse strains maintained at Jackson Labs.
Several studies have reported strain-dependent differences in cardiovascular function between inbred mouse strains by various in vitro and in vivo methods (4, 7, 23, 37, 40, 47). The majority of these strain-dependent differences in cardiac physiology have been carried out on a small subset of inbred strains in the absence of disease-related injury/stress. Previous reports have also demonstrated the potent effects of genetic background on modifying the cardiac phenotype of genetically modified mice (20, 21, 50). For instance, two distinct transgenic lines expressing hypertrophic cardiomyopathy-linked mutant tropomyosin E180G showed drastically different phenotypes, one displaying relatively mild diastolic dysfunction (29) and the other developing overt hypertrophic cardiomyopathy and heart failure (36). Excluding possible differences in animal care and housing, the main difference between these transgenic mice is their genetic background; one mouse was created on the FVB/N background, the other on the C57BL/6 background. Although the precise mechanism underlying this effect is not understood, the phenotypic variation between these two transgenic lines contributes to the impetus for this study.
The purpose of this study was to compare the cardiac function of eight commonly used inbred mouse strains at rest and following physiologically relevant stress/injury. The rationale for conducting these studies is twofold. First, the large number of inbred strains used in medical research necessitates a consideration for the physiological differences that arise from genetic divergence between strains. Secondly, the fixed genetic divergence between inbred mouse strains serves as a potential substrate for the identification of reproducible quantitative traits that differ between inbred strains. Eight common inbred strains were used, six of which are listed on Jackson Laboratory's list of the most popular inbred mouse strains (http://jaxmice.jax.org/findmice/popular.html). The techniques used to measure cardiac function for this study are the Langendorff perfused isolated heart preparation and in vivo cardiac hemodynamic function as measured by catheter-based conductance micromanometry. The Langendorff preparation is a well-established technique for assessing function of a heart performing isovolumic contractions at a controlled end diastolic pressure (26, 45). In vivo hemodynamic analysis provides pressure and volume measurements of hearts in the intact animal where physiological loading and autonomic innervation remain intact (33). Additionally, we studied strain-dependent differences in cardiac function following physiologically relevant stresses. Specifically, isolated hearts were subjected to global ischemia and reperfusion. Mice instrumented for in vivo hemodynamic measurements were subjected to β-adrenergic blockade and an acute hypoxic challenge. Strain-dependent differences in cardiac function were found both at baseline and following physiologically relevant stress. Collectively, this study highlights strain-dependent differences between inbred mice that provide insight into the marked physiological divergence reported for cardiac transgenic mice generated on differing backgrounds. The identification of strain-dependent quantitative hemodynamic traits may form the foundation of future studies aimed at identifying QTL that modify cardiac function.
MATERIALS AND METHODS
Mouse strains.
All mouse strains analyzed in this study were acquired from Jackson labs. Mouse strains and stock numbers were as follows: C57BL/6J (000664), FVB/NJ (001800), DBA/2J (000671), BALB/cJ (000651), C3H/HeJ (000659), 129X1/SvJ (000691), C57BL/10SnJ (000666), and 129S1/SvlmJ (002448). Male mice ages 4–6 mo were used in this study. The procedures used in this study are in agreement with the guidelines of the University of Minnesota and approved by the University of Minnesota Committee on the Use and Care of Animals.
Isolated heart model and ischemia/reperfusion.
Ex vivo measurements of left ventricular function were carried out as previously described (10). Mice were injected with 300 units sodium heparin and anesthetized with pentobarbital sodium. The heart and lungs were then removed following thoracotomy and placed immediately in ice-cold Hanks' buffered salt solution without calcium and magnesium. The lungs and thymus were trimmed away to expose the aorta, which was then cannulated. Hearts were then perfused at a constant pressure of 80 mmHg with modified Krebs-Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM glucose, 25 mM NaHCO3, 2.5 mM CaCl2, 0.5 mM EDTA) warmed to 37°C and brought to pH = 7.4 by bubbling with 95% 02, 5% CO2. Hearts were paced at 7 Hz, and changes in left ventricular pressure were monitored by insertion of a water-filled balloon with an in-line pressure transducer into the left ventricle. Within the left ventricle, the balloon was inflated to an end diastolic pressure of 3–8 mmHg. Following 10–15 min of stabilization time, hearts were subjected to global no-flow ischemia for 20 min. Hearts were not paced during ischemia. Hearts were then reperfused for 60 min, and pacing was reinitiated at 8 min following the end of ischemia. Data were collected at a sampling rate of 400 Hz and analyzed using Chart 6 software (ADInstruments).
In vivo conductance micromanometry.
Measurements of real-time in vivo cardiovascular hemodynamics were obtained using conductance micromanometry as previously described (34, 35, 53, 54). Mice were anesthetized and ventilated via a tracheal cannulation and ventilated via a pressure controlled ventilator with 2% isoflurane at a peak inspiratory pressure of 15 cm H2O and a respiratory rate of 60 breaths/min. With aid of a dissecting microscope, the heart was exposed via a thoracotomy. A 1.4 French Millar pressure-volume catheter (PVR-1045; Millar Instruments, Houston, TX) was then placed into the left ventricular chamber via an apical stab. Left ventricular pressure and volume measurements were collected at a sampling rate of 1 kHz. Data were analyzed with Ponemah software, P3 Plus (DSI International, St. Paul, MN). Transient inferior vena cava (IVC) occlusions were performed to obtain the end systolic and end diastolic pressure-volume relationships. IVC occlusions were performed at baseline and at the end of the esmolol infusion. After baseline hemodynamics (ventilated with isoflurane and O2) were obtained, mice received a continuous infusion of esmolol (188 μg·kg−1·min−1) for 5 min to block all adrenergic responsiveness. After esmolol infusion, mice were infused with dobutamine (42 μg·kg−1·min−1) to enable recovery of baseline function (Supplemental Table S1).1 After dobutamine infusion cardiac function was allowed to return to baseline levels. Mice were then exposed to an acute hypoxic challenge (7% O2 balanced in nitrogen) and monitored until cardiac pump failure. End-point cardiac decompensation was defined as the point when peak left ventricular systolic pressure (LVSP) dropped <60 mmHg. At this point mice were recovered with 100% O2 to obtain instrument calibration.
Statistics.
All results are expressed as means ± SE. Significance was established as P < 0.05. All multivariable assays were assessed using a one-way analysis of variance with Dunnett's post hoc test comparing all groups to C57BL/6J. Survival after the in vivo acute hypoxic challenge was assessed by the Fisher exact test.
RESULTS
Baseline ex vivo cardiac function.
