Graphical abstract
The synthesis of α-l-fucosyl ceramide derivatives and their biological evaluation is described.
Keywords: CD1d, iNKT, Antigen, Ceramide, Lipid
Abstract
Several l-fucoglycolipids are associated with diseases such as cancer, cystic fibrosis and rheumatoid arthritis. Activation of iNKT cells is known to lead to the production of cytokines that can help alleviate or exacerbate these conditions. α-Galactosyl ceramide (α-GalCer) is a known agonist of iNKT cells and it is believed that its fucosyl counterpart might have similar immunogenic properties. We herein report the synthesis of α-l-fucosyl ceramide derivatives and describe their biological evaluation. The key challenge in the synthesis of the target molecules involved the stereoselective synthesis of the α-glycosidic linkage. Of the methods examined, the per-TMS-protected glycosyl iodide donor was completely α-selective, and could be scaled up to provide gram quantities of the azide precursor 11, from which a range of N-acylated α-l-fucosyl ceramides were readily obtained and evaluated for ex vivo expansion of human iNKT cells.
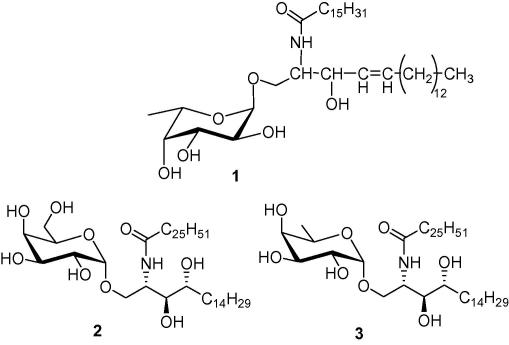
Both d- and l-fucose (6-deoxy galactose) are widely found in nature.1 Of interest, l-fucose is predominantly found in the α-configuration in the lipopolysaccharides (LPS) of Gram-negative bacteria and animal glycosphingolipids.1,2 With the exception of the monohexylceramide, α-l-fucosylceramide 1, initially isolated from metastatic human adenocarcinoma,3 most fucosphingolipids are usually ceramide oligosaccharides.2,4 Many of these fucolipids are antigenic5–7 and play a role in tumour cell biology.8–10 Conversely, the synthetic glycolipid, α-galactosyl ceramide (α-GalCer)11 (2), is a strong agonist of iNKT cells when bound to CD1d, triggering the release of diverse cytokines, including both Th1 and Th2 cytokines.12–14 It is believed that the release of Th1 cytokines may contribute to antitumour and antimicrobial functions while that of Th2 cytokines may help alleviate autoimmune diseases15–17 such as multiple sclerosis18 and arthritis.19
α-GalCer and its derivatives have proved to be and remain invaluable tools in understanding the functioning of CD1d and iNKT cells in a wide range of immune responses. Crystal structures of the bound α-GalCer–CD1d complex20–22 showed that the hydroxyl group at C-6 in the sugar head is not crucial for binding to the T-cell receptor (TCR) as opposed to C-2, C-3 and C-4. Hence, the α-d-fucosylceramide 323 was shown to be a potent inducer of IFN-γ in mice in vivo, while a slightly different derivative, synthesised by Motoki et al.24 showed strong lymphocytic proliferation stimulatory effects in vitro in mice.
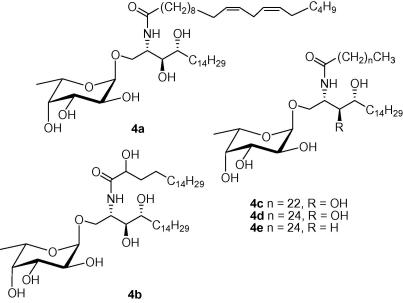
While there are a few examples describing the synthesis of α-l-fucosylceramide 4b25 and other derivatives in the literature,26,27 their biological activities are yet to be fully explored. As part of various ongoing studies, and because of the aforementioned reasons, we have synthesised compounds 4a–4d for biological evaluation and comparative studies.
Fan et al.25 reported the synthesis of compound 4d, while Okamoto et al.27 reported that of compound 4e. Both groups obtained their target compounds as an α, β mixture (3:1 ratio) from different glycosyl donors and acceptors (Scheme 1). On the other hand, in the original reported synthesis of the fucosylceramide 1, only the α-anomer was isolated when another ceramide acceptor was used under different reaction conditions.
