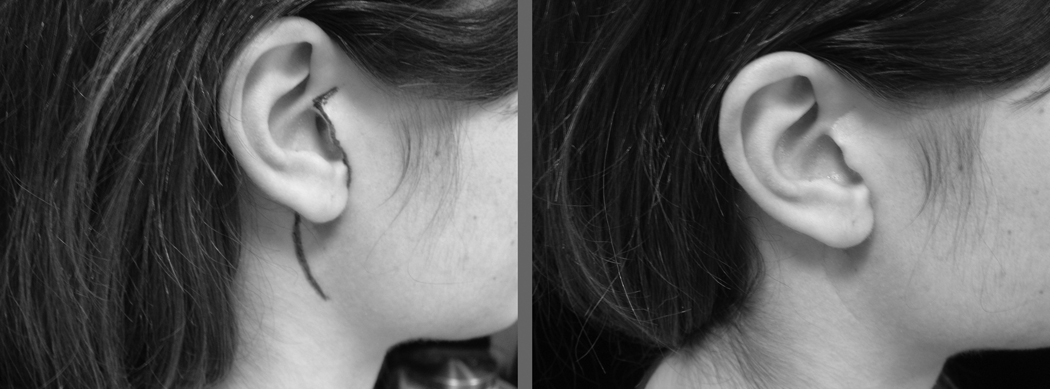Abstract
The head and neck region poses a challenging arena for oncologic surgery. Diseases and their treatment can affect a myriad of functions, including sight, hearing, taste, smell, breathing, speaking, swallowing, facial expression and appearance. This review discusses several areas where refinements in surgical techniques have led to improved patient outcomes. This includes surgical incisions, neck lymphadenectomy, transoral laser microsurgery, minimally invasive thyroid surgery, and the use of vascularized free flaps for oromandibular reconstruction.
Keywords: Neck Dissection, Minimally Invasive Thyroidectomy, Transoral Laser Microsurgery, Facial Incisions, Oromandibular Reconstruction
Introduction
The head and neck region poses a particularly challenging arena for oncologic surgery. Vital structures such as the carotid arteries, cranial nerves, trachea and esophagus are all in close proximity to the primary tumor or regional lymph node metastases. Furthermore, the oral cavity and oropharynx serve as a common channel for breathing, speaking, and swallowing. The larynx serves as a gateway to carry out these functions while protecting the airway and lungs from aspiration. In addition, the functions of vision, hearing, taste, smell, facial expression and appearance can be affected by disease or its treatment. Advances in surgical approaches and techniques have led to significant improvements in oncologic, functional, and aesthetic outcomes for surgery in the head and neck. In this review, we will discuss how refinements in several areas have resulted in lower morbidity and improved function and aesthetic appearance, without adversely affecting oncologic outcomes. The areas to be reviewed are refinements of surgical incisions, management of regional cervical lymph nodes, transoral laser microsurgery for tumors of the oropharynx, larynx and hypopharynx, minimally invasive surgery of the thyroid and parathyroid glands, and the use of vascularized free flaps for oromandibular reconstruction.
Surgical Incisions
Traditionally employed surgical incisions for various procedures in the head and neck have produced significant aesthetic deformity and often an unacceptable surgical scar and facial appearance. The three important areas where traditional incisions have transitioned into more aesthetically-oriented incisions leading to essentially no aesthetic deformity are: (1) the modified Weber-Ferguson incision for maxillectomy; (2) the tragal incision for parotidectomy; and (3) the use of transverse incisions for neck dissection along an upper neck skin crease. The traditional incision for maxillectomy for decades has been the classic Weber-Ferguson incision, which begins by dividing the upper lip at the midline, follows the nose along the vestibule of the nasal cavity, and then along the nasolabial fold up to the medial canthus of the eye. The subciliary extension continues the incision adjacent to the tarsal margin up to the lateral canthus. This incision produces significant aesthetic deformity resulting in unequal facial appearance. Respecting nasal subunits and facial relaxed skin tension lines allows the surgeon to modify this incision, resulting in marginal aesthetic impact on the patient.
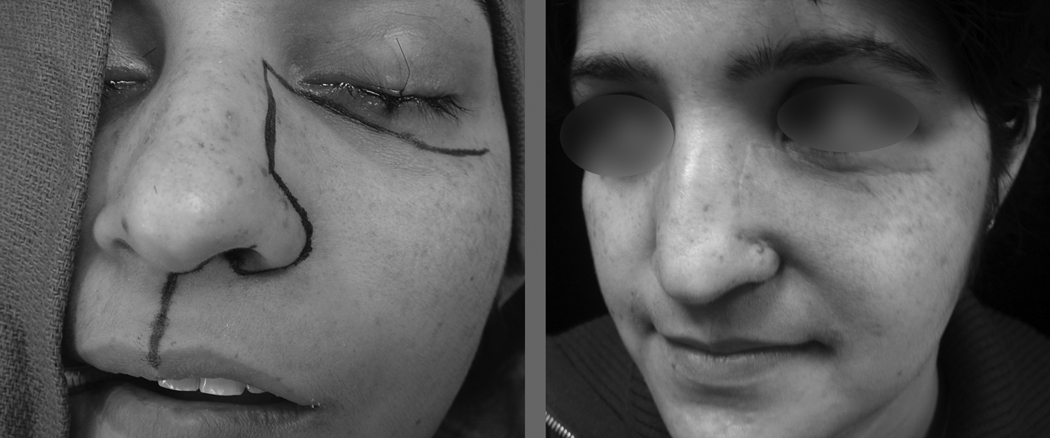
The modified Weber-Ferguson incision begins in the midline of the filtrum of the upper lip and extends from the vermilion border up to the columella. At that point, it takes a right angle turn into the floor of the nasal cavity, exiting the floor of the nasal cavity along the ala of the nose. The incision then follows the nasofacial groove all the way up to the upper end of the ala of the nose at the nasal bulb. The incision again makes a right angle turn on the lateral aspect of the nose, and extends along the lateral nasal dorsal subunit to the root of the nose. Next, the incision makes a lateral turn into an appropriate skin crease in the infraorbital region up to the zygoma. In the vertical portion of the incision, the soft tissue is elevated over the underlying bony framework in the usual manner. During the lateral extension only the skin is elevated in the infraorbital region, leaving the orbicularis oculi muscle on the patient. After the skin incision is elevated, the orbicularis oculi muscle is elevated cephalad as a separate layer up to the orbital rim. Elevation of this part of the flap must be done extremely delicately to avoid perforating the thin skin. Employing this incision, the aesthetic impact on the patient is marginal, if any. The outline of the incision and the postoperative appearance of a patient following maxillectomy are shown in (Figure 1).
Figure 1.
Preoperative (left) and postoperative (right) appearance of the modified Weber-Ferguson incision.
The traditional incision for superficial parotidectomy is the Blair incision[1] which is placed in the preauricular skin crease, then curves around the lobule of the ear over the mastoid process and finally along the upper part of the neck. This incision leaves significant aesthetic deformity, particularly in the young patient. Clearly, in the older patient where a preauricular skin crease already exists, one should follow that skin crease. However, in the young patient without any skin creases the parotidectomy incision begins at the upper end of the helix just cephalad to the tragal cartilage. The incision then follows along the edge of the tragus up to the lobule of the ear, at which point it curves around the lobule of the ear up to the mastoid process. It then takes a horseshoe curve and follows the very lateral end of an upper neck skin crease. Extreme care should be exercised in elevating the skin over the tragus since the skin may easily be perforated. This elevation of the skin is performed with a sharp scalpel until the soft tissue of the subcutaneous fat is reached. At that point, the usual technique is employed in elevation of the rest of the flap. The placement of the incision and postoperative appearance of a young patient who has undergone superficial parotidectomy is shown in Figure 2.