To quantify strain-specific differences in ex vivo cardiac function, hearts from eight inbred mouse strains were isolated and perfused retrograde through the aorta. To simplify data analysis, all inbred strains were compared with the C57BL/6J strain. In general, baseline ex vivo whole heart function of the inbred strains tested was similar to C57BL/6J (Table 1). One notable exception was FVB/NJ, which showed significantly higher left ventricular developed pressure (LVDP) than C57BL/6J (Table 1, P < 0.05). Additionally, FVB/NJ mice showed significantly higher coronary flow rates than C57BL/6J mice at baseline (Supplemental Table S2, P < 0.05). There were no significant differences between C57BL/6J and other inbred strains as measured by the maximum derivative of left ventricular pressure, another measure of systolic function (Table 1). There were no significant differences in diastolic function at baseline between C57BL/6J and other inbred strains as measured by the minimum derivative of left ventricular pressure and the rate constant tau (Table 1). A previous report showed that BALB/c mice have highly variable contractile function in the isolated working heart preparation associated interanimal variability in α-skeletal actin expression (19). However, in our hands the variance of LVDP for BALB/cJ mice was statistically similar to all other strains by Bartlett's analysis of variance (data not shown).
Table 1.
Baseline functional data for ex vivo isolated hearts
| C57BL/6J | FVB/NJ | DBA/2J | BALB/cJ | C3H/HeJ | 129X1/SvJ | C57BL/10SnJ | 129S1/SvImJ | |
|---|---|---|---|---|---|---|---|---|
| LVEDP, mmHg | 3.6 ± 0.4 | 3.1 ± 0.5 | 4.3 ± 0.5 | 5.5 ± 1.3 | 4.7 ± 1.2 | 4.5 ± 0.2 | 3.7 ± 0.3 | 3.9 ± 0.3 |
| LVESP, mmHg | 126.3 ± 3.3 | 147.7 ± 5.9* | 130.6 ± 7.0 | 132.4 ± 3.8 | 125.6 ± 2.7 | 121.7 ± 3.7 | 125.9 ± 1.8 | 124.1 ± 3.4 |
| LVDP, mmHg | 122.8 ± 3.6 | 144.6 ± 5.9* | 126.3 ± 7.2 | 126.9 ± 4.5 | 120.9 ± 2.3 | 117.2 ± 3.7 | 122.2 ± 1.8 | 120.2 ± 3.5 |
| −dP/dt, mmHg/s | −3456 ± 185 | −4137 ± 148 | −3618 ± 310 | −3929 ± 216 | −3681 ± 223 | −3386 ± 267 | −3781 ± 157 | −3184 ± 157 |
| +dP/dt, mmHg/s | 4614 ± 396 | 4697 ± 200 | 4429 ± 445 | 4708 ± 284 | 4338 ± 291 | 4263 ± 345 | 4321 ± 147 | 3654 ± 162 |
| Tau, ms | 22.0 ± 2.0 | 24.1 ± 0.7 | 24.4 ± 1.1 | 21.9 ± 1.6 | 22.5 ± 2.0 | 24.2 ± 1.5 | 22.6 ± 0.5 | 25.9 ± 0.6 |
Baseline functional data for isolated hearts of inbred mouse strains. Systolic function indicated by left ventricular end-systolic pressure (LVESP), left ventricular developed pressure (LVDP), and the maximum derivative of left ventricular pressure (+dP/dt). Diastolic function indicated by left ventricular end-diastolic pressure (LVEDP), minimum derivative of left ventricular pressure (−dP/dt), and the rate constant tau. All values expressed as means ± SE.
P < 0.05 compared with C57BL/6J mice; n = 5–8 for each group.
Effects of ischemia and reperfusion on ex vivo cardiac function.
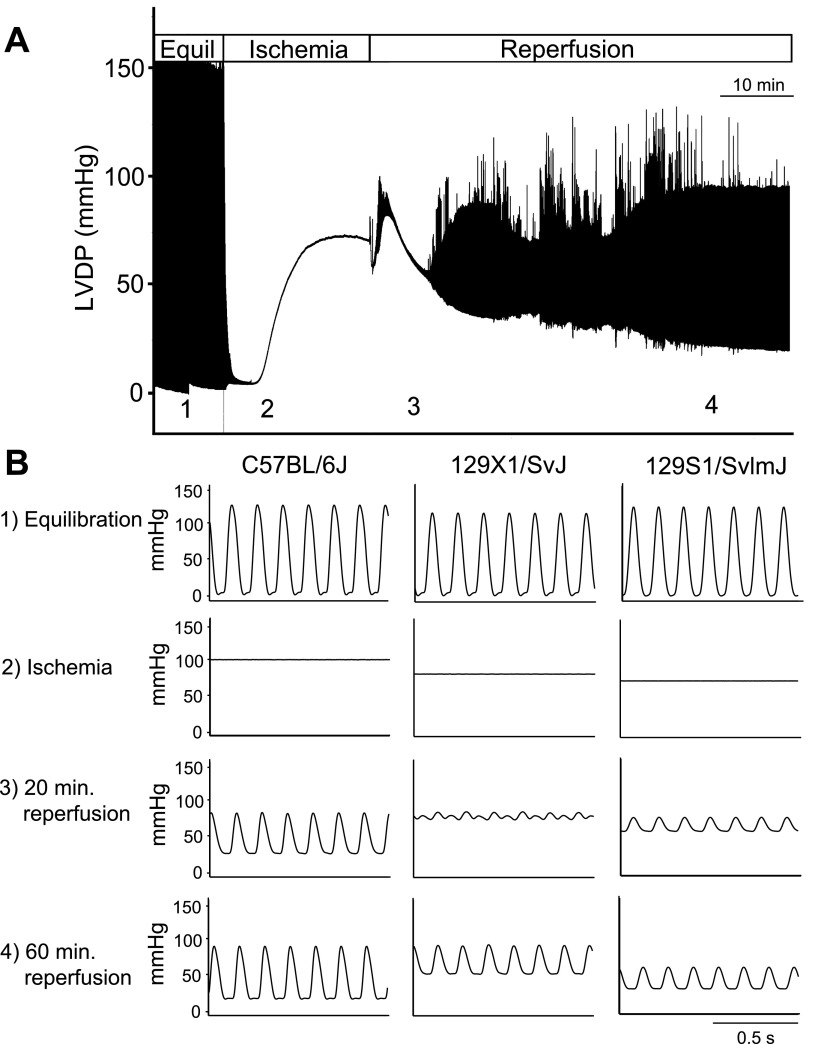
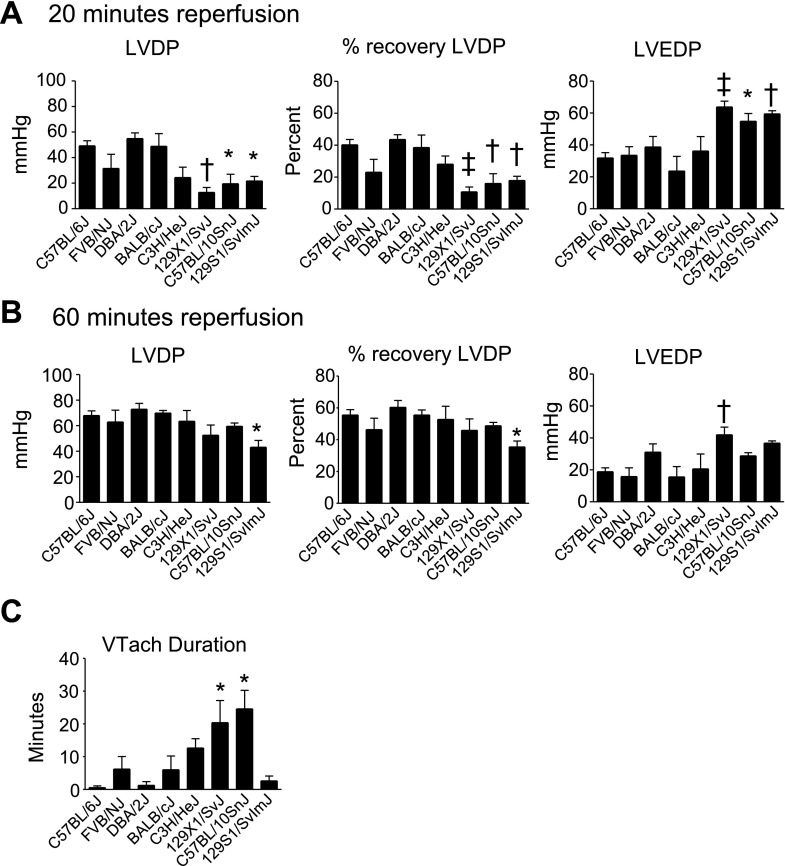