Scheme 1.
(a) Me2S, 2-Cl-pyridine, Tf2O, CH2Cl2; (b) dimethyl(methylthio)sulfonium triflate (DMTST), CH2Cl2, 0 °C-rt.
We recently reported the synthesis28 of α-GalCer and other derivatives where we used N-iodosuccinimide (NIS) and triflic acid (TfOH) as a promoter, and benzoate protecting groups on the sphingosine base. Along with the nature of the protecting groups on both the glycosyl acceptor and donor, the choice of promoter, as well as temperature and reaction times can affect the stereoselectivity of the glycosylation reaction. In this study, we proposed to investigate whether our glycosyl acceptor 7 and reaction conditions could offer a better α-selectivity in the case of l-fucose.
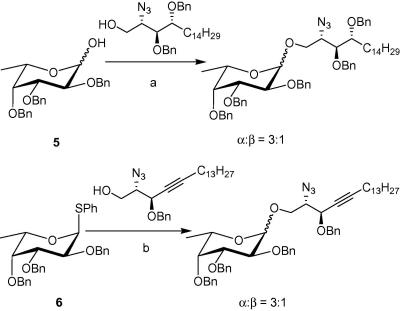
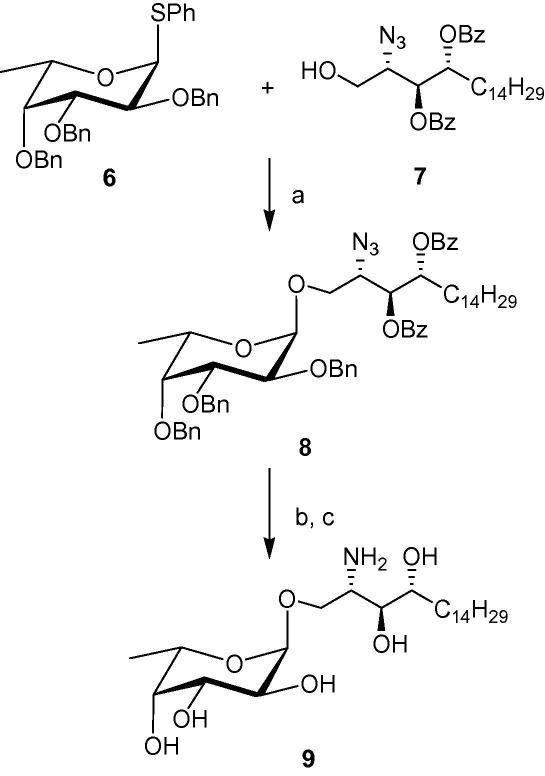
The phytosphingosine acceptor 729 was synthesised from (2S,3S,4R)-2-azido-1,3,4-octadecanetriol30 as described before. Given the previous success of a thiogalactoside in a similar glycosylation reaction with compound 728 we chose to use the thiofucosyl donor 6, rather than compound 5. The thioglycoside 6 was thus obtained from commercially available l-fucose after standard procedures, as previously reported.31
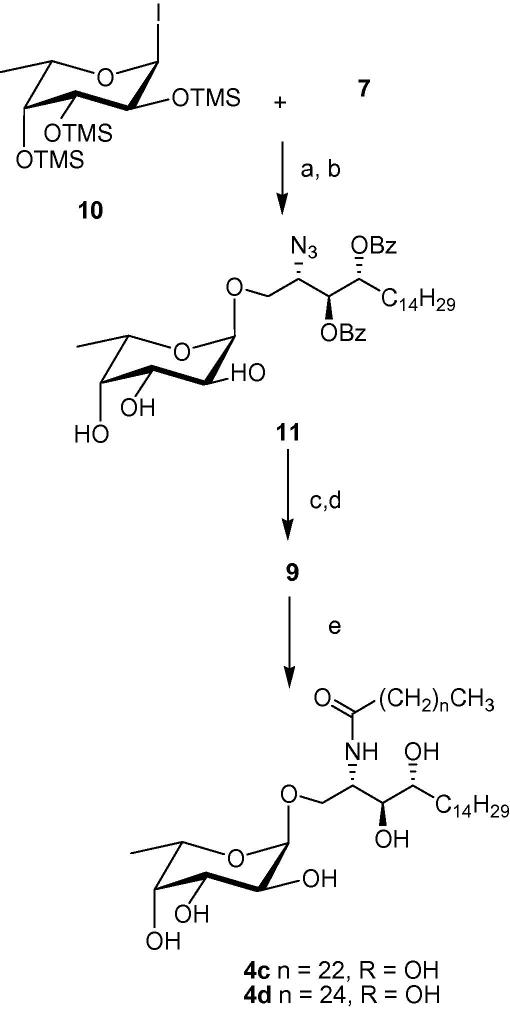
With both the acceptor and donor in hand, we then proceeded to the critical glycosylation reaction. Interestingly, NIS/TfOH activation of 1 g of the thioglycoside 6 (Scheme 2) in anhydrous CH2Cl2 at −78 °C afforded the glycosylated compound 8, almost exclusively as the α-anomer (α:β ratio = 9:1), in 68% yield after 2 h. Our results show a definite improvement in selectivity from the previously reported syntheses. Because the formation of the α-anomer is favoured by the anomeric effect, we rationalise that the latter is a governing factor under our reaction conditions. Both the lower temperature and the different reactivity of the acceptor 7 could potentially influence the stereoselectivity of the glycosylation reaction. These factors will have to be more thoroughly investigated in a later study. Subsequent Zemplen’s deprotection of the benzoate protecting groups produced the azide intermediate in quantitative yields. Tandem hydrogenation of the azido group and hydrogenolysis of the benzyl ethers in methanol then produced the amine 9 as a white solid, which exhibited spectroscopic data consistent with the literature.25