Figure 2.
Preoperative (left) and postoperative (right) appearance of the modified Blair incision.
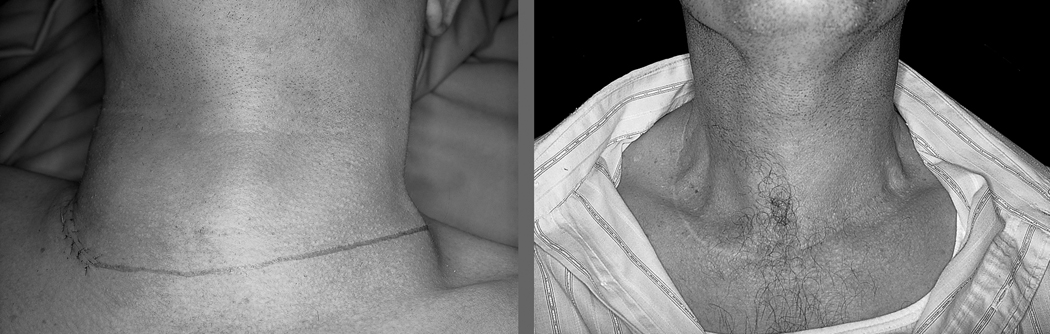
The standard incisions employed for classical radical neck dissections range from a double trifurcate incision popularized by Martin, followed by single trifurcate incisions reported by Kocher, Crile, Schobinger, and others. All of these incisions with a vertical component in the neck give a significant aesthetic deformity. The current practice for neck dissection is to employ only a transverse incision along a suitable skin crease. If one needs to reach the submental level across the midline to the opposite side of the neck, dissecting along the same skin crease permits elevation of the upper flap up to the mental region. Similarly, to gain access to Level IIB, the transverse incision is not curved towards the mastoid process but carried on laterally along the same skin crease, allowing easy access to the region of the mastoid process. This incision is adequate for a supraomohyoid neck dissection as well as a comprehensive neck dissection. Thyroidectomy in conjunction with a comprehensive neck dissection is best performed using a single transverse incision along the skin crease at the level of the cricoid cartilage. This incision may extend from the anterior border of the trapezius muscle on one side to that on the other side. The entire procedure of total thyroidectomy, central compartment neck dissection and bilateral comprehensive lateral neck dissection can be easily performed through this single transverse incision. The placement of the incision and postoperative appearance of a patient undergoing bilateral neck dissections and total thyroidectomy is shown in Figure 3. Attention to detail with aesthetic outcome of surgical procedures on the head and neck is crucial to patient satisfaction.
Figure 3.
Preoperative (left) and postoperative (right) appearance of the incision for total thyroidectomy and bilateral neck dissection.
Knowledge of cutaneous vascular anatomy, the facial lines of tension, the nasal subunits, and facial and cervical skin creases permits one to select aesthetically acceptable incisions for conduct of oncologic surgical procedures in the head and neck. Patients who have undergone surgical procedures with the above-mentioned modified incisions enjoy a better quality of life due to a satisfactory and acceptable aesthetic outcome for their personal image.
Neck Dissection
Neck dissection is a well established, oncologically sound surgical procedure which has stood the test of time for over one hundred years. Its evolution from the radical neck dissection (RND) as a therapeutic procedure for neck metastasis in the early part of the last century to the selective neck dissection (SND) performed today in both elective and therapeutic settings reflect the continuing surgical refinement due to improved understanding of biological progression of metastatic cancer. Here we discuss the rationale for the evolution of neck dissection from a radical operation with significant functional and aesthetic morbidity to a selective operation with much reduced morbidity with equivalent oncologic outcome.
In the 19th century, it was well recognized that head and neck tumors metastasized to the neck. In the late 1800’s, Butlin and others recommended removal of lymph nodes in the neck for treatment of tongue cancer. [2] Iin the early 1900’s, Crile is credited with publishing the first series describing radical neck dissection for oncologic control. [3, 4] This surgery involved en bloc removal of all lymph nodes (Levels I–V) and included resection of the sternocleidomastoid muscle (SCM), Internal Jugular Vein (IJV), and Spinal Accessory Nerve (SAN). In his series of 132 patients, RND proved to be an effective operation, though with significant morbidity. Multiple authors over the next 50 years reported using RND for head and neck tumors with some success [5, 6], and the RND served as an effective but functionally morbid operation. In 1951, Hayes Martin [7] reported on 1450 neck dissection cases over 22 years at Memorial Hospital. This seminal paper described his technique and philosophy of RND, which served as a guide for surgeons for the next few decades.
Although effective, the RND was associated with significant morbidity. Nahum, et al. noted a recurring pattern of pain and ipsilateral arm and shoulder weakness in patients after RND. He termed this constellation of sequelae “Shoulder Syndrome.” [8] A more recent comparison of morbidity after different types of neck dissection illustrates the impact of RND. Using a validated quality of life (QOL) instrument, Terrell, et al. compared QOL scores for RND versus modified radical neck dissection (MRND, discussed below). Significantly worse pain with a higher need for analgesics was noted in the RND treated group. [9]
The morbidity of RND led surgeons to consider whether sparing non-lymphoid tissues (i.e., the SCM, IJV and SAN) could be performed while still retaining the same oncologic control. This surgery, the removal of all neck lymph nodes (Levels I–V) while sparing one or more of structures, was termed modified radical neck dissection. Credit for successfully performing this operation is given to Suarez[10], who first published it in the Spanish literature. Multiple studies by Bocca [8, 11] and others [12] later showed that MRND could be just as effective as RND in the clinically N0 neck, while still preserving critical structures such as the SCM, IJV, and SAN.
Over the 60 years from 1900 to 1960, treatment of regional cervical lymph nodes for node positive and N0 neck evolved from a radical en bloc operation to one that respected critical structures while preserving oncologic outcomes, at least in the N0 neck. However, when such operations were performed in the node positive neck, the local recurrence rate was still very high (29%). [11] Between the 1970’s and 1990’s, surgeons considered whether complete lymphadenectomy of Levels I–V was necessary. Multiple studies began noting different patterns of metastatic spread based on primary tumor site. Therefore, addressing only the at-risk nodal basins was felt to be as effective as comprehensive MRND in the N0 neck.
Lindberg, from UT MD Anderson Cancer Center, in 1972 described the distribution of nodal metastasis for oral cavity, oropharynx, supraglottic larynx, hypopharynx, and nasopharynx carcinomas.[13] Byers reported a review of 428 selective neck dissection cases from the same institution to better understand metastatic patterns of spread. Nineteen subsites were analyzed. In general, tumors of the oral cavity primarily drained to nodes in Levels I–III, while tumors of the oropharynx, larynx, and hypopharynx drained primarily to Levels II–IV. Oral cavity lesions rarely drained to Level V, and oropharyngeal and laryngeal lesions rarely drained to Level I. Only base of tongue tumors had any significant drainage to Level V. [14] Similar findings were also reported by Shah [15], who reviewed 1119 RND performed at Memorial Sloan-Kettering Cancer Center. Again, oral cavity lesions primarily affected Levels I–III first, while oropharynx, hypopharynx, and larynx primarily affected Levels II–IV. Depending on the subsite, Level IV was involved by oral cavity lesions 7–29% of the time, but Level V was involved without disease present at other levels.
