Abstract
Skeletal muscle tissue exhibits a remarkable capacity to regenerate after injury and to adapt its properties in response to altered functional demands or environmental pressure. This potential renders skeletal myocytes especially attractive candidates to be used in therapeutic strategies. Besides the well-described adaptability of skeletal myocytes in terms of contractile function and metabolic profile, more recent research has revealed that the electrophysiological properties of myocytes are also subject to significant changes both under physiological conditions and in pathophysiological situations. A better understanding of skeletal myocyte plasticity, its regulation and its forced induction could improve existing therapeutic approaches and may pave the way for new therapeutic strategies.
Introduction
In adult skeletal muscle, undifferentiated progenitor cells, the so-called satellite cells, reside between the sarcolemma and the basal lamina of adult skeletal muscle fibres. After muscle injury, normally quiescent satellite cells become activated and regenerate the muscle. Moreover, when overloaded, the muscle adapts by increasing its size and strength through satellite cell-mediated mechanisms [1]. Satellite cells are, to a certain extent, committed to develop a muscle phenotype [2]; however, in vitro, myoblasts (descendants of activated satellite cells) can be converted into other cell types such as osteoblasts [3] or adipocytes [4]. Myoblasts are already utilised for therapeutic strategies, for example, intra-cardiac myoblast transplantation therapy after heart injury [5]. Recently, various populations of stem cells have been isolated from skeletal muscle that may provide an alternative pool of progenitor cells in addition to, and clearly distinct from, satellite cells [2,6].
Largely independent of satellite cells [7,8•] fully differentiated skeletal muscle fibres also exhibit a remarkable capacity to adapt in response to altered functional demands. Their phenotypic profile (i.e. fibre type: fast glycolytic, fast glycolytic/oxidative or slow oxidative) is affected by innervation, neuromuscular activity, exercise training, mechanical loading/unloading, hormones and ageing [9]. Fibre type conversions involve changes in molecular, structural, metabolic and contractile properties [10], and the cellular signalling mechanisms involved are beginning to be understood [11]. Besides the well-described adaptability of skeletal myocytes in terms of contractile function and metabolic profile [12], more recent research has revealed that the electrophysiological properties of myocytes also significantly change in the course of fibre type conversion. The first part of this short review will summarise this topic. The second part will highlight the relevance of skeletal myocyte plasticity and its forced induction for various therapeutic approaches. The review will focus on papers published not earlier than 2005 but will also consider older papers if they are essential.
Main text
Electrophysiological adaptations in skeletal myocytes under physiological conditions
The electrophysiological properties of excitable cells, such as neurons or skeletal myocytes, are determined by the expression and function of membrane ion channels. Over the past few years, it has become increasingly clear that ion channel gene expression is dynamically controlled not only in developing, but also in fully differentiated cells of various origins. For example, the expression of various sodium channel genes is highly dynamic in neurons, where it accompanies transitions between different physiological states (e.g. low-frequency versus high-frequency action potential firing states) [13]. In contrast to neuronal tissues, little is known about ion channel plasticity in skeletal muscle. The current knowledge will be reviewed in the following section.
