Abstract
The adaptive immune response meets the needs of the organism to generate effector cells capable of controlling pathogens, but also leads to production of memory cells, which mediate more effective protection during rechallenge. In this review we focus on the generation, maintenance and function of memory T cells, with a special emphasis on the increasing evidence for great diversity among functional memory T cell subsets.
Introduction: Do we have a definition of effector and memory T cells?
During a typical immune response to an acute pathogen, antigen specific cells are activated, proliferate vigorously and expand extensively. This expansion phase yields a large population of effector T cells, most of which will die in the subsequent contraction phase of the response (Fig. 1). However, the expansion phase also yields cells that will eventually form the memory cell pool - primed cells maintained long term after immunization. Immune memory indicates a qualitatively and/or quantitatively distinct immune response upon successive (but interrupted) exposures to antigen. This leads to an improved secondary immune response compared to the primary response – “improved” in this context usually means greater in magnitude, faster, more sensitive to low doses of antigen and more effective in the diversity or complexity of secondary effectors (Kaech and Wherry, 2007) (Harty and Badovinac, 2008; Williams and Bevan, 2007).
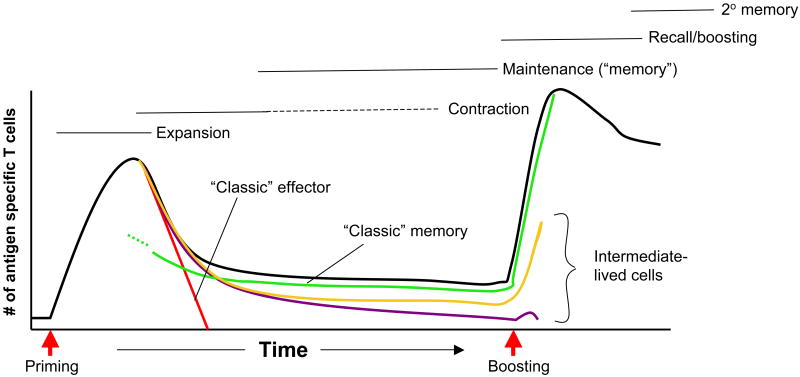
Figure 1. Making lasting memories.
The schematic shows numbers of antigen specific T cells (black line) at various stages after priming and boosting of a prototypical acute immune response. During the primary response, the fate of typical effector (red line) and memory (green line) cells is shown. Also shown are populations of intermediate longevity whom may also contribute to protection form re-infection (maroon and gold lines).
The memory cell pool is not monolithic but rather contains diverse and pliable populations. Two broad categories of memory cells are effector-memory (Tem) and central-memory (Tcm) (being CD62LloCCR7lo and CD62LhiCCR7hi, respectively) (Sallusto et al., 1999). This characterization, together with earlier studies, suggest that phenotypically defined subsets possessed distinct functional properties (Sallusto et al., 1999) (Hamann et al., 1997), but subsequent work has only added to the range of phenotypic and functional memory subsets which can be identified. So, are there features we can use to clearly define memory cells (and their subsets), and distinguish these from effector cells? A minimal definition of memory cells would be the population that persists long term after antigen clearance (whereas “typical” effector cells do not). However, there is no consensus on the minimum longevity sufficient to classify as “memory”, and this definition includes no functional properties. A commonly used additional criteria is the ability of “typical” memory (but not effector) cells to undergo recall proliferation and differentiation into secondary effector and memory populations. Use of these two parameters (longevity and proliferative potential) allows demarcation of idealized naïve, effector, Tem and Tcm cell populations (Fig 2a). Tem cells are generally considered to have a more limited lifespan and weaker proliferative potential, compared to their Tcm cell counterparts. However, analysis of actual post-activation populations illustrates much greater diversity in survival and recall potentials (Fig 2b) as well as diverse subsets defined by other phenotypic and functional markers in lymphoid and non-lymphoid sites (see, e.g. (Hikono et al., 2007; Masopust et al., 2006b). Some cells represented in Fig 2b arise in certain tissue sites, or distinct stages of the immune response but this chart helps illustrate that memory subsets, as well as the properties of “effector” and “memory” cells, are not sharply distinct. Nor will one pair of criteria accurately define the populations potentially generated in an immune response: a different chart (with just as much diversity) could be produced plotting other features – such as cytolytic activity versus longevity for CD8+ T cell populations. Without appreciating the diversity of the post-activation T cell pool, we may miss critical elements in the armaments induced during adaptive immunity. Our goal here is to discuss how different populations of post-activation T cells are generated, and how this diverse pool may all contribute to distinct aspects of recall immune responses and (a distinct feature) protective immunity. This review will focus exclusively on T cells and, because of space constraints will focus mainly on findings from acute antigen exposure models. The effects of chronic and persistent infections on T cell memory have been discussed in recent reviews (Harty and Badovinac, 2008; Kaech and Wherry, 2007; Williams and Bevan, 2007).