These data clearly suggested different and predictable nodal drainage patterns for different head and neck primary sites. The oral cavity and its subsites (hard palate, tongue, floor of mouth, buccal mucosa) primarily drained to Levels I, II, and III. The oropharynx, larynx and hypopharynx primarily drained to Levels II, III, IV. Tumors of the nasopharynx, base of tongue, supraglottic larynx, soft palate, and tonsil were at elevated risk for contralateral drainage.[13]
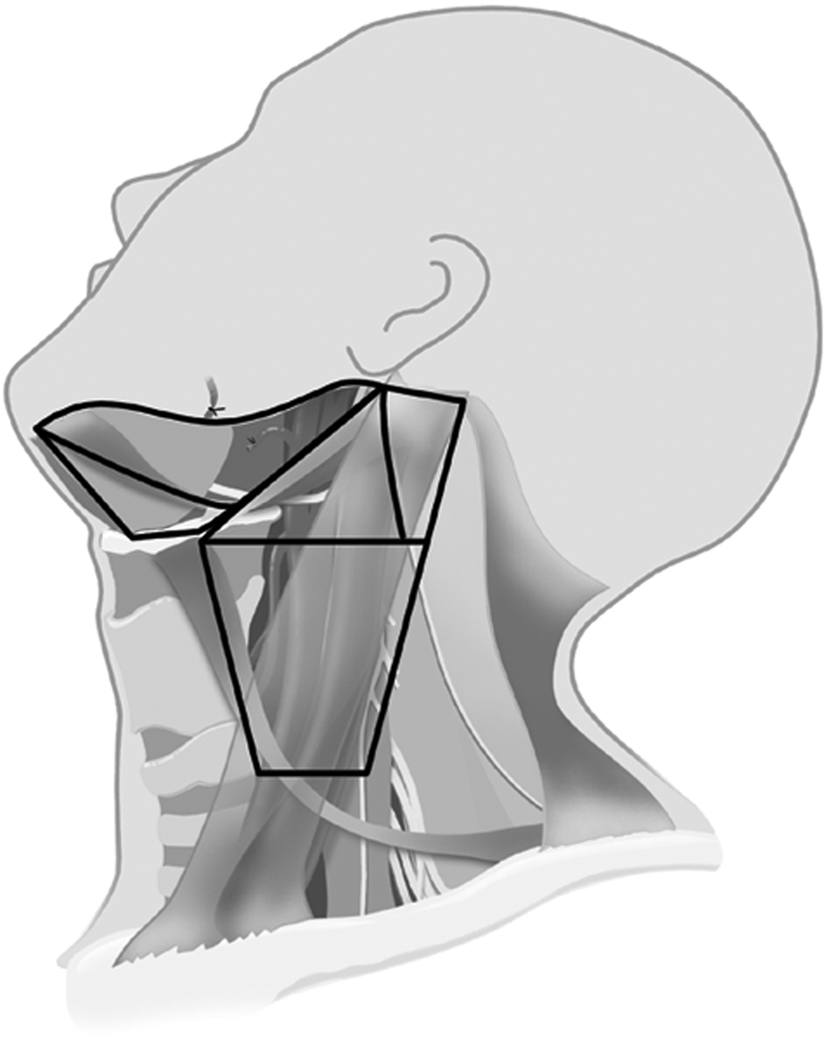
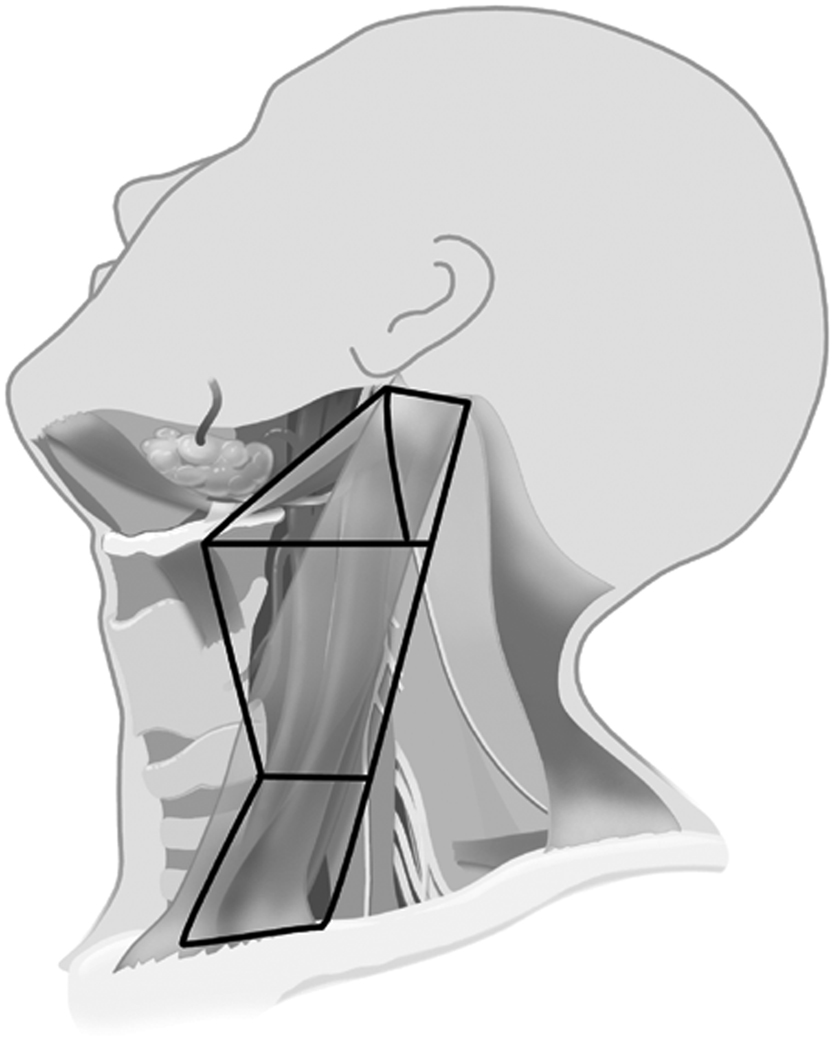
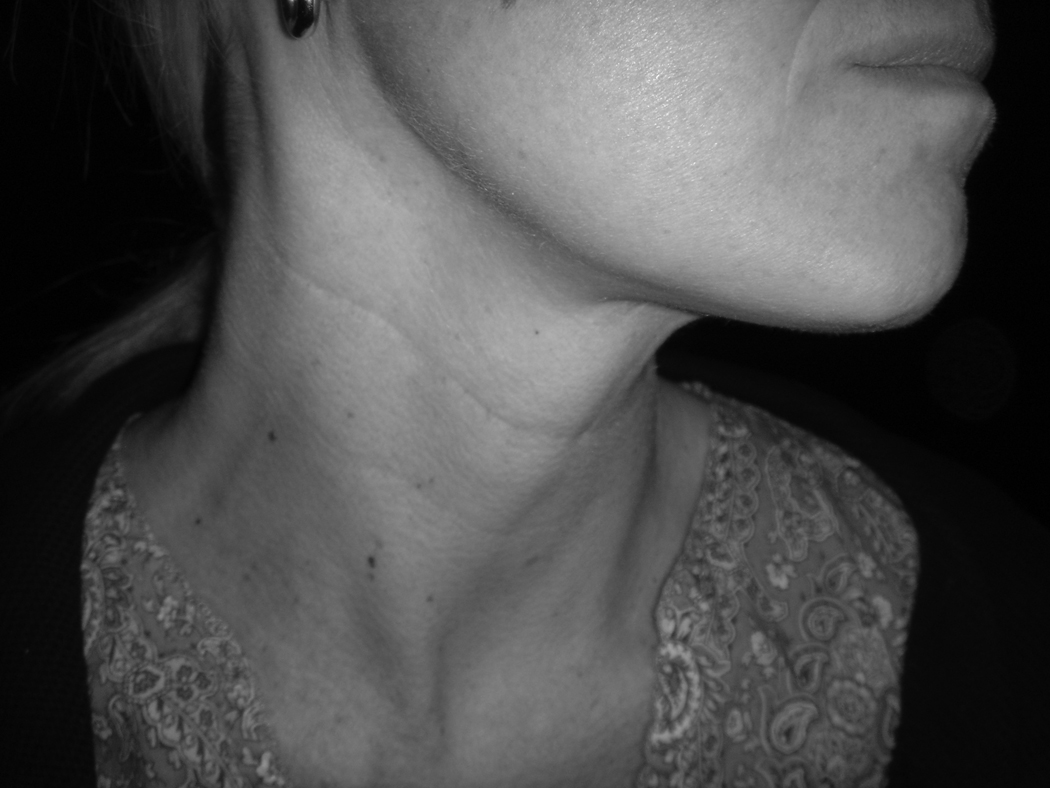
These data supported the idea of performing an SND in elective treatment for the N0 neck at risk for micrometastasis. Weiss, et al. [16] performed a computer-based decision analysis to determine when elective nodal dissection should be performed. He recommended that if the probability of occult metastasis was greater than 20%, then elective neck dissection was warranted. Support showing SND to be as effective as MRND for elective management was demonstrated in two major studies from the Brazilian Head and Neck Study Group. In one prospective study on oral cavity cancer, supraomohyoid neck dissection (SOHND) versus MRND was compared for oncologic control. No difference was found in rates of recurrence or 5-year actuarial survival rates.[17] In a second trial, patients with larynx cancer were randomized to receive either MRND or SND (Levels II–IV) for elective management. Again, no significant difference was found in oncologic control. The rates of 5-year overall survival, neck recurrence, and complications were similar in both groups. [18] This data and others strongly outlined the situations and type of operation for elective management of the N0 neck in head and neck cancer. This most recent approach is much less morbid and invasive because it addresses only those lymph node basins at greatest risk, a far cry from the RND proposed a hundred years ago. Examples of two types of SND, the SOHND and jugular neck dissection (JND) can be seen in figures 4 and 5 respectively. An example of postoperative appearance following SOHND is shown in figure 6.