Scheme 2.
(a) NIS/TfOH, CH2Cl2, −78 °C to −20 °C, 68%; (b) NaOMe/MeOH, 92%; (c) H2, Pd, 76%.
In an attempt to improve the stereoselectivity and circumvent the sometimes problematic removal of the benzyl ethers by hydrogenolysis, we embarked on a different glycosylation route. Recently, the use of glycosyl iodide donors has been revived by Gervay-Hague’s group.32,33 They have demonstrated that glycosylation reactions employing glycosyl iodides and promoted by tetrabutyl ammonium iodide (TBAI) are generally quite stereoselective and fast. It has been hypothesized that TBAI catalyses the isomerization of the α-glycosyl iodide to the β-anomer,34 thereby leading to the formation of an α-glycoside. They have successfully adapted this strategy to the synthesis of α-GalCer35 and other α-fucosylglycosides.36 Furthermore, the replacement of the benzyl ethers with trimethylsilylethers (TMS) made Gervay-Hague’s synthetic strategy even more attractive. TMS protected sugars are easy to generate and their deprotection requires very mild acidic conditions, compatible with the glycosidic linkage.
The per-O-trimethylsilyl-α-l-fucosylpyranosyl iodide 10 was generated by the reaction of the per-O-tetramethylsilyl-α-l-fucose with one equivalent of iodotrimethylsilane35 and then added to the phytosphingosine acceptor 7 which was premixed with diisopropylethylamine (DIPEA) and TBAI (Scheme 3). After 2 days at room temperature, the solvent was evaporated and the TMS protecting groups were removed by treatment with an acidic resin in MeOH. Compound 11 was obtained as the α-anomer exclusively in an overall yield of 62%. The formation of the desired α-linkage was confirmed by the H-1 and C-1 signals in 1H and 13C NMR. Methanolysis, followed by hydrogenation of the azide then afforded compound 9.
Scheme 3.
(a) TBAI, DIPEA, CH2Cl2, rt; (b) Dowex 50WX8-200, MeOH, rt, 62% over two steps; (c) NaOMe/MeOH, quantitative; (d) H2, Pd, MeOH, 80%; (e) C25H51COCl or C23H47COC1, THF, NaOAc, 78–80%.