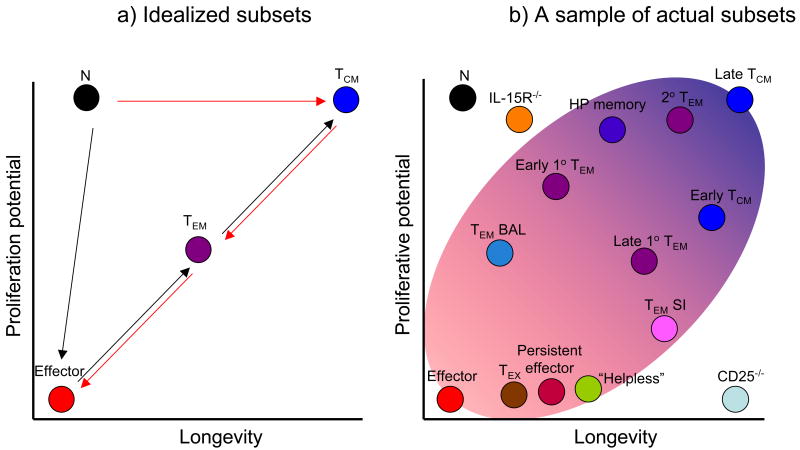
Figure 2. Embracing diversity.
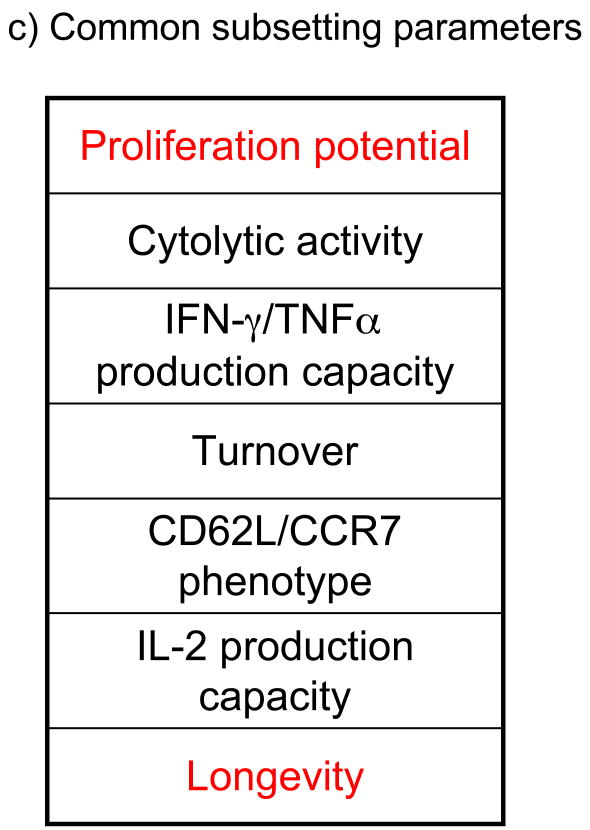
(left) The chart shows the relative longevity and recall expansion potential of “idealized” naïve, effector, Tcm and Tem cell populations, as they are typically discussed, as well as conflicting models of lineage differentiation. (right) The same chart is shown with inclusion of some populations found during actual immune responses. The shaded area indicates a distribution “cloud” of cells with effector-through-memory like properties. Many populations in this cloud would typically be designated “Tem” but, as indicated, there is also diversity among the “Tcm” cell pool. Shown are memory populations identified in various tissue sites, including Tem cells from lung bronchoalveolar lavage (BAL) (Ely et al., 2003) and small intestine (SI)(Masopust et al., 2006b) and Tem and Tcm cells isolated from spleen at different memory time points (“Early” and “Late” Tcm and Tem) (Roberts et al., 2005). Also shown are CD8+ T cells primed without sufficient CD4+ T cell help (“Helpless”), memory-like cells made through lymphopenia-driven homeostatic proliferation (“HP” memory cells), T cells driven to “exhaustion” by chronic antigen exposure (“Tex”) and effector cells maintained into the long-lived phase (“Persistent effector”) (references provided in the main text). The position of CD8+ memory T cells produced in IL-15- (or IL-15Ra-) deficient mice, and of CD25-deficient CD8+ memory T cells is also illustrated, though it is not yet clear whether such cells are representative of populations generated in natural responses (hence these cells fall outside the “cloud”). Relative positions of populations are not intended to be precise, but merely to indicate the diversity of groups identified using only these two parameters. The box indicates further layers of complexity would be revealed if additional functional parameters (some of which are listed here) were included in the subset definitions.