Figure 4.
Neck levels included in a supraomohyoid neck dissection – Levels IA, IB, IIA, IIB, III.
Figure 5.
Neck levels included in a jugular neck dissection – Levels IIA, IIB, III, IV.
Figure 6.
Example postoperative appearance after supraomohyoid neck dissection.
In the node positive neck, a similar evolution has taken place. Early on, RND was the only operation considered for the node positive neck. In the 1980’s and 1990’s, surgeons began considering whether a more conservative approach may have the same oncologic results. This concept was based on the observation that Level V metastases were exceedingly rare. [19] Byers reviewed a series of 967 neck dissections[20]. In this series, a subset of patients had undergone SND for N1 disease. He noted a 7% recurrence rate when these patients were treated with surgery alone and concluded that that this was adequate treatment for the N1 neck. He also noted that patients with lymph node extracapsular spread benefitted from postoperative radiation therapy. Andersen, et al. [21] also showed that MRND resulted in good regional control in their review of 129 MRND (Comprehensive neck dissection Type I, preserving only the SAN) for node positive disease at Memorial Sloan-Kettering Cancer Center. They noted only a 6% failure rate and also noted poorer outcomes with extracapsular spread. In particular, MRND was able to preserve the SAN while remaining oncologically effective. These and many other studies suggested that node positive necks could also be treated with more conservative neck surgery, reducing the morbidity of treatment.
Our understanding of neck lymph node drainage patterns and the sequential progression of nodal metastasis for head and neck cancer has supported the evolution of management of these tumors. While the RND was a breakthrough in the treatment of regional disease, it clearly came at the price of patient morbidity. As our understanding of lymph node drainage has become more refined, this has changed the surgery for regional disease from RND to MRND to SND. For the node positive neck, MRND Type I (preserving the spinal accessory nerve) has been shown to be as effective as RND for treatment. This is a vivid example of how refinements in surgical technique coupled with improved understanding have led to less morbid treatment of head and neck disease, while maintaining comparable oncologic control.
Minimally Invasive Thyroid and Parathyroid Surgery
Thyroidectomy has seen tremendous changes since the surgery was nearly banned by the French Academy of Medicine in the 1800’s. [22] Today, thyroidectomy is routinely performed with low morbidity and very low mortality. The introduction of minimally invasive thyroid surgery (MITS) attempts to further improve on this operation by addressing aesthetic outcomes and postoperative pain.
Traditional thyroidectomy through a low cervical incision is a well established procedure with very low morbidity and quite acceptable aesthetic outcome. Over the last decade, surgeons have evaluated the safety of discharging patients the same day after lobectomy [23–25], the need for postoperative drains[26–28], and the feasibility of thyroidectomy under local anesthesia.[25, 29] It is not surprising that as thyroid surgery has become less invasive, a variety of “minimally invasive” techniques have also been considered. These include Minimally Invasive Video Assisted Thyroid Surgery (MIVAT) [30], the mini-lateral approach[31, 32], and even extracervical approaches such as the trans-axillary approach and indirect breast approach [33, 34]. In fact, “natural orifice” surgery via a transoral approach to thyroidectomy has recently been reported in a cadaver study. [35]
All minimally invasive thyroid approaches retain similar features and have the goal of minimizing the size of the incision and postoperative pain. The thyroid is approached from an incision smaller than the traditional incision. In some variations, the incision is placed ipsilateral to the side of the lesion, rather than at the midline. Video-assisted endoscopic techniques are often employed to enhance visualization. In all cases, however, identification of the recurrent laryngeal nerve, usually the superior laryngeal nerve, and preservation of the parathyroid glands are still key portions of the operation. Careful patient selection is also important. MITS appears to be best suited for solitary thyroid nodules less than 3 cm in size. Prior neck surgery, morbid obesity, poor anatomy, and multinodular disease are all considered relative contraindications to this surgery. [31]
Multiple evidence-based reviews of minimally invasive thyroid surgical procedures were recently published in the World Journal of Surgery. [31, 33, 34, 36] The strongest evidence in support of the safety and efficacy of MITS were five randomized prospective trials comparing MITS in the form of MIVAT to conventional thyroidectomy. [30, 36–40] Over the five trials, 275 patients were randomized. All studies showed no significant difference in permanent recurrent laryngeal nerve injury, hematoma, mortality, or infection. To evaluate whether the same completeness of resection is achieved with both approaches, Miccoli, et al. compared MIVAT versus conventional surgery for patients with documented thyroid cancer on fine needle aspiration in two studies [40, 41]. Postoperative completeness of resection was assessed by comparing Iodine-131 uptake in the thyroid bed postoperatively. They found no significant differences, thereby demonstrating comparable completeness of resection. The advantages of MIVAT over conventional surgery was apparent in these studies. Patients undergoing MIVAT noted improved cosmesis [30, 37, 39] and less postoperative pain [30, 39] in multiple studies.
These data demonstrate that MITS matches the safety and efficacy of conventional thyroidectomy while easing the postoperative recovery and improving cosmesis. Although operative time [38] is still longer than conventional surgery, this should improve as surgeon experience grows. Taken together, thyroid surgery has advanced greatly from its early days as a morbid operation. Great strides have been made in improving its safety such that mortality from this surgery now approaches zero. Although MITS represents the next step in the continued refinement of this technique, caution must be exercised in selection of cases for MITS. At present, patients with thin necks and small intrathyroidal tumors without nodal metastasis are considered the best candidates for MITS. The learning curve for this technique is steep and a significant volume is essential to gain expertise.