Finally, N-acylation with the fully saturated fatty acids, tetracosanoic acid (C24:0) and hexacosanoic acid (C26:0), was achieved via reaction of the corresponding acid chloride with the free amine 9 in a 1:1 mixture of THF and saturated sodium acetate solution (Scheme 3). Target compounds 4c and 4d were obtained as white solids after concentration of the organic phase and purification of the residue by flash chromatography. The spectroscopic data of the final compounds were consistent with the literature.25 While compound 4a was obtained by heating amine 9 with the N-hydroxysuccinimide activated ester of C20:2 fatty acid in a mixture of pyridine: water (9:1) at 50 °C overnight, compound 4b was obtained via dicyclohexylcarbodiimide (DCC) activated coupling using procedures described previously.37,38
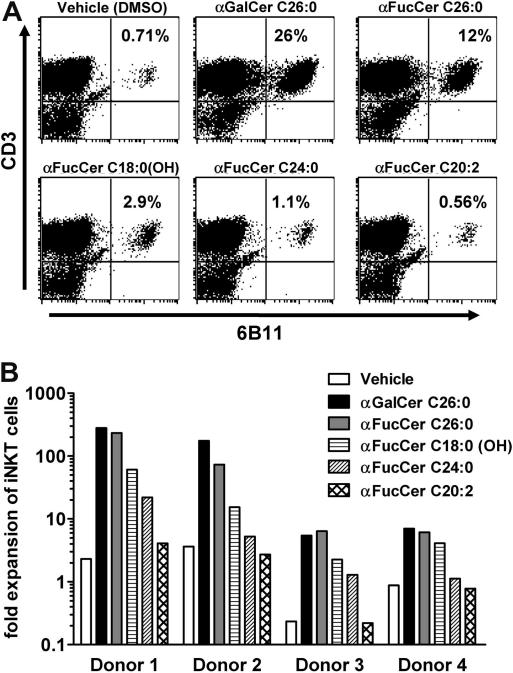
To assess the biological activity of the α-l-fucosylceramides and compare these to α-GalCer (KRN7000, 2), we assessed the ability of each compound to induce the expansion of iNKT cells in samples of human peripheral blood mononuclear cells (PBMC) during an eight-day in vitro culture.39 The results showed that both the percentages and absolute numbers of iNKT cells in cultures were increased by stimulation with α-l-fucosylceramides with C26:0 (4d) > C18:0 (OH) (4b) > C24:0 (4c). The α-l-fucosylceramide containing a C26:0 fatty acid (4d) was the most active of the fucosyl series, and stimulated iNKT cell expansions in some donors that approached those seen with the prototype iNKT cell agonist KRN7000 (2). In contrast, the α-l-fucosylceramide containing the C20:2 fatty acid (4a) was found to lack detectable iNKT cell stimulating activity in any of the donors tested (Fig. 1B). Representative profiles obtained by flow cytometry of cultures from one normal blood donor are shown in Figure 1A. This analysis was carried out with PBMC from four separate donors (Fig. 1B). Although, differences were observed for the levels of iNKT cell expansion between different donors, all donors responded significantly to two of the α-l-fucosylceramide analogues (4b and 4d).
Figure 1.
Ex vivo expansion of human iNKT cells by α-l-fucosylceramides. PBMC from four different donors were stimulated with the indicated glycolipids at a concentration of 250 nM in the presence of low levels of exogenous IL-2 and IL-7. At day 8, cultures were harvested and analysed by flow cytometry using monoclonal antibodies specific for CD3 and for the invariant TCRα chain expressed by iNKT cells (6B11). (A) Dot plots showing relative levels of CD3+ 6B11+iNKT cells are shown for one representative donor. Numbers in upper right quadrant indicate percentages of total lymphocytes that are iNKT cells. (B) Absolute numbers of iNKT cells in the cultures were determined by flow cytometry using fluorescent counting beads, and the values of iNKT cell fold expansion were determined by dividing by the input number of iNKT cells.
In summary, in the current study we have developed an efficient method for the synthesis of a series of biologically active α-l-fucosylceramides.40 The second method, employing the per-O-trimethylsilyl-α-l-fucosylpyranosyl iodide 10, proved to be superior with a better α-selectivity and reasonably good yield in the glycosylation reaction. Given the marked difference in the stereochemistry of the α-fucosyl head group of these glycolipids compared to α-galactosyl group of strong iNKT cell activators like KRN7000, it is surprising that the compounds in the current series show such substantial activity. This would seem to further reinforce the notion that the TCR of iNKT cells, in spite of its relative limited variability, is nevertheless able to interact efficiently with a broad range of structurally diverse ligands. It is noteworthy that the C20:2 N-acyl variant of α-fucosylceramide (4a) showed no detectable iNKT cell stimulating activity, given that the α-galactosyl version of this compound is an extremely potent iNKT cell agonist.41 This suggests that modification of the carbohydrate moiety can significantly alter the influence of the lipid moiety of the ligand on CD1d presentation and iNKT cell responses. Although the mechanism for this remains unclear, it is an important consideration for synthetic strategies that seek to combine biologically active alterations of the carbohydrate and lipid moieties of iNKT cell ligands.