Sodium channels
Fast muscle fibres exhibit larger action potentials than slow fibres [14]. This is due to the higher density of voltage-gated sodium channels in fast muscle fibres (e.g. reference [15]). Moreover, fast and slow muscle fibres also clearly differ in basic functional properties of their sodium currents. For example, fast and slow sodium current inactivation occurs at more positive potentials and is less voltage-dependent in slow fibres [15,16]. Importantly, these basic differences in sodium channel expression levels and gating properties affect cellular excitability. The high channel density of fast fibres reduces the refractory period for action potential generation. Thus, fast fibres, but not slow fibres, are able to fire at high rates [17]. Moreover, the relative resistance of slow fibres to slow inactivation may enable them to remain tonically active. On the contrary, pronounced slow inactivation in fast fibres limits the duration these fibres can fire at high rates to prevent injury associated with prolonged high-frequency contraction [16]. Taken together, Ruff's exciting work has made it clear that cellular excitability substantially differs between the skeletal muscle fibre types. The molecular mechanisms underlying these differences, however, remained unknown. Using a simple in vitro approach, we recently showed [18] that basic functional parameters of sodium currents in differentiated skeletal myocytes of the mouse were significantly altered after their fibre type had been partly transformed from fast to slow. In accordance with Ruff's work, the slow fibre phenotype showed an increased resistance to slow inactivation. This was most probably due to enhanced expression of the cardiac sodium channel isoform Nav1.5 versus the adult skeletal muscle isoform Nav1.4 [18]. Importantly, these changes in the expression of sodium channel isoforms may represent a mechanism to generate differences in cellular excitability between fast and slow muscle fibres. However, convincing evidence for considerable expression of Nav1.5 in adult slow skeletal muscle is still lacking. As an alternative mechanism, the different resting potentials of fast and slow fibres [19] may influence the functional properties of their sodium channels [20•], and thereby alter excitability.
Calcium channels
Similar to sodium channel expression, the expression of L-type calcium channels is also subject to changes during fibre type conversion. [21,22], using in vivo chronic low frequency electrical stimulation, showed that fast-to-slow fibre type conversion downregulated the expression of Cav1.1 (skeletal muscle isoform), whereas upregulated the expression of Cav1.2 (cardiac isoform) calcium channels. In accordance, significant expression of Cav1.2 was detected at protein level in adult slow skeletal muscle [22,23]. Moreover, the abundance of calcium channel auxiliary subunits (α2, β) shows fibre type-specific differences [22]. The functional consequences of these shifts in calcium channel expression remain unclear. The finding that not only the cardiac voltage-gated calcium channel, but also the cardiac isoform of the ryanodine receptor (RyR2), is upregulated during fast-to-slow conversion [22] suggests a possible role of cardiac-like excitation-contraction coupling in fast-to-slow transformed and slow skeletal muscle [24]. A clear functional role of calcium influx in skeletal muscle was recently exposed by [25,26] who showed that calcium influx becomes relevant for muscle contractility during ageing.
Chloride channels
Chloride channels conduct at hyperpolarised potentials and thereby influence the resting potential and excitability of skeletal muscle fibres [19]. Owing to the higher sarcolemmal chloride conductance of fast fibres, their resting potential is significantly shifted towards hyper-polarised potentials compared with that of slow fibres [19]. In accordance with a higher chloride conductance in fast fibres, slow-to-fast fibre type conversion, induced by hindlimb unloading, increases the chloride conductance in rat skeletal muscle fibres [27,28,29•] by a reduction in PKC activity, which normally silences chloride channels in slow fibres [29]. Most interestingly, an increased chloride conductance precedes the transitions of myosin heavy chain isoforms, the classical markers of skeletal muscle fibre type, during conversion [27,28]. This suggests a possible involvement of ion channel (i.e. chloride channel) activity in initiating fibre type conversions. Accordingly, inhibition of the ryanodine receptor 1 calcium channels induces a fast-to-slow conversion [30].
Besides the voltage-gated ion channels described above, also other ion channel types change their expression levels and/or properties in the course of fibre type conversion (e.g. references [31-33]). Although impacts on cellular excitability are likely, the functional consequences of these changes are less clear and not further discussed. Finally, skeletal myocytes also undergo electrophysiological adaptations in pathophysiological situations. For example, in dystrophic compared with normal mouse muscle, the functional properties of voltage-gated calcium channels are altered [34]. At present, however, it is too early to finally judge how electrophysiological adaptations contribute to the pathology of skeletal muscle diseases. By contrast, in neuronal tissues, dysregulation of ion channel expression has been causally associated with a variety of pathophysiological settings, the so-called ‘transcriptional channelopathies’ [35].
