Abstract
Introduction. We present a case of an 89-year-old female who attended our surgical endocrine clinic with a 3-month history of a left-sided neck lump. There was no past medical history of thyroid disease. Methods. Following examination and further investigation, including core biopsy, a diagnosis of plasma cell granuloma of the thyroid was made. Biochemical testing of thyroid function and Thyroid Peroxidase Antibody was in-keeping with an associated Hashimoto's thyroiditis. Results. The patient was treated conservatively with thyroxine and regularly seen in clinic. TSH levels improved and the lump showed signs of regression. Conclusion. Plasma cell granuloma of the thyroid is rare with only 16 previously reported cases. We present a new approach to management without the use of surgery or steroids. The literature is reviewed comparing clinico-pathological features and management of other reported cases.
1. Case Report
An 89-year-old female presented with a 3-month history of a left-sided neck lump. The lump had been steadily increasing in size during this time. There was no history of shortness of breath, dysphagia, or stridor and no history of voice change. The patient had a past medical history of vascular dementia, hypertension, and B12 deficiency secondary to pernicious anaemia. Regular medications included aspirin, bendrofluazide, and 3 monthly injections of hydroxocobalamin. There was no past medical history of thyroid disease or neck irradiation and no family history of autoimmune disease.
On examination the patient was frail and clinically euthyroid. Examination of the neck revealed a large firm, irregular mass in the upper pole of the left thyroid lobe with a background of multinodular goitre. The lump measured 6.1 cm × 5.5 cm with calipers on presentation. There was no evidence of lymphadenopathy and the trachea was central with no signs of stridor.
Initial assessment was suggestive of lymphoma or poorly differentiated carcinoma. In order to increase diagnostic accuracy, a needle core biopsy was taken rather than fine needle aspiration. Two passes were made using a 14-guage needle. TFTs were checked revealing a TSH of 17.6 μIU/L (0.4–5.5 μIU/L) with a Thyroid Peroxidase Antibody (TPA) of 557 IU/ml (0–50 IU/ml) and free T4 of 12.5 pmol/L (11–26 pmol/L). Full blood count, liver function tests, and urea and electrolytes were all in the normal range. These results were in-keeping with hypothyroidism due to Hashimoto's thyroiditis.
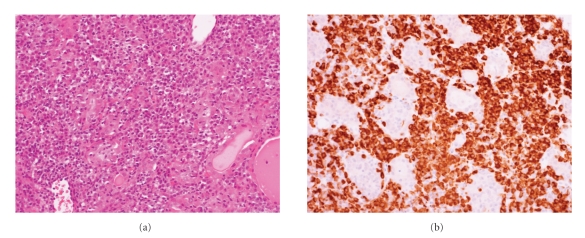
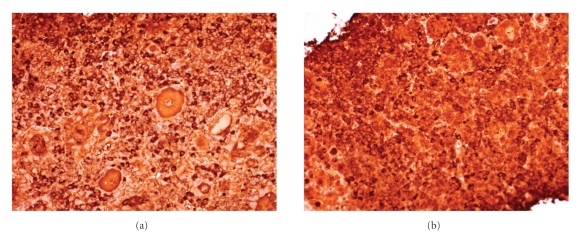
Two core biopsies both measuring 15 mm were obtained for histological examination. This showed a heavy plasma cell infiltrate and admixed B- and T-lymphocytes (Figure 1). The plasma cell infiltrate was polyclonal (Figure 2) and expressed CD79a, CD138, and MUM-1. There was no evidence of anaplastic carcinoma or other primary thyroid carcinoma. There were no morphological features to suggest Riedels thyroiditis.
Figure 1.
Core biopsy showing plasma cells confirmed with staining for CD79a ((a) H&E (b) CD79a both ×200).
Figure 2.
Staining for kappa (a) and lambda (b) light chains to confirm polyclonality (both ×200).
The histological findings were therefore consistent with a plasma cell granuloma of the thyroid with underlying Hashimoto's thyroiditis.