To study strain-dependent differences in the response to a physiologically relevant stress, isolated hearts were subjected to 20 min of global ischemia followed by 60 min of reperfusion (Figs. 2, 3). During reperfusion, three strains showed reduced cardiac function compared with C57BL/6J: 129X1/SvJ, 129S1/SvImJ, and C57BL/10SnJ. Each of these strains showed reduced systolic function during early (20 min) reperfusion compared with C57BL/6J as shown by reduced LVDP and % recovery of baseline LVDP (Figs. 2 and 3A, P < 0.05). Additionally, these strains showed reduced diastolic function compared with C57BL/6J as shown by increased left venticular end-diastolic pressure (LVEDP) (Figs. 2 and 3A, P < 0.05). During late (60 min) reperfusion, 129S1/SvImJ showed reduced systolic function compared with C57BL/6J as shown by reduced LVDP and recovery of baseline LVDP (Figs. 2 and 3B, P < 0.05). 129X1/SvJ showed reduced diastolic function compared with C57BL/6J during late reperfusion as shown by increased LVEDP (Figs. 2 and 3B, P < 0.05). Additionally, the duration of sustained ventricular tachycardia (VTach) during reperfusion was also recorded as a measure of ischemic injury (17). C57BL/6J rarely entered into VTach during reperfusion. However, 129X1/SvJ and C57BL/10SnJ entered into VTach for >20 min during reperfusion (Fig. 3C, P < 0.05 compared with C57BL/6J). Because VTach generally occurs early in reperfusion, this finding suggests that depressed systolic function of 129X1/SvJ and C57BL/10SnJ during early reperfusion is caused by increased heart rate. This is supported by the finding that systolic function of 129X1/SvJ and C57BL/10SnJ was similar to C57BL/6J once VTach had resolved during late reperfusion. 129S1/SvImJ rarely entered into VTach, suggesting that reduced systolic function in these mice was due to reduced contractility following ischemic injury. In addition to 129X1/SvJ, C57BL/10SnJ, and 129S1/SvImJ, several other strains showed more subtle differences in cardiac function compared with C57BL/6J during ex vivo ischemia and reperfusion. These data can be found in Supplemental Fig. S1.
Fig. 2.
Effects of ischemia and reperfusion on isolated hearts of inbred mouse strains. A: representative compressed pressure tracing from an experiment in which an isolated heart was subjected to global ischemia and reperfusion. Equil, baseline equilibration; LVDP, left ventricular developed pressure. B: representative traces from C57BL/6J, 129X1/SvJ, 129S1/SvImJ hearts during 1) baseline equilibration, 2) ischemic contracture (at 20 min of ischemia, just before reperfusion), 3) 20 min following initiation of reperfusion, 4) 60 min following initiation of reperfusion.
Fig. 3.
Ex vivo cardiac function of isolated hearts measured during reperfusion. A: LVDP, percent recovery of baseline LVDP, and left ventricle end diastolic pressure (LVEDP) of isolated hearts during early (20 min) reperfusion. B: LVDP, percent recovery of baseline LVDP, and LVEDP of isolated hearts during late (60 min) reperfusion. C: duration of sustained ventricular tachycardia (VTach) during reperfusion. Values are expressed as means ± SE; n = 5–8 for each group. *P < 0.05, †P < 0.01, ‡P < 0.01 compared with C57BL/6J.
Additionally, to demonstrate the stability and reproducibility of these findings, Supplemental Fig. S2 shows the results of an initial study carried out with cohorts of FVB/NJ, C3H/HeJ, and BALB/cJ mice. As above, isolated hearts from these mice were subjected to ex vivo ischemia and reperfusion. The findings of this initial study were comparable with those of the main study, supporting the reproducibility of our findings.