It is worth noting that parathyroidectomy has undergone a similar evolution. In the past, four gland parathyroid exploration for hyperparathyroidism was the standard of treatment. [42] Multiple technological and surgical advancements have since resulted in a shift away from this traditional treatment. Localization studies, whether through sestamibi scanning[43], ultrasound, or CT scanning [44] have resulted in dramatically improved accuracy in determining the location of parathyroid adenomas. Improvements in surgical technique using video-assisted technologies and smaller incisions have resulted in a more focused approach to parathyroidectomy, resulting in lower morbidity through a smaller incision [45]. Finally, the measurement of intraoperative parathyroid hormone (PTH) levels in parathyroid surgery now allows confirmation of a successful operation before the patient is awakened from anesthesia. [46, 47] These advances have paved the way for minimally invasive parathyroidectomy (MIP) to become a low morbidity procedure with a shorter recovery time, yet retaining a high rate of success due to the use of intraoperative PTH monitoring. This evolution has paralleled the development of MITS and together they are a good example of how surgical innovation has improved patient outcomes.
Fibula and Free Flaps in Oromandibular Reconstruction
Advanced neoplasms of the oral cavity often require composite resection (“Commando” operation) to achieve clear surgical margins. The resulting bony and soft tissue defect has posed a challenging reconstructive problem. The transition from delayed secondary reconstruction to primary reconstruction using vascularized bone flaps over the last thirty years has yielded significant functional and aesthetic improvements.
The shape and contour of the mandible contribute significantly to the shape and expression of the lower third of the face. Indeed mandibular projection is often addressed in cosmetic surgery to complement the aesthetics of the nose and other midfacial areas [48]. In oncologic surgery, resection of the anterior mandibular arch or hemi-mandible may be necessary to obtain clear oncologic margins. These resections can result in significant functional and aesthetic sequelae. For example, loss of the anterior mandibular arch was termed an “Andy Gump” deformity, after the well known cartoon character.
In addition, bone and soft tissue loss directly affect facial appearance and expression as well as mastication and deglutition. When chewing, significant load forces are applied to the mandible to reduce the food to a soft bolus. Loss of mandibular continuity results in ineffective chewing because those load forces can no longer be transferred appropriately. Loss of bone also results in misaligned dental occlusion, which further limits effective chewing. The dead space generated from soft tissue loss also prevents effective manipulation of the oral food bolus causing trapping. Retained food is not only a nidus for infection but results in halitosis and difficult oral care. Finally, loss of sensation and motor function of the lip after resection affects deglutition due to loss of oral competence. Food cannot be propelled posteriorly and swallowed. Instead, it escapes through the incompetent os. The net result of these surgeries without proper reconstruction is long term dependency on an altered diet, bad breath, and an unsightly appearance.
Given these considerations, reconstruction of oromandibular bony and soft tissue defects has always been an important consideration following oncologic resection. In the 1970’s, primary bony reconstruction was avoided in favor of delayed (secondary) reconstruction. At that time, primary bone reconstruction was limited to non-vascularized reconstruction using corticocancellous bony chips harvested from the iliac crest placed in a prosthetic mesh. The placement of either a foreign body or non-vascularized bone in a saliva-contaminated wound resulted in frequent wound complications 50% of the time. [49, 50] Delayed reconstruction was therefore preferred.
However, this delay was not without its problems. The interval healing time resulted in dental occlusive drift, local scarring, and fibrosis of the muscles of mastication secondary to loss of mandibular continuity. In addition, many of these patients had received postoperative radiation. This made for a challenging secondary operation. Furthermore, due to the scarring and fibrosis, postoperative masticatory function rarely approached preoperative function.
Thirty years later, the regular use of vascularized free flaps for primary reconstruction has dramatically changed outcomes following oromandibular resection, with the fibula free flap (FFF) as the workhorse flap for mandible reconstruction. The fibula was reported by Taylor from Australia [51] and was popularized by Hidalgo [52] from Memorial Sloan-Kettering Cancer Center. The FFF is supplied by the peroneal artery and can supply up to 25cm of bone, as well as a small paddle of skin and soft tissue. Through osteotomies and plating, the fibula can be shaped into the contour of the resected mandible. Reconstruction is now performed at the time of ablative surgery with immediate inset into the oral cavity.
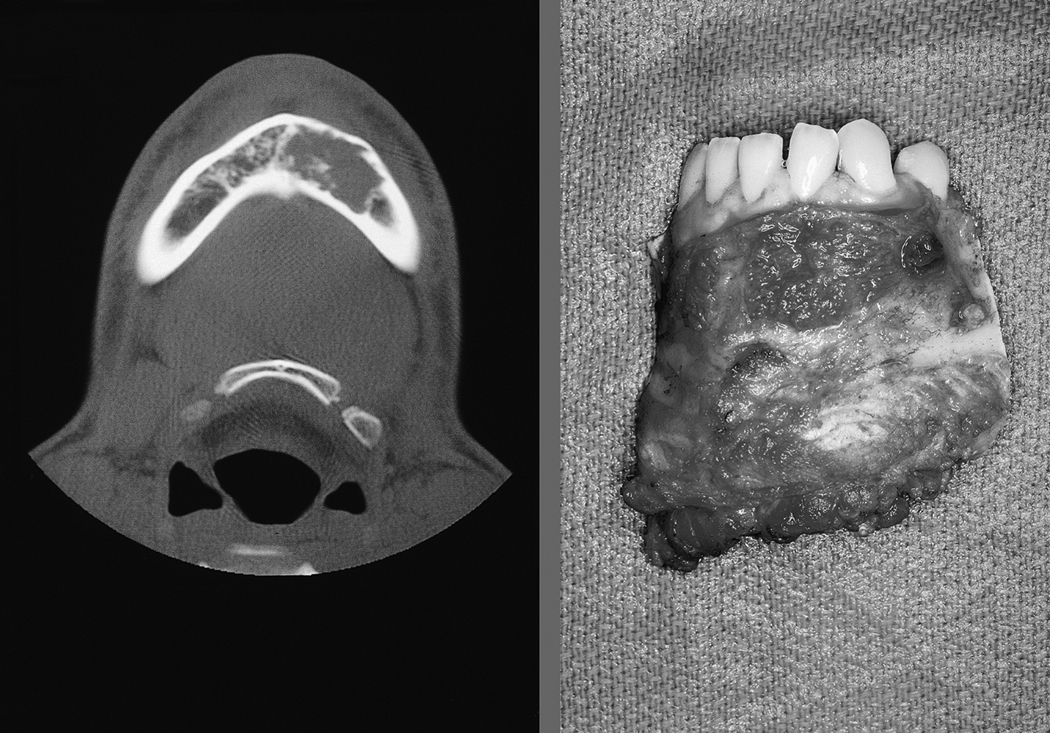
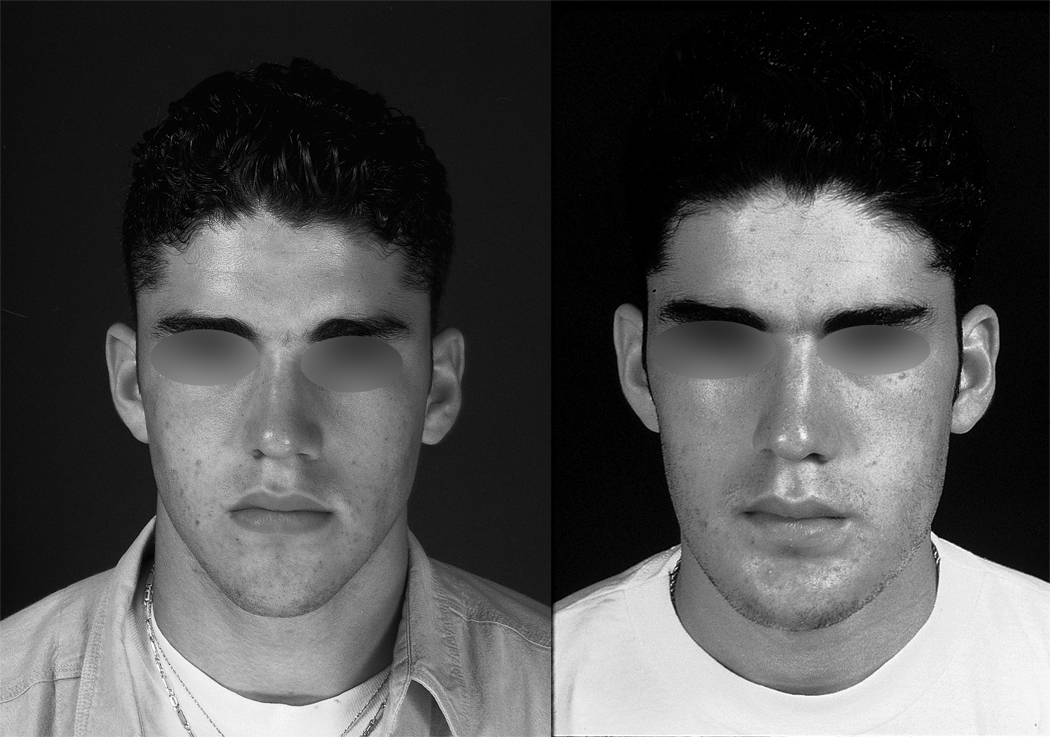
This advancement in technique addresses many of the shortcomings outlined earlier. The use of a vascularized flap allows its survival in a saliva-contaminated wound. Immediate reconstruction avoids the scarring and fibrosis seen from delayed reconstruction. Overall aesthetic appearance is improved because the flap re-creates the resected bone. Finally, bony continuity is restored, allowing eventual use of dentures or placement of osseointegrated dental implants. [53] Most patients can expect to resume a regular diet after healing from surgery. An example of the postoperative appearance following FFF is seen in Figure 7.