Acknowledgements
G.S.B. acknowledges support in the form of a Personal Research Chair from Mr. James Badrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Council and The Wellcome Trust (084923/B/08/7). S.A.P. and G.B. were supported by NIH/NIAID grant AI45889. Core resources that facilitated flow cytometry were supported by the Einstein Center for AIDS Research (AI 051519) and the Einstein Cancer Center (CA 13330). The NMR spectrometers used in this research were funded in part through Birmingham Science City with support from Advantage West Midlands (AWM) and part-funded by the European Regional Development Fund (ERDF).
References and notes
- 1.Flowers M. Adv. Carbohydr. Chem. Biochem. 1981;39:279. doi: 10.1016/s0065-2318(08)60208-5. [DOI] [PubMed] [Google Scholar]
- 2.Vanhooren P.T., Vandamme E.J. J. Chem. Technol. Biotechnol. 1999;74:479. [Google Scholar]
- 3.Watanabe K., Matsubara T., Hakomori S.-I. J. Biol. Chem. 1976;251:2385. [PubMed] [Google Scholar]
- 4.Hakomori S. Adv. Cancer Res. 1978;52:257. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 5.Kannagi R., Fukushi Y., Tachikawa T., Noda A., Shin S., Shigeta K., Hiraiwa N., Fukuda Y., Inamoto T., Hakomori S.-I., Imura H. Cancer Res. 1986;46:2619. [PubMed] [Google Scholar]
- 6.Magnani J.L., Steplewski Z., Koprowski H., Ginsburg V. Cancer Res. 1983;43:5489. [PubMed] [Google Scholar]
- 7.Singhal A.K., Orntoft T.F., Nudelman E., Nance S., Schibig L., Stroud M.R., Clausen H., Hakomori S. Cancer Res. 1990;50:1375. [PubMed] [Google Scholar]
- 8.Hakomori S., Nudelman E., Levery S.B., Kannagi R. J. Biol. Chem. 1984;259:4672. [PubMed] [Google Scholar]
- 9.Hattori H., Uemura K., Taketomi T. Biochim. Biophys. Acta. 1981;666:361. doi: 10.1016/0005-2760(81)90295-2. [DOI] [PubMed] [Google Scholar]
- 10.Hattori H., Uemura K., Ogata H., Katsuyama T., Taketomi T., Kanfer J.N. Cancer Res. 1987;47:1968. [PubMed] [Google Scholar]
- 11.Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E., Koseki H., Taniguchi M. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 12.Crowe N., Uldrich A.P., Kyparissoudis K., Hammond K.J.L., Hayakawa Y., Sidobre S., Keating R., Kronenberg M., Smyth M.J., Godfrey D.I. J. Immunol. 2003;171:4020. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 13.Burdin N., Brossay L., Kronenberg M. Eur. J. Immunol. 1999;29:2014. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Carnaud C., Lee D., Donnars O., Park S.H., Beavis A., Koezuka Y., Bendelac A. J. Immunol. 1999;163:4647. [PubMed] [Google Scholar]
- 15.Taniguchi M., Harada M., Kojo S., Nakayama T., Wakao H. Annu. Rev. Immnunol. 2003;21:483. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey D.I., MacDonald H.R., Kronenberg M., Smyth M.J., Van K.L. Nat. Rev. Immunol. 2004;4:231. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Aseguinolaza G., Van Kaer L., Bergmann C.C., Wilson J.M., Schmieg J., Kronenberg M., Nakayama T., Taniguchi M., Koezuka Y., Tsuji M. J. Exp. Med. 2002;195:617. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto K., Miyake S., Yamamura T. Nature. 2001;413:513. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 19.Chiba A., Oki S., Miyamoto K., Hashimoto H., Yamamura T., Miyake S. Arthritis Rheum. 2004;50:305. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 20.Zajonc D.M., Cantu C., Mattner J., Zhou D., Savage P.B., Bendelac A., Wilson I.A., Teyton L. Nat. Immunol. 2005;6:810. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch M., Stronge V.S., Shepherd D., Gadola S.D., Mathew B., Ritter G., Fersht A.R., Besra G.S., Schmidt R.R., Jones E.Y., Cerundolo V. Nat. Immunol. 2005;6:819. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 22.Borg N.A., Wun K.S., Kjer-Nielsen L., Wilce M.C.J., Pellicci D.G., Koh R., Besra G.S., Bharadwaj M., Godfrey D.I., McCluskey J., Rossjohn J. Nature. 2007;448:44. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 23.Tashiro T., Nakagawa R., Inoue S., Shiozaki M., Watarai H., Taniguchi M., Mori K. Tetrahedron Lett. 2008;49:6827. [Google Scholar]