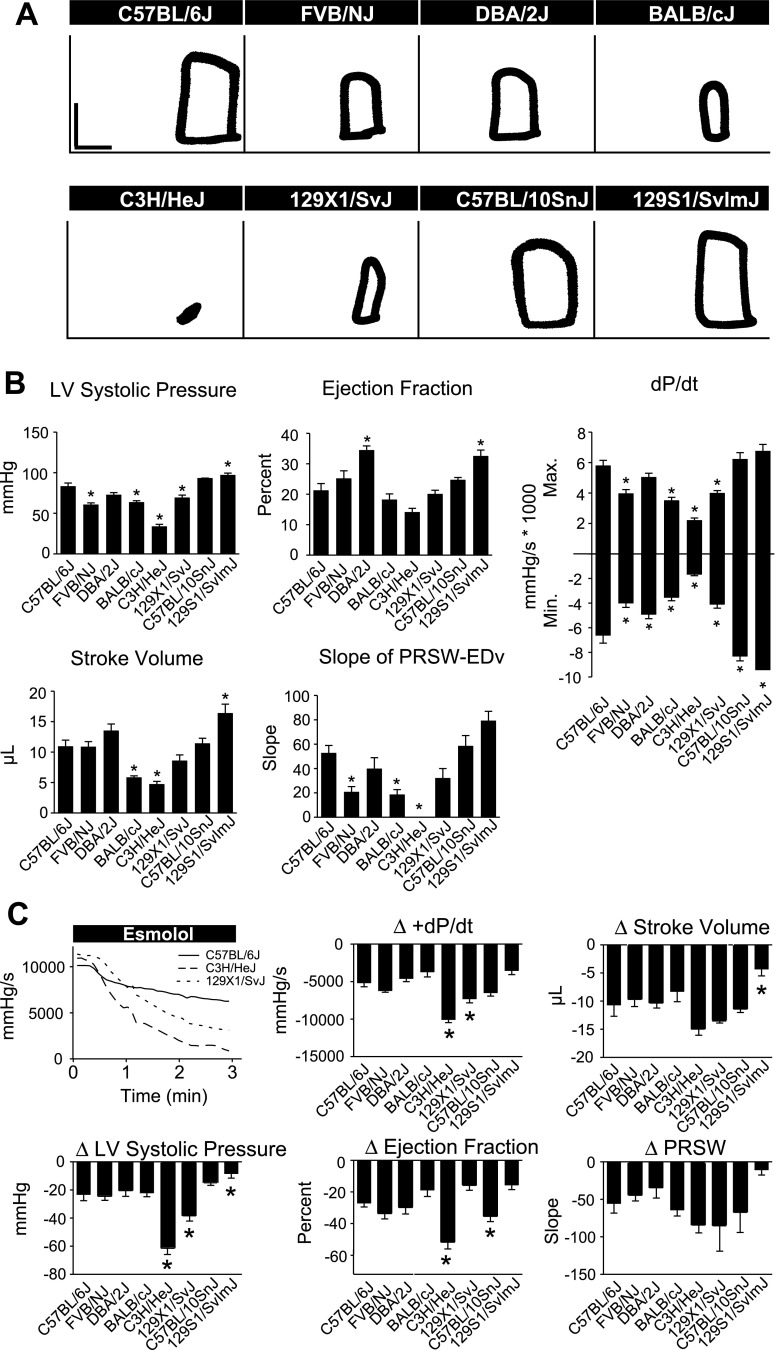
In vivo baseline hemodynamic function.
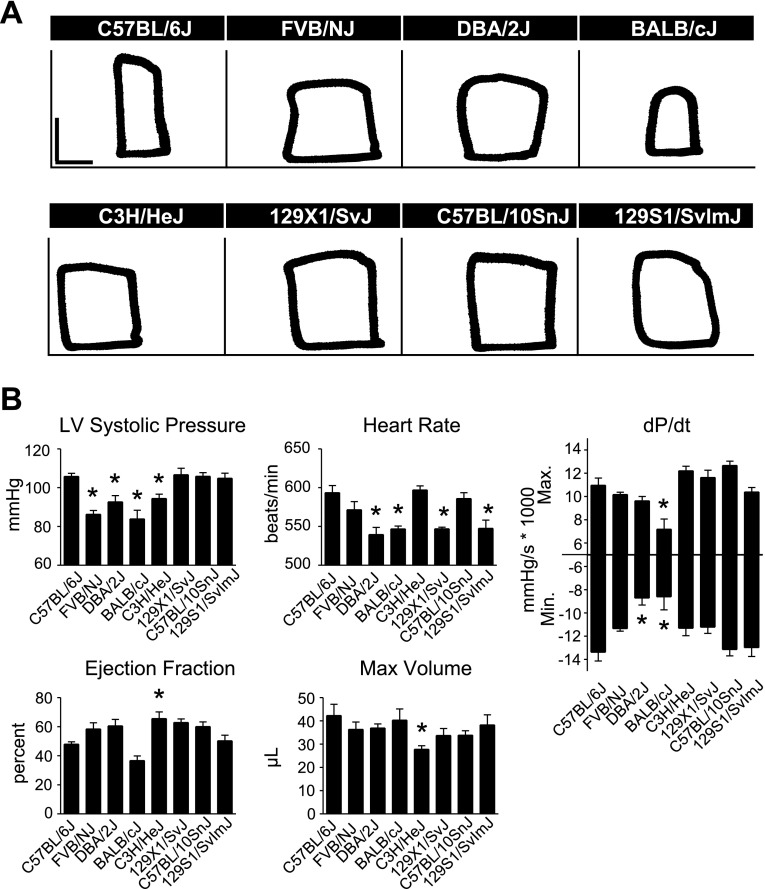
To study differences in real-time in vivo hemodynamic function between inbred strains, mice were instrumented with a 1.4 French Millar conductance micromanometry catheter. This methodology allows for precise, real-time assessment of cardiac function by simultaneous measurement of left ventricular pressure and volume (33). There were significant differences in systolic and diastolic function between C57BL/6J and other strains at baseline, with C57BL/6J generally displaying higher cardiac function than the other strains tested (Fig. 4, Supplemental Table S3). In measures of systolic function, C57BL/6J mice showed increased LVSP compared with FVB/NJ, DBA/2J, BALB/cJ, and C3H/HeJ mice, as well as increased maximal dP/dt compared with BALB/cJ mice (Fig. 4, P < 0.05). C3H/HeJ mice showed a greater ejection fraction (EF) than C57BL/6J. However, this may have been a manifestation of smaller cardiac dimensions in C3H/HeJ mice as they showed reduced heart weight and maximum volume compared with C57BL/6J mice (Supplemental Table S4, Fig. 4; P < 0.05). C57BL/6J mice also displayed greater diastolic function compared with other inbred strains, as shown by a more negative minimum dP/dt compared with DBA/2J and BALB/cJ (Fig. 4, P < 0.05).
Fig. 4.
Baseline in vivo hemodynamic function of inbred mouse strains. A: representative raw pressure-volume (PV) loops for each strain acquired by conductance micromanometry at baseline (PV loops: vertical scale bar, 50 mmHg; horizontal scale bar, 10 μl). B: mean data for each strain for various cardiac hemodynamic parameters derived from real-time PV analysis. For all groups n = 7–9. Values are expressed as means ± SE. *P < 0.05 compared with C57BL/6J.
In vivo hemodynamic function during β-adrenergic blockade.