The choice between “effector” and “memory” differentiation: One cell to yield them all?
Activation of T cells generates a pool with potential to enter the effector or the memory cell population. A key question, intensely investigated in recent years, is what influences the effector versus memory decision. The simplest model of all would be that effector cells and memory cells differentiate from distinct subsets of naïve T cells. However, adoptively transferred single naïve CD8+ T cells can give rise to both effector and diverse memory populations (Stemberger et al., 2007), a result reinforced by an alternative innovative method of “cellular barcoding” (Schepers et al., 2008) (Schumacher personal communication). Such studies, by necessity, utilized T cell receptor (TCR) transgenic T cells, so it is unclear whether these results will always be generalizable. Indeed, recent reports argue that sub-optimal TCR stimulation can lead to T cell elimination after the effector phase (Teixeiro et al., 2009; Williams et al., 2008), suggesting that there may be instances where individual clones contribute to the effector but not memory pools (and, potentially, the reverse).
An extension of the “one cell-multiple fates” model has been proposed by Reiner's group based on their studies of asymmetric cell division during the initial T cell response (Chang et al., 2007). This report found differential segregation of receptors and signaling molecules between the first two daughter cells of activated naive T cells, and suggested they may differ in their capacity to produce effector versus memory pools (Fig 3). At its most extreme, this model would indicate the fate of the effector versus memory “lineages” is sealed early in the response, driven by differential gene expression programs. In contrast to this strict model, recent work argues gene expression of “effector-like” molecules (such as Granzyme B in CD8+ T cells and IFN-γ in CD4+ T cells) in cells that later join the memory pool (as well as in effector cells) (Bannard et al., 2009; Harrington et al., 2008; Lohning et al., 2008; Maris et al., 2003). These and other reports fit best with a form of linear differentiation model in which activated T cells all pass through an “early effector” phase during which genes for several effector molecules are expressed. However, this does not exclude the possibility that cells produced by the first few divisions have different capacities to join the effector versus memory pool later on (Fig 3). In addition, however, “environmental” cues such as inflammatory cytokines can dramatically affect subsequent differentiation of the activated T cell population (Fig. 3), as will be discussed next.
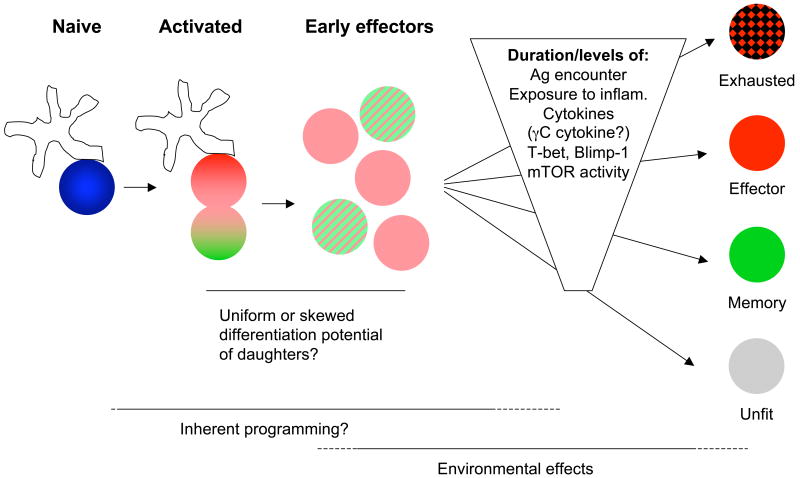
Figure 3. Walking the tightrope: factors affecting differentiation of activated T cells.