Relevance of skeletal myocyte plasticity and its forced induction for therapeutic approaches
Myoblast transplantation therapy
Intra-cardiac transplantation of skeletal myoblasts is a new therapy to regenerate the injured or diseased heart [5]. The fact that skeletal myocytes have different properties than cardiomyocytes, in addition to their limited transdifferentiation capacity after transplantation in vivo [36], very probably limits the efficacy of myoblast transplantation therapy. In particular, specific electrophysiological features of the skeletal myocyte (functional ion channel properties, lack of connexin 43 expression) do not allow for proper cardiac-like impulse conduction (e.g. reference [17]) and, thus, may prevent recovery of heart function by synchronous contraction of the skeletal graft with cardiac host tissue. Here, it is worthwhile to reconsider the electrophysiological differences between slow and fast muscle fibres reviewed above. In contrast to fast fibres, slow fibres exhibit some cardiac-like electrophysiological properties, that is cardiac sodium channel [18] and calcium channel [22,23] expression. It is therefore tempting to speculate that myoblasts derived from slow skeletal muscle, which, in the rat, are pre-determined to generate slow fibres [37], are better suitable for intra-cardiac transplantation than fast muscle-derived myoblasts. Indeed, in an animal model of dilated cardiomyopathy, transplanted myoblasts expressing slow, but not fast, myosin heavy chain isoforms persisted in the graft and expressed connexin 43 [38•]. In analogy, the efficacy of myoblast transplantation to restore dystrophin in dystrophic slow skeletal muscle was significantly increased if slow instead of fast muscle-derived myoblasts were used [39]. This suggests that careful matching between donor myoblasts and host muscle is essential. The whole concept, however, requires that human satellite cells derived from slow muscle are, like in rodents [37], pre-programmed to develop slow myocytes, which has been called into question [40].
In addition, forced induction of cardiac-like electrophysiological properties in skeletal myocytes may be another strategy to improve their performance in the heart. Using a simple in vitro approach, we recently showed [41•] that cardiac Nav1.5 sodium channel expression is induced in skeletal myocytes by paracrine action of cardiomyocytes. Importantly, this suggests that skeletal myocytes can be forced to express cardiac electrophysiological properties. However, cardiac-like impulse conduction in the skeletal graft would, besides cardiac sodium channels, also require the expression of other cardiac ion channels, for example calcium and potassium channels. An additional prerequisite would be significant electrical coupling between the transplanted cells and host cardiomyocytes that may not occur in vivo [36]. The latter problem could be overcome by genetic modification of myoblasts to overexpress the cardiac gap junction protein connexin 43 [42].
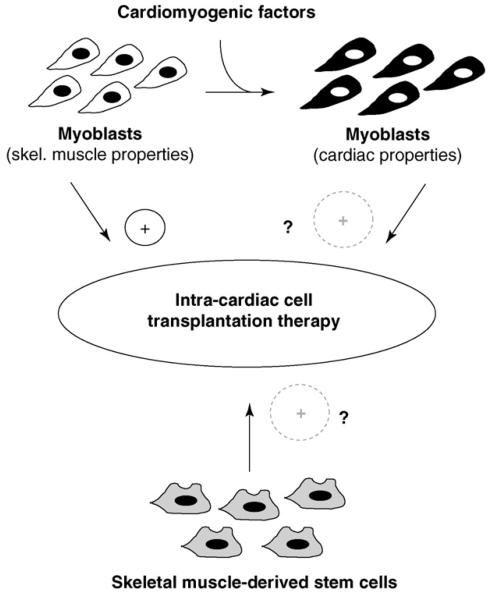
Future approaches of intra-cardiac myoblast transplantation may benefit from the use of factors that induce cardiac properties in skeletal myocytes (Figure 1). Such factors could be applied in the course of transplantation and/or in the initial postoperative weeks. Alternatively, it may be possible to boost the endogenous release of such factors by cardiocytes. The ultimate goal is to ‘reprogram’ myoblasts before transplantation to induce cardiomyogenic function [43]. Finally, skeletal muscle stem cells [2] other than satellite cell-derived myoblasts may be considered a promising alternative cell source for transplantation into the failing heart (Figure 1).