We next investigated strain-dependent differences in the effects of adrenergic signaling on cardiac function. Here, in vivo hemodynamic function was recorded during an acute infusion of the β-blocker esmolol. This manipulation allows the dissection of the role of adrenergic signaling in maintaining normal cardiovascular performance in different mouse strains. Comparison of the response to esmolol infusion revealed strain dependent variation in cardiac function during β-blockade (Fig. 5, Supplemental Table S5). In the absence of β-adrenergic signaling, and similar to baseline in vivo functional measurements, C57BL/6J mice maintained higher levels of cardiac function compared with most, but not all, of the inbred strains tested (Fig. 5B). As described above, there were significant differences in cardiac function between inbred strains at baseline. To account for these differences in baseline function when assessing the effects of β-adrenergic blockade on cardiac function, the difference between functional parameters at baseline and following 3 min of esmolol infusion was determined (Fig. 5C). Esmolol infusion caused a general depression in cardiac function of all strains tested. However, there were strain-dependent differences in the degree to which β-blockade depressed cardiac function. In particular, esmolol infusion had a marked effect on the cardiac function of C3H/HeJ mice (Fig. 5C). All C3H/HeJ mice underwent severe cardiac decompensation within 3 min of the start of esmolol infusion as shown by greater reductions in LVSP, EF, and maximum dP/dt compared with C57BL/6J (Fig. 5C, P < 0.05). The effects of β-blockade were also more severe on 129X1/SvJ compared with C57BL/6J as shown by greater reductions in maximum dP/dt and LVSP (Fig. 5C, P < 0.05). C57BL/10SnJ showed a greater decrease in EF compared with C57BL/6J followed esmolol infusion as well (Fig. 5C, P < 0.05).
Fig. 5.
In vivo hemodynamic function of inbred mouse strains during β-blockade. A: representative raw PV loops for each strain acquired by conductance micromanometry after 3 min of esmolol infusion (PV loops: vertical scale bar, 50 mmHg; horizontal scale bar, 10 μl). B: absolute values for cardiac function during esmolol infusion. Mean data for each strain for various cardiac hemodynamic parameters derived from real-time PV loop analysis at 3 min following the start of esmolol infusion. C: representative changes in the positive pressure derivative (+dP/dt) during the time course of esmolol infusion showing marked differences in adrenergic tone between strains (top left). Mean data for each strain for various cardiac hemodynamic parameters derived from real-time PV analysis. For all groups n = 7–9. Values are expressed as means ± SE. *P < 0.05 compared with C57BL/6J.
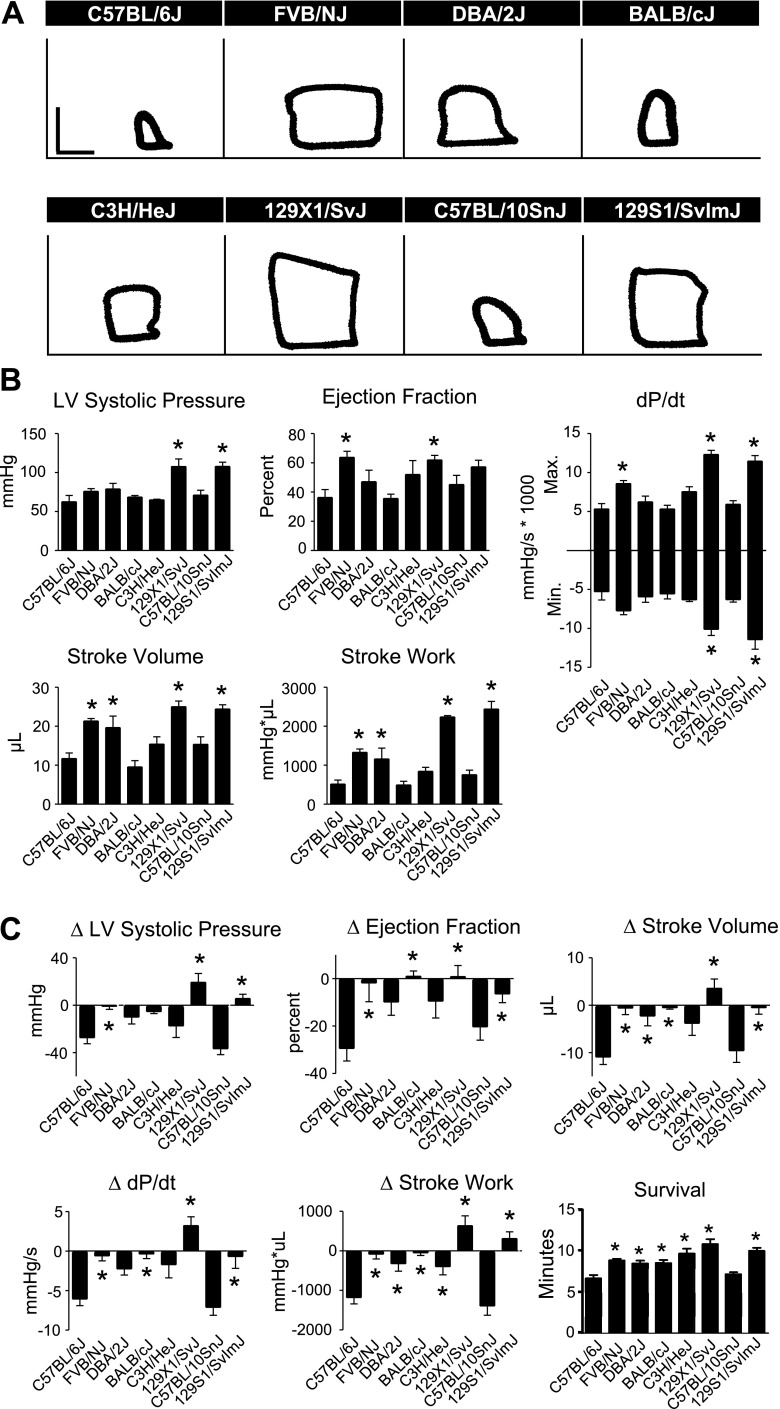
In vivo hemodynamic function during an acute hypoxic challenge.
To assess the capacity for inbred mouse strains to respond to an acute cardiac stress, mice instrumented with a pressure-volume catheter were exposed to an acute hypoxic challenge by ventilation with 7% oxygen (10). Significant differences in cardiac function and survival were observed between C57BL/6J and other inbred strains during this challenge (Fig. 6). To assess cardiac function during hypoxia, hemodynamic parameters were compared at 6 min, 40 s into the hypoxic challenge at which time C57BL/6J mice underwent cardiac decompensation as defined in materials and methods. Unlike our findings at baseline and during esmolol infusion, C57BL/6J mice showed relatively poor cardiac function during an acute hypoxic challenge compared with other inbred strains (Fig. 6B and Supplemental Table S6). To account for differences in baseline function when determining the strain-dependent differences in the response to hypoxia, the difference between functional parameters at baseline and following acute hypoxia was determined (Fig. 6C). Unlike the effects of β-blockade, acute hypoxia had much more severe effects on the cardiac function of C57BL/6J mice than most other inbred strains tested. 129X1/SvJ, 129S1/SvImJ, and FVB/NJ maintained contractility to a greater degree compared with C57BL/6J during acute hypoxia as shown by less negative change in LVSP following hypoxia (Fig. 6C, P < 0.05). Additionally, FVB/NJ, BALB/cJ, 129X1/SvJ, and 129S1/SvImJ showed greater maintenance of EF, stroke volume, and maximum dP/dt following hypoxia compared with C57BL/6J (Fig. 6C, P < 0.05). FVB/NJ, DBA/2J, BALB/cJ, C3H/HeJ, 129X1/SvJ, and 129S1/SvImJ showed greater maintenance of stroke work following hypoxia compared with C57BL/6J (Fig. 6C, P < 0.05). Analysis of survival during acute hypoxia also emphasizes the functional deficit of C57BL/6J mice compared with other inbred strains. C57BL/6J mice showed significantly shorter survival during hypoxia compared with DBA/2J, FVB/NJ, C3H/HeJ, BALB/cJ, 129X1/SvJ, and 129S1/SvImJ (Fig. 6C, P < 0.05). C57BL/10SnJ was the only strain that did not perform significantly better than C57BL/6J during acute hypoxia.