- 24.Motoki K., Morita M., Kobayashi E., Uchida T., Akimoto K., Fukushima H., Koezuka Y. Biol. Pharm. Bull. 1995;18:1487. doi: 10.1248/bpb.18.1487. [DOI] [PubMed] [Google Scholar]
- 25.Fan G.T., Pan Y.-S., Lu K.-C., Cheng Y.-P., Lin W.-C., Lin S., Lin C.-H., Wong C.-H., Fang J.-M., Lin C.-C. Tetrahedron. 2005;61:1855. [Google Scholar]
- 26.Yoshino T., Watanabe K., Hakomori S.-I. Biochemistry. 1982;21:928. doi: 10.1021/bi00534a018. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto N., Kanie O., Huang Y.-Y., Fujii R., Watanabe H., Shimamura M. Chem. Biol. 2005;12:677. doi: 10.1016/j.chembiol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Veerapen N., Brigl M., Garg S., Cerundulo V., Cox L.R., Brenner M.B., Besra G.S. Bioorg, Med. Chem. Lett. 2009;19:4288. doi: 10.1016/j.bmcl.2009.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akimoto K., Natori T., Morita M. Tetrahedron Lett. 1993;34:5593. [Google Scholar]
- 30.Van den Berg R.J.B.H.N., Boltje T.J., Verhagen C.P., Litjens R.E.J.N., Van der Marel G.A., Overkleeft H.S. J. Org. Chem. 2006;71:836. doi: 10.1021/jo0520240. [DOI] [PubMed] [Google Scholar]
- 31.Komba S., Ishida H., Kiso M., Hasegawa A. Bioorg. Med. Chem. 1996;4:1833. doi: 10.1016/s0968-0896(96)00165-4. [DOI] [PubMed] [Google Scholar]
- 32.Gervay-Hague J., Ngyuen T., Hadd M. Carbohydr. Res. 1997;300:119. [Google Scholar]
- 33.Meloncelli P.J., Martin A.D., Lowary T.L. Carbohydr. Res. 2009;344:1110. doi: 10.1016/j.carres.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Lam S.N., Gervay-Hague J. Carbohydr. Res. 2002;337:1953. doi: 10.1016/s0008-6215(02)00227-6. [DOI] [PubMed] [Google Scholar]
- 35.Du W., Kulkarni S.S., Gervay-Hague J. Chem. Commun. 2007;23:2336. doi: 10.1039/b702551c. [DOI] [PubMed] [Google Scholar]
- 36.Bhat A.S., Gervay-Hague J. Org. Lett. 2001;13:2081. doi: 10.1021/ol0160405. [DOI] [PubMed] [Google Scholar]
- 37.Goff R.D., Gao Y., Mattner J., Zhou D., Yin N., Cantu C., Teyton L., Bendelac A., Savage P.B. J. Am. Chem. Soc. 2004;126:13602. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 38.Xia C., Zhang W., Zhang Y., Woodward R.L., Wang J., Wang P.G. Tetrahedron. 2009;65:6390. [Google Scholar]
- 39.Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-hypaque centrifugation of leucocyte concentrates obtained from healthy volunteer blood donors. For ex vivo expansion of human iNKT cells, 4 million PBMC were cultured in wells of 24-well tissue culture plates in RPMI-1640 medium with 10% foetal calf serum and recombinant IL-2 (60 IU/mL) and IL-7 (5 ng/mL) (Peprotech). Glycolipids were solubilised in 100% DMSO and added directly to culture media to achieve a final concentration of 250 nM. Control wells received an amount of DMSO vehicle identical to that added with the glycolipids (0.00125%). Cultures were incubated for 8 days at 37 °C in a 5% CO2 humidified incubator. Cultures were harvested and the cells stained with fluorochrome labelled monoclonal antibodies specific for CD3 and the iNKT cell TCR (6B11, BD Biosciences). Samples were also stained with propidium iodide to exclude dead cells, and a known number of Caltag fluorescent counting beads (Invitrogen) were added to the samples to allow quantitation of absolute cell numbers. The total number of iNKT cells (CD3+, 6B11+, PI negative lymphocytes) was calculated by normalising according to counting beads and the fold expansion values were calculated based on the initial number of iNKT cells. Data were collected on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences).