After stimulation of naïve T cells by antigen bearing APC, the first cell division is asymmetric and may produce cells with distinct potential to become effector or memory cells (indicated by shading of daughter cells). However, the products of initial stages of the expansion phase (“Early effectors”) probably all express key effector molecules (including Granzyme B in CD8+ T cells and IFN-γ in CD8+ T and Th1 cells), yet lack later effector markers. This early effector pool may have full or only limited potential to become any of the differentiated populations shown on the right. The production of effector and memory populations during the remainder of the expansion phase is also conditioned by the duration and intensity of various signals. These include signaling associated with the TCR and also cytokine receptors. Such signals may dictate expression of key transcription factors including T-bet and Blimp-1, and also control the metabolic status of the activated cell (including that regulated by mTOR activity). Unusually strong or persistent stimuli may generate “exhausted” T cells, whereas inadequate stimuli produces “unfit” cells. In between these extremes, effector and memory differentiation may be driven by cumulative cues operating on the early effector cell (or by selectively favoring outgrowth/survival of cells with a bias toward the effector or memory fate).
Everything in moderation: better memory by avoiding over-exertion?
A key question is when competent memory T cell precursors emerge after activation of naïve T cells. Studies on CD8+ T cells well before the peak of expansion after primary acute LCMV infection suggest low expression of the molecule KLRG-1 identifies cells containing the memory precursor pool (Sarkar et al., 2008). Although the reliability of KLRG-1 expression as a memory precursor marker in other infection models is unclear, this approach has been useful to study memory cell generation in the LCMV system. As early as 4.5 days after primary LCMV infection, some KLRG-1lo cells have already reacquired the ability to make interleukin-2 (IL-2) and go on to show enhanced survival and recall proliferation potential (Sarkar et al., 2008). These cells also show enhanced ability to subsequently upregulate IL-7Rα, which is important for memory T cell survival (Buentke et al., 2006; Kaech et al., 2003; Schluns et al., 2000) and to differentiate into a long-lived pool of Tcm cells. So what conditions favor generation and/or survival of memory precursors? Current models suggest a delicate balance between sufficient but not over-exuberant responses to various stimuli is the key to good memory.
An appealing model for effector or memory choice builds around the idea that sustained stimulatory signals drive cells into a short-lived effector pool, whereas more transient (but sufficient) signals favor generation of memory cells (Intlekofer et al., 2006; Kaech et al., 2002; Lanzavecchia and Sallusto, 2002). The signals discussed here include TCR encounter with peptide-MHC but also the integration of stimuli through various adhesion, costimulatory, and cytokine receptors.
Studies in which the intensity of inflammatory cues is controlled suggest restrained exposure to inflammatory cytokines enhances generation of memory-like cells (Haring et al., 2006; Kaech and Wherry, 2007). Inflammatory cytokines such as type I interferon (IFN-I) and IL-12 operate as “signal 3” (with TCR and costimulation) to promote CD8+ T cell effector differentiation (Mescher et al., 2006). Studies suggest intense and/or sustained exposure to IL-12 preferentially promotes CD8+ T cell differentiation toward the effector rather than memory fate (Joshi et al., 2007; Pearce and Shen, 2007). Other inflammatory cues probably work indirectly rather than on the T cell itself, and the impact of cytokine deficiencies can be complex. An instructive example is IFN-γ, deficiency of which leads to a substantial block in CD8+ T cell contraction (Badovinac et al., 2000), yet testing the effects of deficiency of IFN-γ receptor selectively on responding CD8+ T-cells suggests the cytokine offers a competitive advantage for memory T cell generation, in at least some responses (Whitmire et al., 2007).