Fig. 6.
In vivo hemodynamic function of inbred mouse strains during acute hypoxia. A: representative raw PV loops for each strain acquired by conductance micromanometry at 6:40 into the hypoxia challenge (PV loops: vertical scale bar, 50 mmHg; horizontal scale bar, 10 μl). B: absolute values for cardiac function during acute hypoxia. Mean data for each strain for various cardiac hemodynamic parameters derived from real-time PV analysis at 6 min, 40 s following the start of esmolol infusion. C: the mean change in cardiac function from the start of hypoxia to 6:40 into the hypoxic challenge indicating the delta values for given hemodynamic parameters during hypoxia compared with baseline. Mean survival values for each strain during the acute hypoxic challenge showing significant differences between groups compared with C57BL/6J (bottom right). For all groups n = 7–9. Values are expressed as means ± SE. *P < 0.05 compared with C57BL/6J.
DISCUSSION
To our knowledge this is the first detailed comparative analysis of in vivo and ex vivo heart performance between several commonly used inbred strains. In the past decade, there have been tremendous advances in cardiac biology enabled by exquisite cardiac transgenesis and gene targeting studies in mice (1, 12, 14, 15, 18, 32, 52). Cardiac transgenic, knockout, and other genetically modified mice have been created and backcrossed to a wide array of inbred mouse strains, including those tested in this study. As the number of genetically modified mouse models used in cardiovascular research continues to grow, so to does the need to identify and appreciate the differences in cardiovascular physiology that arise from the genetic diversity within these inbred mouse strains.
One main finding of this study is that genetic divergence between commonly used inbred mouse strains significantly affects cardiac function. All inbred strains showed functional divergence from C57BL/6J to some degree. In ex vivo preparations, most inbred strains were similar to C57BL/6J at baseline, which is in general agreement with previously published findings on baseline ex vivo cardiac function (37). However, following ischemic injury 129X1/SvJ, C57BL/10SnJ, and 129S1/SvImJ showed significantly reduced cardiac function compared with C57BL/6J. C57BL/10SnJ and 129X1/SvJ showed reduced function that was associated with a higher incidence of sustained VTach during the early periods of reperfusion compared with C57BL/6J. This is in agreement with previously published findings that isolated C57BL/6 hearts are relatively resistant to arrhythmia (55). This suggests that C57BL/10SnJ and 129X1/SvJ differ from C57BL/6J in their propensity for arrhythmogenicity, and not in contractility, following ischemic injury. Conversely, 129S1/SvImJ showed reduced cardiac function throughout reperfusion in the absence of VTach, suggesting that ischemic injury compromises contractility of these mice without promoting arrhythmia. In vivo hemodynamic analyses also showed strain-dependent differences in cardiac function between C57BL/6J and other inbred strains. The most striking of these was the effect of esmolol on C3H/HeJ mice. As expected, β-adrenergic blockade reduced cardiac function of all mice to some degree. However, esmolol infusion caused severe cardiac decompensation of C3H/HeJ within 3 min, whereas other inbred strains maintained a reduced, but sustainable, level of cardiac function. Additionally, C57BL/6J mice showed greater susceptibility to hypoxia compared with all inbred strains with the exception of the closely related C57BL/10SnJ. This finding under hypoxic challenge is in contrast to the majority of findings in this study, where C57BL/6J performed as well or better than other inbred strains in cardiac performance tests. A recently published study by Shah and colleagues (42) reports a similar finding when comparing the cardiac function of four inbred strains of mice in vivo by echocardiography and in vitro by recording sarcomere shortening and intracellular calcium handling. Compared with other strains tested (BALB/c, C57BL/6 and FVB), 129 mice showed reduced contractility as measured by echocardiography but increased calcium handling and sarcomere shortening in vitro. Collectively, these findings suggest that the mechanism by which cardiac performance is modified by genetic divergence between strains are complex and may be governed by specific genetic loci that respond differentially to regulate cardiac function depending on the conditions being used to assess cardiac function.
Although a priori we predicted cardiac function to be similar between in vivo and ex vivo preparations, it is not surprising that differences were observed given the significant differences between these assays (11, 33). Specifically, the absence of physiological loading conditions and autonomic innervation in the ex vivo Langendorff preparation are possible reasons for these differences. Additionally, these techniques also differ in their methods of anesthesia. Prior to the extraction and perfusion of the heart for ex vivo functional measurements mice are anesthetized with pentobarbital. Pentobarbital is known to depress cardiac function in vivo (39, 44). However, once the heart is isolated and perfused, it is generally believed that the effects of pentobarbital are quickly washed out from the heart (11). This is supported by the lack of ex vivo functional differences between C57BL/6J and DBA/2J hearts, despite previously published findings that DBA mice are highly susceptible to pentobarbital compared with C57BL/6 mice (31). Conversely, during in vivo measurement of cardiac function, mice were constantly anesthetized with isoflurane. Isoflurane has been shown to have strain-dependent cardiodepressive effects in vivo (8, 9, 28, 30, 46). Additionally, isoflurane anesthesia has been shown to be cardioprotective from ischemic injury through mechanisms that are not fully understood but are thought to mimic ischemic preconditioning (25). Collectively, these previously published findings may provide insights to explain the discrepancies in cardiac function between ex vivo and in vivo preparations. Specifically, the cardiodepressive effects of isoflurane may differentially affect each inbred strain, confounding our comparison of their intrinsic cardiac function. Additionally, given that isoflurane has been shown to be cardioprotective during ischemia, it stands to reason that isoflurane may have a similar effect during hypoxia. If so, and if the magnitude of this effect is strain-dependent, then this may be another mechanism to explain the differences between our ex vivo and in vivo findings.