- 40.Selected data for new compounds. Compound 8: 1H NMR (CDCl3): δ 7.92–8.04 (10H, m, Ar-H), 7.10–7.64 (15H, m, Ar-H), 5.42–5.50 (2H, m, H-3Cer, H-4Cer), 5.02 (1H, d, J1,2 = 3.6 Hz, H-1), 4.72–4.84 (6H, m, 3 × CH2Ph), 3.98 (1H, dd, J2,3 = 11.5 Hz, H-2), 3.75–3.90 (3H, m, H-4, H-3, H-1aCer), 3.55 (1H, m, H-5), 3.68 (1H, dd, J1a,1b = 10.4, J1b,2 = 8.6 Hz, H-1bCer), 1.88 (2H, m, H-5aCer, H-5bCer), 1.27 (3H, d, J5,6 = 6.4 Hz, H-6), 1.24 (24H, m, CH2), 0.89 (3H, t, CH3); 13C NMR (75 MHz, CDCl3): δ 99.5 (C-1); HRMS calcd for C59H73N3O9 [M+Na]+: 990.5245, found 990.5248. Compound 9: 1H NMR (CD3OD): δ 4.73 (1H, d, J1,2 = 3.7 Hz, H-1), 3.91–3.96 (2H, m, H-3, H-4), 3.68–3.72 (1H, dd, J1a,1b = 9.9, J1b,2 = 3.9 Hz, H-1aCer), 3.61–3.68 (2H, m, H-4, H-3Cer, H-4Cer), 3.52–3.55 (1H, dd, J2,3 = 6.0 Hz, H-2), 3.43–3.49 (1H, m, H-5), 3.39–3.42 (1H, dd, J1b,2 = 8.6 Hz, H-1bCer), 3.35 (1H, m, H-2Cer), 1.89 (2H, m, H-5aCer, H-5bCer), 1.28 (3H, d, J5,6 = 6.4 Hz, H-6), 1.24 (24H, m, CH2), 0.89 (3H, t, CH3); 13C NMR (75 MHz, CD3OD): δ 102.5 (C-1); HRMS calcd for C24H49NO7 [M+Na]+: 486.3403, found 486.3309. Compound 11: 1H NMR (CDCl3): δ 7.36–7.86 (10H, m, Ar-H), 5.60 (1H, dd, J3,4 = 4.8, J3,2 = 6.8 Hz, H-3Cer), 5.51 (1H, ddd, J4,5a = 4.2, J4,5b = 8.5 Hz, H-4Cer), 4.82 (1H, d, J1,2 = 2.8 Hz, H-1), 3.88–4.00 (3H, m, H-2, H-4, H-5), 3.62–3.80 (3H, m, H-3, H-2Cer, H-1aCer), 3.71 (1H, dd, J1a,1b = 10.4, J1b,2 = 6.5 Hz, H-1bCer), 1.76–1.91 (2H, m, H-5aCer, H-5bCer), 1.10–1.43 (24 H, m, CH2), 1.07 (3H, d, J5,6 = 6.6 Hz, H-6), 0.85 (3H, t, CH3); 13C NMR (75 MHz, CDCl3): δ 100.9 (C-1); HRMS calcd for C38H55N3O10 [M+Na]+: 720.3836, found 720.3811. Compound 4a: HRMS calcd for C44H83NO8 [M+Na]+: 776.6013, found 776.6011. Compound 4b: HRMS calcd for C42H83NO9 [M+Na]+: 768.5962, found 768.5941. Compound 4c: HRMS calcd for C48H95NO8 [M+Na]+: 836.6956, found 836.6953. Compound 4d: HRMS calcd for C50H99NO8 [M+Na]+: 864.7268, found 864.7240.
- 41.Yu K.O., Im J.S., Molano A., Dutronc Y., Illarionov P.A., Forestier C., Fujiwara N., Arias I., Miyake S., Yamamura T., Chang Y.T., Besra G.S., Porcelli S.A. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3383. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]