Due to patient frailty and comorbidities, operative intervention was deemed inappropriate. The patient was regularly reviewed in the clinic having been started on Thyroxine. TSH levels improved with modification of T4 dosage. The neck lump remained static for several months until eventually showing signs of regression. The lump measured 4.5 cm × 3 cm 10 months after presentation and 8 months following start of treatment with thyroxine. The patient remained asymptomatic with respect to breathing and swallowing.
2. Discussion
Plasma cell granuloma of the thyroid gland is rare with only 16 previously reported cases. It predominantly affects women with the majority of cases reporting patients over the age of 35 years. Plasmacytoma of the thyroid is more common and the two can be differentiated histologically by assessing for clonality.
Plasma cell granuloma (PCG) is well documented in the literature, first being described in 1973 by Bahadori and Liebow [1]. It is a nonmalignant lesion characterised by proliferation of polyclonal plasma cells with varying degrees of myofibroblastic proliferation [2]. The polyclonal nature is important in distinguishing PCG from plasmacytoma. Immunohistochemistry can also be used to demonstrate the polyclonality of the plasma cells. Lesions of this type are mainly found in the lungs with other recorded cases occurring in the liver [3], stomach [4], pancreas [5], bladder [6], and kidney [7]. Hurthle cell metaplasia found on histology has been documented in some cases [8–10] of PCG of the thyroid but this is not universal. Macroscopically, there are also some variations in the literature but the lesions are usually firm with a white/grey colour. Often the specimen has been as part of a lobectomy or total thyroidectomy.
The aetiology of plasma cell granulomas is not completely understood. It has been suggested that it may be secondary to a chronic inflammatory process causing abnormalities of plasma cell differentiation. Many of the cases of thyroid plasma cell granulomas demonstrate an association with an autoimmune disease such as Hashimoto's thyroiditits and diabetes mellitus [10–14]. This is largely anecdotal and, although there is no strong evidence to link the two disease processes, it can be supported by evidence of the cellular infiltrate expressing antithyroid peroxidase antibodies and response to immunosuppressant medications.
Treatment of these lesions varies in the literature with the majority of patients undergoing some form of surgical intervention with either total/subtotal thyroidectomy or lobectomy (Table 1). Corticosteroid usage and other immunosuppressive therapies such as cyclophosphamide and azathioprine have also been used to treat these lesions with some degree of improvement. In our case, we have shown that these benign lesions can resolve spontaneously without the need for unnecessary surgery or medications with potentially significant side effects. We do, however, appreciate that surgical intervention may be a necessity in a case of a large, rapidly increasing nodule that is compromising a patients airway or is associated with significant symptoms. However, if PCG is confirmed histologically, for example, on core biopsy as in our patient, with no debilitating symptoms, it is reasonable to observe these cases without any intervention either surgical or medical other than treatment of any underlying thyroid dysfunction.
Table 1.
Clinical and pathological features of reported cases of plasma cell granuloma of the thyroid.
| Paper | Age/Sex | Presentation | Thyroid function | Autoimmunity | Pathology | Treatment |
|---|---|---|---|---|---|---|
| Chan et al. 1986 [15] | 35 F | Neck lump, right lobe nodule. Mild tracheal compression | Euthyroid | No | 3 cm white, round nodule. Plasma cell aggregates. Hurtle cells absent. Polyclonal pattern on staining | Right hemithyroidectomy |