Our findings of significant differences in cardiac function between inbred mouse strains are telling of the impact of genetic divergence between strains on cardiovascular physiology. Although strain-dependent differences in physiological function can be challenging when comparing findings of studies in which multiple inbred strains have been used, they can also be taken advantage of to expand our understanding of complex disease processes. Similar to humans, selected cohorts of mice show distinct susceptibility to specific disease processes and these characteristics can be used to inform experimental design. For example, when creating a transgenic mouse that expresses a gene thought to confer resistance to cardiac ischemic injury, it may be beneficial to create or cross this transgenic line to a strain that shows particular susceptibility to cardiac ischemic injury such as 129S1/SvImJ as shown here. Carefully selecting the genetic background of novel genetically engineered mice in this way may maximize the differences between treated and untreated groups when attempting to test a particular therapeutic strategy. Additionally, because disease therapies are designed to treat patients that presumably show increased susceptibility to the disease of interest, it makes sense that research studies be designed in a similar fashion.
Another useful outcome of this study is the identification of quantitative traits that arose from the phenotypic diversity between inbred mouse strains. Future studies could center on tracking and identification of QTL that modify particular cardiac disease-related physiological processes. Previous studies have demonstrated the feasibility of using the genetic homogeneity of inbred mouse strains to isolate and identify physiologically significant QTL (2, 6, 13, 20, 21, 48, 49). In this study, we observed significant functional differences between inbred strains that were evident when subjected to the stresses of ischemia/reperfusion, β-blockade, and acute hypoxia. Because these challenges have direct clinical correlates, we anticipate that future studies focused on identifying the QTL to mechanistically explain observed functional differences between these strains will be of value. For example, a QTL analysis could be designed with C3H/HeJ and C57BL/6J mice to identify loci that cause the marked loss of function in C3H/HeJ mice following esmolol infusion. Such a study may provide new mechanistic and clinically relevant insight into the regulation of cardiac function by β-adrenergic signaling by elucidating alterations in this pathway between these strains. This may be valuable in identifying factors that alter adrenergic signaling in the normal and diseased heart (16, 24). Finally, the finding that under baseline hemodynamic conditions C57BL/6J mice had improved in vivo heart performance compared with other strains, including FVB/N, may help explain earlier reports of marked differences in mutant transgene outcomes between these inbred strains (29, 36).
Collectively, the results of this study emphasize the importance of genetic divergence between inbred mouse strains and the complex manner in which this influences cardiac function. Additionally, we found that differences in cardiac function between inbred strains are modified by both genetic background and the physiological test setting (ex vivo/in vivo) used to assess cardiac function. Finally, in this study we have identified numerous quantitative traits that may serve as the substrate for future studies on genetic modifiers of cardiac function. The demonstration here of reproducible and robust physiological traits, provoked under baseline or pathophysiological challenges, should aid future works attempting to identify new genetic modifiers of heart performance.
GRANTS
This work was supported by National Institutes of Health grants to J. M. Metzger.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Joshua Martindale and DeWayne Townsend for assistance and expertise in isolated heart and in vivo hemodynamic techniques, respectively.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Barbosa ME, Alenina N, Bader M. Induction and analysis of cardiac hypertrophy in transgenic animal models. Meth Mol Med 112: 339–352, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Bauman LE, Sinsheimer JS, Sobel EM, Lange K. Mixed effects models for quantitative trait loci mapping with inbred strains. Genetics 180: 1743–1761, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet 24: 23–25, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Bendall JK, Heymes C, Wright TJ, Wheatcroft S, Grieve DJ, Shah AM, Cave AC. Strain-dependent variation in vascular responses to nitric oxide in the isolated murine heart. J Mol Cell Cardiol 34: 1325–1333, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Hoger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet 23: 141–142, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Blizard DA, Lionikas A, Vandenbergh DJ, Vasilopoulos T, Gerhard GS, Griffith JW, Klein LC, Stout JT, Mack HA, Lakoski JM, Larsson L, Spicer JM, Vogler GP, McClearn GE. Blood pressure and heart rate QTL in mice of the B6/D2 lineage: sex differences and environmental influences. Physiol Genomics 36: 158–166, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blizard DA, Welty R. Cardiac activity in the mouse: strain differences. J Comp Physiol 77: 337–344, 1971. [DOI] [PubMed] [Google Scholar]
- 8. Boban M, Stowe DF, Buljubasic N, Kampine JP, Bosnjak ZJ. Direct comparative effects of isoflurane and desflurane in isolated guinea pig hearts. Anesthesiology 76: 775–780, 1992. [DOI] [PubMed] [Google Scholar]
- 9. Dalal PG, Corner A, Chin C, Wood C, Razavi R. Comparison of the cardiovascular effects of isoflurane and sevoflurane as measured by magnetic resonance imaging in children with congenital heart disease. J Clin Anesth 20: 40–44, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, D'Alecy LG, Ingwall JS, Metzger JM. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med 12: 181–189, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Dhein S, Mohr FW, Delmar M. Practical Methods in Cardiovascular Research. Springer, 2005, p. 1010. [Google Scholar]
- 12. Dillmann WH. Calcium regulatory proteins and their alteration by transgenic approaches. Am J Cardiol 83: 89H–91H, 1999. [DOI] [PubMed] [Google Scholar]
- 13. Doorenbos C, Tsaih SW, Sheehan S, Ishimori N, Navis G, Churchill G, Dipetrillo K, Korstanje R. Quantitative trait loci for urinary albumin in crosses between C57BL/6J and A/J inbred mice in the presence and absence of Apoe. Genetics 179: 693–699, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du XJ. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc Res 63: 510–519, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Eckhart AD, Koch WJ. Transgenic studies of cardiac adrenergic receptor regulation. J Pharmacol Exp Therapeut 299: 1–5, 2001. [PubMed] [Google Scholar]
- 16. Engelhardt S. Beta-adrenergic receptors in heart failure. Heart Fail Clin 1: 183–191, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Ferrier GR, Guyette CM. Ventricular tachycardia in an isolated guinea pig ventricular free wall model of ischemia and reperfusion. J Cardiovasc Pharmacol 17: 228–238, 1991. [DOI] [PubMed] [Google Scholar]
- 18. Gros D, Dupays L, Alcolea S, Meysen S, Miquerol L, Theveniau-Ruissy M. Genetically modified mice: tools to decode the functions of connexins in the heart-new models for cardiovascular research. Cardiovasc Res 62: 299–308, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Hewett TE, Grupp IL, Grupp G, Robbins J. Alpha-skeletal actin is associated with increased contractility in the mouse heart. Circ Res 74: 740–746, 1994. [DOI] [PubMed] [Google Scholar]