Figure 1.
New concepts for improvement of intra-cardiac cell transplantation therapy with skeletal muscle-derived progenitor cells. Skeletal myoblasts derived from satellite cells are already used for intra-cardiac cell transplantation therapy with limited success. Induction of cardiac properties in myoblasts by cardiomyogenic factors may enhance their performance in the heart. In addition, cell therapy may benefit from the use of skeletal muscle stem cells other than satellite cell-derived myoblasts.
Skeletal muscle regeneration
Using a DNA microarray approach [44•] reported transient alterations in the transcript levels of voltage-gated ion channels to go along with the initiation of a regeneration program in skeletal muscle. Importantly, this indicates an involvement of ion channel expression changes in muscle regeneration. Moreover, the regenerative potential of dystrophic mouse muscles was shown to be positively related to their chloride conductance [45]. Consequently, aimed alteration of the electrophysiological properties of skeletal myocytes (e.g. stimulation of chloride conductance) may be a strategy to boost the regeneration of skeletal muscle.
Aimed fibre type conversion to induce therapeutic benefit
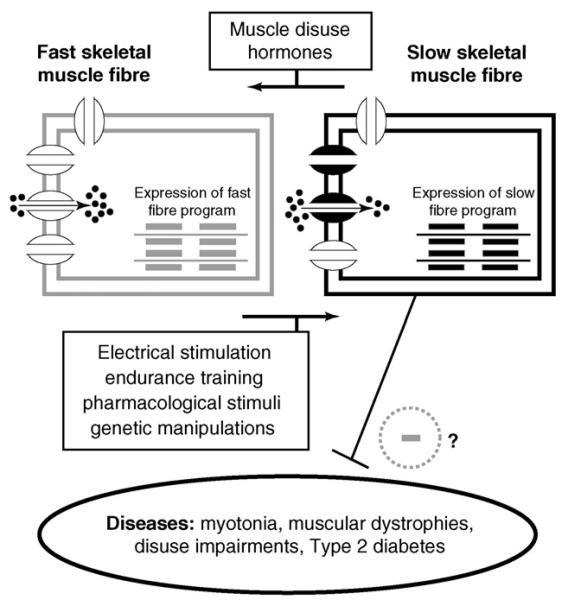
It is obvious that fibre type-specific excitability of skeletal myocytes should influence skeletal muscle diseases that are related to impaired cellular excitability (e.g. myotonias and periodic paralyses). Accordingly, it is easier to trigger myotonia in fast than in slow rat skeletal muscles [46]. If slow muscle is less susceptible to myotonia, aimed fast-to-slow fibre type conversion seems a reasonable therapeutic strategy. There is experimental evidence that also other diseases may benefit from a forced induction of the slow skeletal muscle phenotype: First, a fast-to-slow fibre shift has been suggested to alleviate the progression of muscular dystrophy in a mouse model (mdx) of Duchenne muscular dystrophy [47,48•,49•]. Secondly, promotion of the slow phenotype may protect against the development of insulin resistance and Type 2 diabetes [50,51•]. Finally, slow-to-fast fibre type conversion, accompanied by decreased exercise tolerance, is normally observed in pathophysiological situations of skeletal muscle disuse (e.g. references [1,27]). Forced fast-to-slow conversion may be considered a therapeutic strategy to counteract. In accordance, electrical stimulation of thigh muscles in patients with advanced chronic heart failure resulted in fast-to-slow conversion and significantly improved the patient's physical condition and exercise tolerance [52]. Besides electrical stimulation, various other strategies to achieve clinically relevant promotion of the slow skeletal muscle phenotype are feasible: endurance training [9], ion channel modulation [27,30], beta-2 receptor antagonists (indirect evidence deduced from [53]) and, most promising, activation of signalling pathways that promote the slow fibre phenotype [11,47,49•]. The concept depicted in this paragraph is summarised in Figure 2.
Figure 2.