| De Mascarel et al. 1989 [16] | 35 F | 3 cm nodule in left lobe | Euthyroid | No | 2.2 cm firm lesion. Fibrous tissue with polyclonal plasma cells | Thyroidectomy |
| Ferrer-Garcia et al. 2004 [11] | 41 M | Goiter | Hypothyroid | Hashimoto's | Polyclonal plasma cells with evidence of Hashimoto's thyroiditis. | FNA inconclusive. Total thyroidectomy |
| Fontenot et al. 2008 [17] | 55 F | Enlarging neck swelling, with compressive symptoms | Hypothyroid | No | Firm, fibrotic lesion. Polyclonal plasma cells with the expression of both kappa and lambda light chains | Thyroidectomy |
| Holck, 1981 [18] | 70 F | Neck swelling with breathing difficulties. Right lobe, 3 cm nodule on examination | Hypothyroid | No | Obliteration of parenchyma with mature plasma cells. No Hurtle cell changes | Subtotal thyroidectomy |
| Kojima et al. 2009 [19] | 75 F | Painless left-sided neck swelling | Euthyroid | No | Inflammatory pseudotumour (IPT). Predominantly fibrohistiocytic. Vimentin and CD68 +ve. | Lobectomy |
| Kriegl et al. 2007 [12] | 50 M | Thyroid enlargement with dysphagia | Euthyroid | Hashimoto's | Polyclonal plasma cells with associated Hashimoto's thyroiditis. EBV and HHV8 DNA negative. | Subtotal thyroidectomy |
| Laurent et al. 2004 [13] | 35 F | Dysphagia and asthenia. Normal thyroid on examination. Later painful enlargement of thyroid with signs of tracheal compression | Hypothyroid | ?Hashimoto's (↑ antimicrosomal antibodies, anti-TPO positive) | Numerous plasma cells, macrophages and T lymphocytes and B lymphocytes. Plasma cells polyclonal | Methylprednisolone initially without response. Unable to excise due to fibrosis, biopsy taken. IV methylprednisolone given followed by IV cyclophosphamide and oral azathioprine for 6 months |
| Li Voon Chong et al. 2001 [20] | 29 M | Neck tenderness, dysphagia, odynophagia, and fever. 8 cm mass in left lobe. | Euthyroid | Diabetes Mellitus | Histology proven plasma cell granuloma. Staining showed presence of IgG, IgM, and IgA. | Initial antibiotics. Surgical exploration with multiple biopsies |
| Martinez et al. 2002 [8] | 46 F | Large painless neck mass. History of goitre. | Euthyroid | No | 3 to 15 mm nodules separated by fibrous bundles. Numerous plasma cells with Hurtle cell changes | Total thyroidectomy |
| Mugler et al. 2003 [14] | 46 M | Painless left neck mass. Family history of thyroid Ca. Dominant nodule on examination | Hypothroid | Hashimoto's | 5 × 3 × 3 cm nodule. Changes consistent with thyroiditis, including Hurtle cell changes. Plasma cell aggregation, polyclonal on staining. | Neoplasm could not be ruled out on FNA. Total thyroidectomy |
| Talmi et al. 1989 [21] | 51 F | Painless enlarging nodule in right lobe. | Not known | No | 2 cm white nodule. Mature polyclonal plasma cells. | Lobectomy |
| Yapp et al. 1985 [9] | 61 F | Painless goiter enlargement. | Hypothyroid | No | Polyclonal plasma cells. Hurtle cell changes. Some lymph node enlargement | Total thyroidectomy |
| Zingrillo et al. 1995 [10] | 65 F | Neck swelling and breathing difficulties. 3 × 5 cm nodule in left lobe | Hypothyroid | Hashimoto's | Polyclonal plasma cells with lymphocytic infiltrate. Hurtle cell changes present | Total thyroidectomy |
References
- 1.Bahadori M, Liebow AA. Plasma cell granulomas of the lung. Cancer. 1973;31(1):191–208. doi: 10.1002/1097-0142(197301)31:1<191::aid-cncr2820310127>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Chan JKC. Inflammatory pseudotumor: a family of lesions of diverse nature and etiologies. Advances in Anatomic Pathology. 1996;3:156–171. [Google Scholar]
- 3.Pack GT, Baker HW. Total right heptic lobectomy. Report of a case. Annals of Surgery. 1953;138:253–258. doi: 10.1097/00000658-195308000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaacson P, Buchanan R, Mepham BL. Plasma cell granuloma of the stomach. Human Pathology. 1978;9(3):355–358. doi: 10.1016/s0046-8177(78)80094-x. [DOI] [PubMed] [Google Scholar]