Figure 7.
a) Axial CT scan image of the mandible of a young patient with low grade chondrosarcoma of the mandible (left) and the surgical specimen after segmental resection. (right)
b) Preoperative (left) and postoperative (right) appearance after fibula free flap reconstruction.
A follow-up study of Hidalgo’s experience with the FFF demonstrates the excellent form and function after free flap reconstruction. [54] Twenty patients with a mean follow-up of 11 years were retrospectively evaluated for their outcomes following free flap reconstruction after mandible resection. All but one had undergone FFF reconstruction. Aesthetic outcome, as judged by two observers, was judged to be excellent in 55 percent of patients and good in 20 percent. All patients, except those that had undergone glossectomy as part of the original resection, had easily intelligible speech. Fourteen (70%) patients tolerated a regular diet, with the remaining tolerating a soft diet. Finally, five patients had undergone osseointegrated implants, allowing function without the need for dentures.
Other free flaps that have been successful for oromandibular reconstruction include the iliac crest flap [55], scapular flap [56], and osteocutaneous radial forearm free flap [57]. Through the use of vascularized flaps in primary reconstruction, patients undergoing oromandibular resection can expect reasonable aesthetic outcomes and excellent preservation of oral function. This surgical refinement illustrates a significant step forward in the treatment of oral cancers.
Transoral Laser Microsurgery for Laryngeal Cancer
Laryngeal cancer can be a devastating disease because cancer often affects the larynx’s multiple functions: phonation, deglutition, and breathing. While early (T1a) laryngeal lesions can be resected transorally with phonosurgical instruments as an outpatient, management of advanced cancers (T3 and T4) frequently requires open resection and protracted rehabilitation. Total laryngectomy is still unparalleled in its ability to achieve local control for advanced cancers, but generates a permanent stoma and permanently affects speaking and swallowing. Since its introduction by Strong and Jako in 1972 [58], the CO2 laser has gained wider use in the treatment of larynx cancer. Initially used in early glottic lesions, its role has now expanded to advanced glottic, supraglottic, and hypopharyngeal tumors as well. Transoral Laser Microsurgery (TLM) with the CO2 laser has allowed larynx preservation, even with advanced cancers, to yield superior functional and aesthetic outcomes over traditional approaches.
Prior to the introduction of TLM, surgical management of early laryngeal tumors that extended beyond a single vocal cord (T1b or T2) were limited to an open approach by Vertical Partial Laryngectomy (VPL). Many variations of VPL exist, including vertical anterior, vertical frontolateral, partial and hemi-laryngectomies [59–61], and the choice of operation was dependent on the extent of the tumor. In general, these surgeries approached the larynx by a laryngofissure approach, with resection performed en bloc to include tumor, soft tissue, and associated thyroid cartilage. Reconstruction with local flaps was occasionally necessary to compensate for resected soft tissue. A temporary tracheotomy was frequently needed during the healing period. The impact on function was significant because the partial loss of laryngeal structure affected all laryngeal functions: phonation, swallowing, breathing, and protection from aspiration.
In the 1990’s, Steiner [62] demonstrated that the CO2 laser could be effective in treatment of early laryngeal lesions, without the need for open surgery. He operated on 159 early glottic lesions, of which 60% were T1 tumors and 22% were T2 tumors, using the CO2 laser. Although local recurrences were seen in 10 (6%) of these patients, all patients were salvaged and none of these patients died due to recurrent or distant disease. No patients required a tracheostomy and voice quality was defined as “satisfactory” in 92% of patients. The lack of a tracheostomy and preservation of voice was a significant reduction in morbidity when compared with VPL. This study and others [63] demonstrated that these early laryngeal glottic cancers could be successfully managed by TLM with good local control.
The theory and technique of TLM represented a radical departure from traditional oncologic principles. Instead of resecting the tumor en bloc as in VPL, Steiner and others performed their surgeries by dividing the tumor and removing the tumor in planned segmental excisions. The tumor was first carefully assessed by palpation using phonosurgical instruments, and carefully inspected with the operating microscope for its boundaries and submucosal extension. Depth of invasion by the tumor was assessed by examining its mobility over the underlying tissues. The CO2 laser was then used as a cutting, rather than vaporizing, instrument to divide the tumor into segments that were each resected. Each segment was oriented and margins were assessed. At the end of the case, surgical margins from the defect were also sent. The wound was left to heal by secondary intention with no reconstruction. The net result was an improvement in functional and aesthetic outcomes, while preserving oncologic control as evidenced above. Because the larynx was preserved, voice function and swallowing were better.
Since the work of Steiner and others, TLM has become an accepted treatment option for early glottic lesions. This same technique has also been explored for supraglottic lesions and advanced tumors of the larynx. In 1998 Iro, et al. reported on their experience using TLM for supraglottic tumors. [64] Their results showed that the oncologic outcome following TLM for these tumors is comparable to traditional open procedures as long as negative resection margins could be achieved. Hinni, et al. [65] reported on the use of TLM for advanced disease (T2–T4, Stage III and IV) at both glottic and supraglottic subsites. Their oncologic outcomes for local control were comparable to rates reported for chemoradiation.
It is important to note that TLM only addresses the primary tumor site for local control, and does not address regional neck disease. Especially for supraglottic tumors and advanced cancers, regional metastasis needs to be addressed separately either through neck dissection, radiation therapy, or both.
Taken together, TLM has proved to be a new and effective means of surgical management of laryngeal cancer. The oncologic results are comparable to those achieved with traditional open methods, but patients experience significantly better functional outcomes with less postoperative morbidity. So far, oncologic outcomes with TLM have been on par with radiation and chemoradiation outcomes. Further studies are underway to evaluate posttreatment function after these modalities to better compare surgical and non-surgical modalities for laryngeal cancer treatment. Nevertheless, the introduction of the CO2 laser for larynx cancer has significantly contributed to the surgical options for this disease.