Skeletal muscle fibre type conversion to induce therapeutic benefit. Slow-to-fast and fast-to-slow fibre type conversions can be induced by various stimuli. These conversions include changes in the expression and function of ion channels in the cellular membranes of skeletal muscle cells. Induction of the slow fibre phenotype may inhibit the susceptibility to, and/or the progression of, various human diseases.
Conclusions
Besides satellite cells, also adult skeletal myocytes contribute to the enormous degree of plasticity featuring skeletal muscle. Recent research has revealed that, in addition to the well-described adaptability of skeletal myocytes in terms of contractile function and metabolic profile, also their electrophysiological properties, and thus, their excitability can change. Electrophysiological adaptations occur under physiological conditions, and in diseased muscle, where they may well contribute to the pathology of certain disease states. At present, however, studies that provide a clear causal link between dysregulation of ion channel expression and/or modulation and pathophysiological settings in skeletal muscle are lacking. It can be expected that the existing knowledge in this area will rapidly broaden in the next couple of years especially owing to DNA chip and proteomics technology. Skeletal myoblast and adult skeletal myocyte plasticity comprises great potential to be utilised in therapeutic strategies. A better understanding of skeletal muscle plasticity, its regulation and its forced induction will be necessary to tap its full therapeutic potential.
Acknowledgements
The work was supported by the Austrian Science Fund (FWF, P-15063 and P19352-B11). I want to thank X König, H Todt, L Weigl and E Zebedin for critical comments on the manuscript.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
- 1.Harridge SD. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol. 2007;92:783–797. doi: 10.1113/expphysiol.2006.036525. [DOI] [PubMed] [Google Scholar]
- 2.Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 3.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A. 1995;92:9856–9860. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50:7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 7.Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflugers Arch. 1993;423:255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- 8•.Martins KJ, Gordon T, Pette D, Dixon WT, Foxcroft GR, MacLean IM, Putman CT. Effect of satellite cell ablation on low-frequency-stimulated fast-to-slow fibre-type transitions in rat skeletal muscle. J Physiol. 2006;572:281–294. doi: 10.1113/jphysiol.2005.103366. Using satellite cell ablation by gamma irradiation, the authors demonstrate that fibre type conversions in skeletal muscle can occur independent of satellite cells. In addition, satellite cells seem to play a role in facilitating fibre type conversions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 10.Fluck M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol. 2006;209:2239–2248. doi: 10.1242/jeb.02149. [DOI] [PubMed] [Google Scholar]
- 11.Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- 12.Bottinelli R, Reggiani C, editors. Skeletal Muscle Plasticity in Health and Disease: From Genes to Whole Muscle. Springer; 2006. [Google Scholar]
- 13.Waxman SG, Cummins TR, Black JA, Dib-Hajj S. Diverse functions and dynamic expression of neuronal sodium channels. Novartis Found Symp. 2002;241:34–51. [PubMed] [Google Scholar]
- 14.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speed of their responses. J Physiol (Lond) 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruff RL. Na current density at and away from end plates on rat fast- and slow-twitch skeletal muscle fibers. Am J Physiol. 1992;262:C229–C234. doi: 10.1152/ajpcell.1992.262.1.C229. [DOI] [PubMed] [Google Scholar]
- 16.Ruff RL. Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol Scand. 1996;156:159–168. doi: 10.1046/j.1365-201X.1996.189000.x. [DOI] [PubMed] [Google Scholar]