- 5.Abrebanel P, Sarfaty S, Gal R, Chaimoff C, Kessler E. Plasma cell granuloma of the pancreas. Archives of Pathology and Laboratory Medicine. 1984;108(7):531–532. [PubMed] [Google Scholar]
- 6.Jufe R, Molinolo AA, Fefer SA, Meiss RP. Plasma cell granuloma of the bladder: a case report. Journal of Urology. 1984;131(6):1175–1176. doi: 10.1016/s0022-5347(17)50861-7. [DOI] [PubMed] [Google Scholar]
- 7.Fisch AE, Brodey PA. Plasma cell granuloma of kidney. Urology. 1976;8(1):89–91. doi: 10.1016/0090-4295(76)90066-2. [DOI] [PubMed] [Google Scholar]
- 8.Martinez F, Filipowicz E, Hudnall SD. Plasma cell granuloma of the thyroid: a case report and review of the literature. Archives of Pathology and Laboratory Medicine. 2002;126(5):595–598. doi: 10.5858/2002-126-0595-PCGOTT. [DOI] [PubMed] [Google Scholar]
- 9.Yapp R, Linder J, Schenken JR, Karrer FW. Plasma cell granuloma of the thyroid. Human Pathology. 1985;16(8):848–850. doi: 10.1016/s0046-8177(85)80258-6. [DOI] [PubMed] [Google Scholar]
- 10.Zingrillo M, Tardio B, Bisceglia M. Plasma cell granuloma of the thyroid associated with Hashimoto’s thyroiditis. Journal of Endocrinological Investigation. 1995;18(6):460–464. doi: 10.1007/BF03349746. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer-Garcia JC, Costa-Talens P, Merino-Torres JF, et al. Plasma cell granuloma of the thyroid and Hashimoto’s thyroiditis. Southern Medical Journal. 2004;97(6):598–600. doi: 10.1097/00007611-200406000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Kriegl L, Guetgemann I, Zhou H. Plasma cell granuloma of the thyroid gland mimicking carcinoma: a case report and review of the literature. Pathology Research and Practice. 2007;203(11):813–817. doi: 10.1016/j.prp.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Laurent S, Mouthon L, Longchampt E, et al. Medical cure of plasma cell granuloma of the thyroid associated with Hashimoto’s thyroiditis: a case report and review. Journal of Clinical Endocrinology and Metabolism. 2004;89(4):1534–1537. doi: 10.1210/jc.2003-031355. [DOI] [PubMed] [Google Scholar]
- 14.Mugler K, Gaido L, Ryder J, Said S. Plasma cell granuloma of the thyroid with Hashimoto’s thyroiditis: report of a rare case. Ear, Nose and Throat Journal. 2003;82(1):64–66. [PubMed] [Google Scholar]
- 15.Chan KW, Poon GP, Choi CH. Plasma cell granuloma of the thyroid. Journal of Clinical Pathology. 1986;39(10):1105–1107. doi: 10.1136/jcp.39.10.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Mascarel A, Vergier B, Merlio J-P, Goussot JF, Coindre J-M. Plasma cell granuloma of the adrenal gland and the thyroid: report of two cases. Journal of Surgical Oncology. 1989;41(2):139–142. doi: 10.1002/jso.2930410216. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JW, Levine SN, Adegboyega PA, Cotelingam JD. Plasma cell granuloma of the thyroid: report of case and review of literature. Endocrine Practice. 2008;14(5):611–617. doi: 10.4158/EP.14.5.611. [DOI] [PubMed] [Google Scholar]
- 18.Holck S. Plasma cell granuloma of the thyroid. Cancer. 1981;48(3):830–832. doi: 10.1002/1097-0142(19810801)48:3<830::aid-cncr2820480327>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Kojima M, Suzuki M, Shimizu K, Masawa N. Inflammatory pseudotumor of the thyroid gland showing prominent fibrohistiocytic proliferation. A case report. Endocrine Pathology. 2009;20(3):186–190. doi: 10.1007/s12022-009-9080-4. [DOI] [PubMed] [Google Scholar]
- 20.Li Voon Chong JS, Burrows CT, Cave-Bigley D, Macfarlane IA. A hard thyroid mass due to plasma cell granuloma. International Journal of Clinical Practice. 2001;55(5):335–336. [PubMed] [Google Scholar]
- 21.Talmi YP, Finkelstein Y, Gal R, Zohar Y. Plasma cell granuloma of the thyroid gland. Head and Neck. 1989;11(2):184–187. doi: 10.1002/hed.2880110214. [DOI] [PubMed] [Google Scholar]