Conclusion
The progress in surgical management in each of the five areas outlined above illustrate how advances in technique, technology, and our understanding of biology of tumor progression have led to alteration in oncologic surgery with less morbidity and preservation of function. In the head and neck this is particularly important because of the close proximity of critical structures, the complex functions, and aesthetic concerns. We hope to see the continued evolution of surgical techniques so that our patients may benefit from the reduced morbidity and better outcomes.
Acknowledgments
Jeffrey Liu’s work on this project is supported by Award Number T32CA009685 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Neither author has any disclosures.
Contributor Information
Jeffrey C. Liu, Fellow, Head and Neck Service, Memorial Sloan-Kettering Cancer Center LiuJ2@mskcc.org.
Jatin P. Shah, Check, Head and Neck Service, Memorial Sloan-Kettering Cancer Center shahj@mskcc.org.
Bibliography
- 1.Bailey H. The treatment of tumors of the parotid gland. Br J Surg. 1941;(111):337–346. [Google Scholar]
- 2.Butlin HTSB SW. Disease of the Tongue, etc. London: Cassell and Co; 1900. [Google Scholar]
- 3.GW C. On the surgical treatment of cancer of the head and neck. With a summary of one hundred and twenty-one operations performed upon one hundred and five patients. Trans South Surg Gynecol Assoc. 1905;(18):108–127. [Google Scholar]
- 4.GW C. Excision of cancer of the head and neck. With special reference to the plan of dissection based on one hundred and thirty two operations. J Am Med Assoc. 1906;47:1780–1786. [Google Scholar]
- 5.Ferlito A, et al. Neck dissection: then and now. Auris Nasus Larynx. 2006;33(4):365–374. doi: 10.1016/j.anl.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Givi B, Andersen PE. Rationale for modifying neck dissection. J Surg Oncol. 2008;97(8):674–682. doi: 10.1002/jso.21016. [DOI] [PubMed] [Google Scholar]
- 7.Martin H, et al. Neck dissection. Cancer. 1951;4(3):441–499. doi: 10.1002/1097-0142(195105)4:3<441::aid-cncr2820040303>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Nahum AM, Mullally W, Marmor L. A syndrome resulting from radical neck dissection. Arch Otolaryngol. 1961;74:424–428. doi: 10.1001/archotol.1961.00740030433011. [DOI] [PubMed] [Google Scholar]
- 9.Terrell JE, et al. Pain, quality of life, and spinal accessory nerve status after neck dissection. Laryngoscope. 2000;110(4):620–626. doi: 10.1097/00005537-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Suarez O. El problema de las metastasis linfaticas y alejadas del cancer de laringe e hipofaringe. Rev Otorrinolaringol. 1963;(23):804–808. [Google Scholar]
- 11.Bocca E, et al. Functional neck dissection: an evaluation and review of 843 cases. Laryngoscope. 1984;94(7):942–945. doi: 10.1288/00005537-198407000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Gavilan C, Gavilan J. Five-year results of functional neck dissection for cancer of the larynx. Arch Otolaryngol Head Neck Surg. 1989;115(10):1193–1196. [PubMed] [Google Scholar]
- 13.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446–1449. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10(3):160–167. doi: 10.1002/hed.2890100304. [DOI] [PubMed] [Google Scholar]
- 15.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405–409. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 16.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994;120(7):699–702. doi: 10.1001/archotol.1994.01880310005001. [DOI] [PubMed] [Google Scholar]
- 17.Results of a prospective trial on elective modified radical classical versus supraomohyoid neck dissection in the management of oral squamous carcinoma. Brazilian Head and Neck Cancer Study Group. Am J Surg. 1998;176(5):422–427. doi: 10.1016/s0002-9610(98)00230-x. [DOI] [PubMed] [Google Scholar]
- 18.End results of a prospective trial on elective lateral neck dissection vs type III modified radical neck dissection in the management of supraglottic and transglottic carcinomas. Brazilian Head and Neck Cancer Study Group. Head Neck. 1999;21(8):694–702. doi: 10.1002/(sici)1097-0347(199912)21:8<694::aid-hed3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Davidson BJ, et al. Posterior triangle metastases of squamous cell carcinoma of the upper aerodigestive tract. Am J Surg. 1993;166(4):395–398. doi: 10.1016/s0002-9610(05)80340-x. [DOI] [PubMed] [Google Scholar]
- 20.Byers RM. Modified neck dissection. A study of 967 cases from 1970 to 1980. Am J Surg. 1985;150(4):414–421. doi: 10.1016/0002-9610(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 21.Andersen PE, et al. The role of comprehensive neck dissection with preservation of the spinal accessory nerve in the clinically positive neck. Am J Surg. 1994;168(5):499–502. doi: 10.1016/s0002-9610(05)80110-2. [DOI] [PubMed] [Google Scholar]
- 22.Giddings AE. The history of thyroidectomy. J R Soc Med. 1998;91 Suppl 33:3–6. doi: 10.1177/014107689809133s02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teoh AY, Tang YC, Leong HT. Feasibility study of day case thyroidectomy. ANZ J Surg. 2008;78(10):864–866. doi: 10.1111/j.1445-2197.2008.04681.x. [DOI] [PubMed] [Google Scholar]
- 24.Mishra AK, Agarwal A. Same-day discharge after total thyroidectomy: the value of 6-hour serum parathyroid hormone and calcium levels. Head Neck. 2005;27(12):1112. doi: 10.1002/hed.20289. author reply 1112–3. [DOI] [PubMed] [Google Scholar]
- 25.Inabnet WB, et al. Safety of same day discharge in patients undergoing sutureless thyroidectomy: a comparison of local and general anesthesia. Thyroid. 2008;18(1):57–61. doi: 10.1089/thy.2007.0148. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins C, Mansuri S, Terry RM. How we do it: Dispensing with drains in hemithyroidectomy--a feasibility study. Clin Otolaryngol. 2006;31(5):452–455. doi: 10.1111/j.1749-4486.2006.01199.x. [DOI] [PubMed] [Google Scholar]
- 27.Morrissey AT, et al. Comparison of drain versus no drain thyroidectomy: randomized prospective clinical trial. J Otolaryngol Head Neck Surg. 2008;37(1):43–47. [PubMed] [Google Scholar]
- 28.Ruark DS, Abdel-Misih RZ. Thyroid and parathyroid surgery without drains. Head Neck. 1992;14(4):285–287. doi: 10.1002/hed.2880140405. [DOI] [PubMed] [Google Scholar]
- 29.Lo Gerfo P. Local/regional anesthesia for thyroidectomy: evaluation as an outpatient procedure. Surgery. 1998;124(6):975–978. discussion 978–9. [PubMed] [Google Scholar]
- 30.Miccoli P, et al. Comparison between minimally invasive video-assisted thyroidectomy and conventional thyroidectomy: a prospective randomized study. Surgery. 2001;130(6):1039–1043. doi: 10.1067/msy.2001.118264. [DOI] [PubMed] [Google Scholar]
- 31.Alvarado R, et al. Minimally invasive thyroid surgery for single nodules: an evidence-based review of the lateral mini-incision technique. World J Surg. 2008;32(7):1341–1348. doi: 10.1007/s00268-008-9554-4. [DOI] [PubMed] [Google Scholar]
- 32.Sywak MS, et al. A randomized controlled trial of minimally invasive thyroidectomy using the lateral direct approach versus conventional hemithyroidectomy. Surgery. 2008;144(6):1016–1021. doi: 10.1016/j.surg.2008.07.024. discussion 1021–2. [DOI] [PubMed] [Google Scholar]