- 17.Ruff RL. Cells use the singular properties of different channels to produce unique electrical songs. Biophys J. 1998;74:2745–2746. doi: 10.1016/S0006-3495(98)77982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zebedin E, Sandtner W, Galler S, Szendroedi J, Just H, Todt H, Hilber K. Fiber type conversion alters inactivation of voltage-dependent sodium currents in mouse C2C12 skeletal muscle cells. Am J Physiol Cell Physiol. 2004;287:C270–C280. doi: 10.1152/ajpcell.00015.2004. [DOI] [PubMed] [Google Scholar]
- 19.Bretag AH. Muscle chloride channels. Physiol Rev. 1987;67:618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- 20•.Filatov GN, Pinter MJ, Rich MM. Resting potential-dependent regulation of the voltage sensitivity of sodium channel gating in rat skeletal muscle in vivo. J Gen Physiol. 2005;126:161–172. doi: 10.1085/jgp.200509337. This carefully performed electrophysiological study suggests that changes in membrane resting potential influence the functional properties of sodium channels. This represents a possible mechanism to regulate muscle excitability in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereon Y, Navarro J, Hamilton M, Booth FW, Palade P. Chronic stimulation differentially modulates expression of mRNA for dihydropyridine receptor isoforms in rat fast twitch skeletal muscle. Biochem Biophys Res Commun. 1997;235:217–222. doi: 10.1006/bbrc.1997.6753. [DOI] [PubMed] [Google Scholar]
- 22.Froemming GR, Murray BE, Harmon S, Pette D, Ohlendieck K. Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers. Biochim Biophys Acta. 2000;1466:151–168. doi: 10.1016/s0005-2736(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 23.Pereon Y, Dettbarn C, Lu Y, Westlund KN, Zhang JT, Palade P. Dihydropyridine receptor isoform expression in adult rat skeletal muscle. Pflugers Arch. 1998;436:309–314. doi: 10.1007/s004240050637. [DOI] [PubMed] [Google Scholar]
- 24.Reggiani C, te KT. RyR isoforms and fibre type-specific expression of proteins controlling intracellular calcium concentration in skeletal muscles. J Muscle Res Cell Motil. 2006;27:327–335. doi: 10.1007/s10974-006-9076-3. [DOI] [PubMed] [Google Scholar]
- 25.Payne AM, Zheng Z, Gonzalez E, Wang ZM, Messi ML, Delbono O. External Ca(2+)-dependent excitation—contraction coupling in a population of ageing mouse skeletal muscle fibres. J Physiol. 2004;560:137–155. doi: 10.1113/jphysiol.2004.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne AM, Messi ML, Zheng Z, Delbono O. Motor neuron targeting of IGF-1 attenuates age-related external Ca2+-dependent skeletal muscle contraction in senescent mice. Exp Gerontol. 2007;42:309–319. doi: 10.1016/j.exger.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierno S, Desaphy JF, Liantonio A, De Bellis M, Bianco G, De Luca A, Frigeri A, Nicchia GP, Svelto M, Leoty C, et al. Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse. Brain. 2002;125:1510–1521. doi: 10.1093/brain/awf162. [DOI] [PubMed] [Google Scholar]
- 28.Desaphy JF, Pierno S, Liantonio A, De Luca A, Didonna MP, Frigeri A, Nicchia GP, Svelto M, Camerino C, Zallone A, Camerino DC. Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: effects on the electrical properties and myosin heavy chain profile. Neurobiol Dis. 2005;18:356–365. doi: 10.1016/j.nbd.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 29•.Pierno S, Desaphy JF, Liantonio A, De Luca A, Zarrilli A, Mastrofrancesco L, Procino G, Valenti G, Conte CD. Disuse of rat muscle in vivo reduces protein kinase C activity controlling the sarcolemma chloride conductance. J Physiol. 2007;584.3:983–995. doi: 10.1113/jphysiol.2007.141358. The authors show that slow-to-fast fibre type conversion, induced by hindlimb unloading, increases the chloride conductance in rat skeletal muscle fibres by a reduction in PKC activity, which normally silences chloride channels in slow fibres. Here, fibre type-specific ion channel modulation generates changes in excitability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan T, Jiang H, Li H, DiMario JX. Inhibition of ryanodine receptor 1 in fast skeletal muscle fibers induces a fast-to-slow muscle fiber type transition. J Cell Sci. 2004;117:6175–6183. doi: 10.1242/jcs.01543. [DOI] [PubMed] [Google Scholar]