- 20. Heydemann A, Ceco E, Lim JE, Hadhazy M, Ryder P, Moran JL, Beier DR, Palmer AA, McNally EM. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J Clin Invest 119: 3703–3712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heydemann A, Huber JM, Demonbreun A, Hadhazy M, McNally EM. Genetic background influences muscular dystrophy. Neuromuscul Disord 15: 601–609, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., Jr Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet 13: 1999–2010, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, Pao YH, Nadeau JH. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics 79: 679–685, 2002. [DOI] [PubMed] [Google Scholar]
- 24. Insel PA, Hammond HK. Beta-adrenergic receptors in heart failure. J Clin Invest 92: 2564, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landoni G, Bignami E, Oliviero F, Zangrillo A. Halogenated anaesthetics and cardiac protection in cardiac and non-cardiac anaesthesia. Ann Card Anaesth 12: 4–9, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Langendorff O. Untersuchungen am überlebenden Säugetierherzen. Pflügers Arch 61: 291–332, 1895. [Google Scholar]
- 27. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2010 Update: a report from the American Heart Association. Circulation 121: 948–954, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Merin RG, Bernard JM, Doursout MF, Cohen M, Chelly JE. Comparison of the effects of isoflurane and desflurane on cardiovascular dynamics and regional blood flow in the chronically instrumented dog. Anesthesiology 74: 568–574, 1991. [DOI] [PubMed] [Google Scholar]
- 29. Michele DE, Gomez CA, Hong KE, Westfall MV, Metzger JM. Cardiac dysfunction in hypertrophic cardiomyopathy mutant tropomyosin mice is transgene-dependent, hypertrophy-independent, and improved by beta-blockade. Circ Res 91: 255–262, 2002. [DOI] [PubMed] [Google Scholar]
- 30. Mogil JS, Smith SB, O'Reilly MK, Plourde G. Influence of nociception and stress-induced antinociception on genetic variation in isoflurane anesthetic potency among mouse strains. Anesthesiology 103: 751–758, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Nabeshima T, Ho IK. Pharmacological responses to pentobarbital in different strains of mice. J Pharmacol Exp Therapeut 216: 198–204, 1981. [PubMed] [Google Scholar]
- 32. Nilles KM, London B. Knockin animal models of inherited arrhythmogenic diseases: what have we learned from them? J Cardiovasc Electrophysiol 18: 1117–1125, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protocols 3: 1422–1434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palpant NJ, D'Alecy LG, Metzger JM. Single histidine button in cardiac troponin I sustains heart performance in response to severe hypercapnic respiratory acidosis in vivo. FASEB J 23: 1529–1540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palpant NJ, Szatkowski ML, Wang W, Townsend D, Bedada FB, Koch LG, Britton SL, Metzger JM. Artificial selection for whole animal low intrinsic aerobic capacity co-segregates with hypoxia-induced cardiac pump failure. PloS one 4: e6117, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro JR, Wieczorek DF. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol 33: 1815–1828, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Reichelt ME, Willems L, Hack BA, Peart JN, Headrick JP. Cardiac and coronary function in the Langendorff-perfused mouse heart model. Exp Physiol 94: 54–70, 2009. [DOI] [PubMed] [Google Scholar]
- 38. Ryder-Cook AS, Sicinski P, Thomas K, Davies KE, Worton RG, Barnard EA, Darlison MG, Barnard PJ. Localization of the mdx mutation within the mouse dystrophin gene. EMBO J 7: 3017–3021, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saha DC, Saha AC, Malik G, Astiz ME, Rackow EC. Comparison of cardiovascular effects of tiletamine-zolazepam, pentobarbital, and ketamine-xylazine in male rats. J Am Assoc Lab Anim Sci 46: 74–80, 2007. [PubMed] [Google Scholar]
- 40. Schlager G. Systolic blood pressure in eight inbred strains of mice. Nature 212: 519–520, 1966. [DOI] [PubMed] [Google Scholar]
- 41. Seong E, Saunders TL, Stewart CL, Burmeister M. To knockout in 129 or in C57BL/6: that is the question. Trends Genet 20: 59–62, 2004. [DOI] [PubMed] [Google Scholar]
- 42. Shah AP, Siedlecka U, Gandhi A, Navaratnarajah M, Al-Saud SA, Yacoub MH, Terracciano CM. Genetic background affects function and intracellular calcium regulation of mouse hearts. Cardiovas Res. In press. [DOI] [PubMed] [Google Scholar]
- 43. Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580, 1989. [DOI] [PubMed] [Google Scholar]
- 44. Skolleborg KC, Gronbech JE, Grong K, Abyholm FE, Lekven J. Distribution of cardiac output during pentobarbital versus midazolam/fentanyl/fluanisone anaesthesia in the rat. Lab Anim 24: 221–227, 1990. [DOI] [PubMed] [Google Scholar]
- 45. Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff—still viable in the new millennium. J Pharmacol Toxicol Meth 55: 113–126, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Sonner JM, Gong D, Eger EI., 2nd Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg 91: 720–726, 2000. [DOI] [PubMed] [Google Scholar]
- 47. Stull LB, Hiranandani N, Kelley MA, Leppo MK, Marban E, Janssen PM. Murine strain differences in contractile function are temperature- and frequency-dependent. Pflügers Arch 452: 140–145, 2006. [DOI] [PubMed] [Google Scholar]
- 48. Stylianou IM, Langley SR, Walsh K, Chen Y, Revenu C, Paigen B. Differences in DBA/1J and DBA/2J reveal lipid QTL genes. J Lipid Res 49: 2402–2413, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sugiyama F, Churchill GA, Li R, Libby LJ, Carver T, Yagami K, John SW, Paigen B. QTL associated with blood pressure, heart rate, and heart weight in CBA/CaJ and BALB/cJ mice. Physiol Genomics 10: 5–12, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Suzuki M, Carlson KM, Marchuk DA, Rockman HA. Genetic modifier loci affecting survival and cardiac function in murine dilated cardiomyopathy. Circulation 105: 1824–1829, 2002. [DOI] [PubMed] [Google Scholar]
- 51. Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci USA 88: 2065–2069, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tamargo J, Caballero R, Nunez L, Gomez R, Vaquero M, Delpon E. Genetically engineered mice as a model for studying cardiac arrhythmias. Front Biosci 12: 22–38, 2007. [DOI] [PubMed] [Google Scholar]
- 53. Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther 15: 1086–1092, 2007. [DOI] [PubMed] [Google Scholar]
- 54. Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther 16: 832–835, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Waldeyer C, Fabritz L, Fortmueller L, Gerss J, Damke D, Blana A, Laakmann S, Kreienkamp N, Volkery D, Breithardt G, Kirchhof P. Regional, age-dependent, and genotype-dependent differences in ventricular action potential duration and activation time in 410 Langendorff-perfused mouse hearts. Basic Res Cardiol 104: 523–533, 2009. [DOI] [PubMed] [Google Scholar]
- 56. Ware CB, Siverts LA, Nelson AM, Morton JF, Ladiges WC. Utility of a C57BL/6 ES line versus 129 ES lines for targeted mutations in mice. Transgen Res 12: 743–746, 2003. [DOI] [PubMed] [Google Scholar]
- 57. Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature 420: 520–562, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.