- 33.Tan CT, Cheah WK, Delbridge L. "Scarless" (in the neck) endoscopic thyroidectomy (SET): an evidence-based review of published techniques. World J Surg. 2008;32(7):1349–1357. doi: 10.1007/s00268-008-9555-3. [DOI] [PubMed] [Google Scholar]
- 34.Slotema ET, Sebag F, Henry JF. What is the evidence for endoscopic thyroidectomy in the management of benign thyroid disease? World J Surg. 2008;32(7):1325–1332. doi: 10.1007/s00268-008-9505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benhidjeb T, et al. Natural orifice surgery on thyroid gland: totally transoral video-assisted thyroidectomy (TOVAT): report of first experimental results of a new surgical method. Surg Endosc. 2009;23(5):1119–1120. doi: 10.1007/s00464-009-0347-0. [DOI] [PubMed] [Google Scholar]
- 36.Miccoli P, et al. Minimally invasive video-assisted thyroidectomy for benign thyroid disease: an evidence-based review. World J Surg. 2008;32(7):1333–1340. doi: 10.1007/s00268-008-9479-y. [DOI] [PubMed] [Google Scholar]
- 37.Bellantone R, et al. Video-assisted vs conventional thyroid lobectomy: a randomized trial. Arch Surg. 2002;137(3):301–304. doi: 10.1001/archsurg.137.3.301. discussion 305. [DOI] [PubMed] [Google Scholar]
- 38.Chao TC, Lin JD, Chen MF. Video-assisted open thyroid lobectomy through a small incision. Surg Laparosc Endosc Percutan Tech. 2004;14(1):15–19. doi: 10.1097/00129689-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Lombardi CP, et al. Safety of video-assisted thyroidectomy versus conventional surgery. Head Neck. 2005;27(1):58–64. doi: 10.1002/hed.20118. [DOI] [PubMed] [Google Scholar]
- 40.Miccoli P, et al. Minimally invasive video-assisted thyroidectomy for papillary carcinoma: a prospective study of its completeness. Surgery. 2002;132(6):1070–1073. doi: 10.1067/msy.2002.128694. discussion 1073–4. [DOI] [PubMed] [Google Scholar]
- 41.Miccoli P, et al. Surgical treatment of low- and intermediate-risk papillary thyroid cancer with minimally invasive video-assisted thyroidectomy. J Clin Endocrinol Metab. 2009;94(5):1618–1622. doi: 10.1210/jc.2008-1418. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan EL, Yashiro T, Salti G. Primary hyperparathyroidism in the 1990s. Choice of surgical procedures for this disease. Ann Surg. 1992;215(4):300–317. doi: 10.1097/00000658-199204000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thule P, et al. Preoperative localization of parathyroid tissue with technetium-99m sestamibi 123I subtraction scanning. J Clin Endocrinol Metab. 1994;78(1):77–82. doi: 10.1210/jcem.78.1.8288719. [DOI] [PubMed] [Google Scholar]
- 44.Hopkins CR, Reading CC. Thyroid and parathyroid imaging. Semin Ultrasound CT MR. 1995;16(4):279–295. doi: 10.1016/0887-2171(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 45.Barczynski M, et al. Minimally invasive video-assisted parathyroidectomy versus open minimally invasive parathyroidectomy for a solitary parathyroid adenoma: a prospective, randomized, blinded trial. World J Surg. 2006;30(5):721–731. doi: 10.1007/s00268-005-0312-6. [DOI] [PubMed] [Google Scholar]
- 46.Carneiro-Pla D. Recent findings in the use of intraoperative parathyroid hormone monitoring in parathyroid disease. Curr Opin Oncol. 2009;21(1):18–22. doi: 10.1097/cco.0b013e328319ec2f. [DOI] [PubMed] [Google Scholar]
- 47.Shindo M. Intraoperative rapid parathyroid hormone monitoring in parathyroid surgery. Otolaryngol Clin North Am. 2004;37(4):779–787. doi: 10.1016/j.otc.2004.02.009. ix. [DOI] [PubMed] [Google Scholar]
- 48.Chang EW, et al. Sliding genioplasty for correction of chin abnormalities. Arch Facial Plast Surg. 2001;3(1):8–15. [PubMed] [Google Scholar]
- 49.Komisar A, Warman S, Danziger E. A critical analysis of immediate and delayed mandibular reconstruction using A-O plates. Arch Otolaryngol Head Neck Surg. 1989;115(7):830–833. doi: 10.1001/archotol.1989.01860310068025. [DOI] [PubMed] [Google Scholar]
- 50.Lawson W, et al. Experience with immediate and delayed mandibular reconstruction. Laryngoscope. 1982;92(1):5–10. doi: 10.1288/00005537-198201000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55(5):533–544. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84(1):71–79. [PubMed] [Google Scholar]
- 53.Urken ML, et al. Primary placement of osseointegrated implants in microvascular mandibular reconstruction. Otolaryngol Head Neck Surg. 1989;101(1):56–73. doi: 10.1177/019459988910100111. [DOI] [PubMed] [Google Scholar]
- 54.Hidalgo DA, Pusic AL. Free-flap mandibular reconstruction: a 10-year follow-up study. Plast Reconstr Surg. 2002;110(2):438–449. doi: 10.1097/00006534-200208000-00010. discussion 450–1. [DOI] [PubMed] [Google Scholar]
- 55.Shpitzer T, et al. The free iliac crest and fibula flaps in vascularized oromandibular reconstruction: comparison and long-term evaluation. Head Neck. 1999;21(7):639–647. doi: 10.1002/(sici)1097-0347(199910)21:7<639::aid-hed8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 56.Baker SR, Sullivan MJ. Osteocutaneous free scapular flap for one-stage mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1988;114(3):267–277. doi: 10.1001/archotol.1988.01860150049015. [DOI] [PubMed] [Google Scholar]
- 57.Zenn MR, et al. Current role of the radial forearm free flap in mandibular reconstruction. Plast Reconstr Surg. 1997;99(4):1012–1017. doi: 10.1097/00006534-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Strong MS, Jako GJ. Laser surgery in the larynx. Early clinical experience with continuous CO 2 laser. Ann Otol Rhinol Laryngol. 1972;81(6):791–798. doi: 10.1177/000348947208100606. [DOI] [PubMed] [Google Scholar]
- 59.Mohr RM, Quenelle DJ, Shumrick DA. Vertico-frontolateral laryngectomy (hemilaryngectomy). Indications, technique, and results. Arch Otolaryngol. 1983;109(6):384–395. doi: 10.1001/archotol.1983.00800200030010. [DOI] [PubMed] [Google Scholar]
- 60.Norris CM. Technique of extended fronto-lateral partial laryngectomy. Laryngoscope. 1958;68(7):1240–1250. doi: 10.1002/lary.5540680709. [DOI] [PubMed] [Google Scholar]
- 61.Som ML, Silver CE. The anterior commissure technique of partial laryngectomy. Arch Otolaryngol. 1968;87(2):138–145. doi: 10.1001/archotol.1968.00760060140008. [DOI] [PubMed] [Google Scholar]
- 62.Steiner W. Results of curative laser microsurgery of laryngeal carcinomas. Am J Otolaryngol. 1993;14(2):116–121. doi: 10.1016/0196-0709(93)90050-h. [DOI] [PubMed] [Google Scholar]
- 63.Eckel HE, Thumfart WF. Laser surgery for the treatment of larynx carcinomas: indications, techniques, and preliminary results. Ann Otol Rhinol Laryngol. 1992;101(2 Pt 1):113–118. doi: 10.1177/000348949210100202. [DOI] [PubMed] [Google Scholar]
- 64.Iro H, et al. Transoral laser surgery of supraglottic cancer: follow-up of 141 patients. Arch Otolaryngol Head Neck Surg. 1998;124(11):1245–1250. doi: 10.1001/archotol.124.11.1245. [DOI] [PubMed] [Google Scholar]
- 65.Hinni ML, et al. Transoral laser microsurgery for advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1198–1204. doi: 10.1001/archotol.133.12.1198. [DOI] [PubMed] [Google Scholar]