- 31.O'Reilly C, Pette D, Ohlendieck K. Increased expression of the nicotinic acetylcholine receptor in stimulated muscle. Biochem Biophys Res Commun. 2003;300:585–591. doi: 10.1016/s0006-291x(02)02898-x. [DOI] [PubMed] [Google Scholar]
- 32.Tricarico D, Mele A, Conte CD. Phenotype-dependent functional and pharmacological properties of BK channels in skeletal muscle: effects of microgravity. Neurobiol Dis. 2005;20:296–302. doi: 10.1016/j.nbd.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Tricarico D, Mele A, Lundquist AL, Desai RR, George AL, Jr, Conte CD. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc Natl Acad Sci U S A. 2006;103:1118–1123. doi: 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson BD, Scheuer T, Catterall WA. Convergent regulation of skeletal muscle Ca2+ channels by dystrophin, the actin cytoskeleton, and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2005;102:4191–4196. doi: 10.1073/pnas.0409695102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waxman SG. Transcriptional channelopathies: an emerging class of disorders. Nat Rev Neurosci. 2001;2:652–659. doi: 10.1038/35090026. [DOI] [PubMed] [Google Scholar]
- 36.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalhovde JM, Jerkovic R, Sefland I, Cordonnier C, Calabria E, Schiaffino S, Lomo T. ‘Fast’ and ‘slow’ muscle fibres in hindlimb muscles of adult rats regenerate from intrinsically different satellite cells. J Physiol. 2005;562:847–857. doi: 10.1113/jphysiol.2004.073684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Tezuka A, Kawada T, Nakazawa M, Masui F, Konno S, Nitta SI, Toyo-Oka T. Which skeletal myoblasts and how to be transplanted for cardiac repair? Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2007.11.084. doi: 10.1016/j.bbrc.2007.11.084. Using an animal model of dilated cardiomyopathy, this very recent study shows that transplanted skeletal myoblasts expressing slow, but not fast, myosin heavy chain isoforms persist in the graft and express connexin 43. This suggests that the slow skeletal muscle phenotype is more suitable to regenerate the heart than the fast phenotype. [DOI] [PubMed] [Google Scholar]
- 39.Qu Z, Huard J. Matching host muscle and donor myoblasts for myosin heavy chain improves myoblast transfer therapy. Gene Ther. 2000;7:428–437. doi: 10.1038/sj.gt.3301103. [DOI] [PubMed] [Google Scholar]
- 40.Bonavaud S, Agbulut O, Nizard R, D'honneur G, Mouly V, Butler-Browne G. A discrepancy resolved: human satellite cells are not preprogrammed to fast and slow lineages. Neuromuscul Disord. 2001;11:747–752. doi: 10.1016/s0960-8966(01)00222-x. [DOI] [PubMed] [Google Scholar]
- 41•.Zebedin E, Mille M, Speiser M, Zarrabi T, Sandtner W, Latzenhofer B, Todt H, Hilber K. C2C12 skeletal muscle cells adopt cardiac-like sodium current properties in a cardiac cell environment. Am J Physiol Heart Circ Physiol. 2007;292:H439–H450. doi: 10.1152/ajpheart.00119.2006. Using a simple in vitro approach, this study shows that cardiac Nav1.5 sodium channel expression is induced in skeletal myocytes by paracrine action of cardiomyocytes. This suggests that skeletal myocytes can be forced to express cardiac electrophysiological properties. [DOI] [PubMed] [Google Scholar]
- 42.Stagg MA, Coppen SR, Suzuki K, Varela-Carver A, Lee J, Brand NJ, Fukushima S, Yacoub MH, Terracciano CM. Evaluation of frequency, type, and function of gap junctions between skeletal myoblasts overexpressing connexin43 and cardiomyocytes: relevance to cell transplantation. FASEB J. 2006;20:744–746. doi: 10.1096/fj.05-5088fje. [DOI] [PubMed] [Google Scholar]
- 43.Heng BC, Haider HK, Sim EK, Cao T, Tong GQ, Ng SC. Reprogramming autologous skeletal myoblasts to express cardiomyogenic function. Challenges and possible approaches. Int J Cardiol. 2005;100:355–362. doi: 10.1016/j.ijcard.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 44•.Fluck M, Schmutz S, Wittwer M, Hoppeler H, Desplanches D. Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R4–R14. doi: 10.1152/ajpregu.00833.2004. This microarray analysis exposed a number of mechanistically important pathways involved in muscle fibre type conversion, fibre damage and repair. For example, the authors report that transient alterations in the transcript levels of voltage-gated ion channels go along with the initiation of a regeneration program in skeletal muscle. [DOI] [PubMed] [Google Scholar]
- 45.De Luca A, Pierno S, Camerino DC. Electrical properties of diaphragm and EDL muscles during the life of dystrophic mice. Am J Physiol. 1997;272:C333–C340. doi: 10.1152/ajpcell.1997.272.1.C333. [DOI] [PubMed] [Google Scholar]
- 46.D'Alonzo AJ, McArdle JJ. An evaluation of fast- and slow-twitch muscle from rats treated with 20,25-diazacholesterol. Exp Neurol. 1982;78:46–66. doi: 10.1016/0014-4886(82)90188-1. [DOI] [PubMed] [Google Scholar]
- 47.Chakkalakal JV, Harrison MA, Carbonetto S, Chin E, Michel RN, Jasmin BJ. Stimulation of calcineurin signaling attenuates the dystrophic pathology in mdx mice. Hum Mol Genet. 2004;13:379–388. doi: 10.1093/hmg/ddh037. [DOI] [PubMed] [Google Scholar]
- 48•.Chakkalakal JV, Michel SA, Chin ER, Michel RN, Jasmin BJ. Targeted inhibition of Ca2+/calmodulin signaling exacerbates the dystrophic phenotype in mdx mouse muscle. Hum Mol Genet. 2006;15:1423–1435. doi: 10.1093/hmg/ddl065. The authors show that knockdown of Ca/CaM signalling within slow muscle fibres of the mdx mouse lead to a fibre type shift towards a faster skeletal muscle phenotype and to exacerbation of the dystrophic phenotype. Consequently, promotion of the slow fibre phenotype through CaM-regulated pathways may be a therapeutic strategy to counteract the progression of muscular dystrophies. [DOI] [PubMed] [Google Scholar]
- 49•.Stupka N, Plant DR, Schertzer JD, Emerson TM, Bassel-Duby R, Olson EN, Lynch GS. Activated calcineurin ameliorates contraction-induced injury to skeletal muscles of mdx dystrophic mice. J Physiol. 2006;575:645–656. doi: 10.1113/jphysiol.2006.108472. By overexpression of constitutively active calcineurin-A a in dystrophic skeletal muscle of mdx mice, the authors show that activated calcineurin promotes the slow skeletal muscle phenotype and improves both dystrophic muscle structure and function. Hence, stimulation of the calcineurin signalling pathway may have therapeutic potential for muscular dystrophies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryder JW, Bassel-Duby R, Olson EN, Zierath JR. Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J Biol Chem. 2003;278:44298–44304. doi: 10.1074/jbc.M304510200. [DOI] [PubMed] [Google Scholar]
- 51•.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006;4:407–414. doi: 10.1016/j.cmet.2006.10.003. This study demonstrates that PPARb is required in skeletal muscles for the maintenance of the slow skeletal muscle phenotype. Moreover, ablation of PPARb in mouse skeletal muscles leads to obesity and diabetes. Thus, promotion of the slow phenotype may protect against the development of insulin resistance and Type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 52.Nuhr MJ, Pette D, Berger R, Quittan M, Crevenna R, Huelsman M, Wiesinger GF, Moser P, Fialka-Moser V, Pacher R. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J. 2004;25:136–143. doi: 10.1016/j.ehj.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 53.Zeman RJ, Ludemann R, Easton TG, Etlinger JD. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a beta 2-receptor agonist. Am J Physiol. 1988;254:E726–E732. doi: 10.1152/ajpendo.1988.254.6.E726. [DOI] [PubMed] [Google Scholar]