How changes in the intensity and/or duration of stimuli changes the balance of effector versus memory differentiation is still being discerned. However, evidence points to a key role for differential expression of the transcription factor T-bet, well known for supporting “Type-1” T cell differentiation (Glimcher, 2007). Increasing T-bet expression in activated CD8+ T cells drives differentiation away from memory-like and toward effector-like cells, and T-bet is efficiently induced by stimuli such as IL-12 which promote effector production (Joshi et al., 2007; Pearce and Shen, 2007). Furthermore, recent reports suggest that the transcription factor Blimp-1 is critical for generating mature effector cells (Kallies et al., 2009; Rutishauser et al., 2009). Stimulated Blimp-1 deficient T cells preferentially differentiated into central memory-like cells, curtailing the production of effector cells, a situation which was associated with more efficient immune control of some pathogens (Rutishauser et al., 2009), but not others (Kallies et al., 2009). In B cell differentiation, Blimp-1 and Bcl-6 show mutually antagonistic roles (Calame, 2006), and this may parallel the situation in T cells because Bcl-6 deficient mice exhibit defects in the memory CD8+ T cell pool (Ichii et al., 2002).
For CD8+ T cells, there is evidence that terminating antigen-encounter (“early leavers” in the response) enhances production of memory precursors (Sarkar et al., 2008). Cells which enter the response late (“late comers”) may also have some advantages in differentiation into the memory pool (Catron et al., 2006; D'Souza and Hedrick, 2006; van Faassen et al., 2005).
However, studies show that at least some perception of inflammatory cues is required for memory cell differentiation. CD8+ T cells completely lacking exposure to signal 3 cytokines or deficient for T-bet showed impaired generation of long lived functional memory cells, and T-bet is required for normal expression of CD122 (the beta-chain of the IL-2 and IL-15 receptors) and reactivity to the homeostatic cytokine IL-15 (Intlekofer et al., 2005; Joshi et al., 2007; Shaulov and Murali-Krishna, 2008; Xiao et al., 2009). Indeed, in vitro studies indicate exposure to IL-12 early during T cell priming leads to effective generation of memory CD8+ T cells (Xiao et al., 2009). Also, it is important to note that in several studies where inflammatory cues are shown to promote effector T cell generation, this did not occur at the expense of generating memory cells, suggesting that the memory differentiation pathway can progress in the face of varied production of effector cells (Cui et al., 2009; Joshi et al., 2007). Prematurely truncating exposure to peptide-MHC ligands may lead to a failure in memory (and also effector) differentiation (Fig 3). Activation of CD8+ T cells for only a few hours leads to their becoming “unfit” for successful differentiation (Gett et al., 2003; van Stipdonk et al., 2003; Williams and Bevan, 2004), and shortened encounters with dendritic cells (DCs) (due to blocked ICAM-LFA interactions) produces a similar effect (Scholer et al., 2008). Studies with CD4+ T cells also show that suboptimal and/or short term stimulation leads to production of functionally compromised effector cells and minimal memory generation (Blair and Lefrancois, 2007; Gett et al., 2003; Williams and Bevan, 2004; Williams et al., 2008). At the other extreme, overstimulation of activated T cells (e.g. during chronic infection) can provoke an “exhausted” state, also manifest as impaired function and maintenance (Kaech and Wherry, 2007).
Together, these studies would argue that there is a Goldilocks, “just right” point in which response to TCR cues, inflammatory cytokines (and potentially other cues) supports memory differentiation without pushing the cells too hard into the short lived effector pool (Fig 3). But what does “pushing too hard” mean in molecular terms? Quantitative differences in expression of key transcription factors (e.g. T-bet and Blimp-1, discussed above) may dictate effector versus memory gene expression patterns. In addition, recent reports suggest control of T cell metabolism is important for differentiation towards the long-lived memory pool. T cells need to switch from the anabolic metabolism characteristic of effector cells to adopt the catabolic metabolism of quiescent memory (and naïve) T cells (Jones and Thompson, 2007). Two recent reports show that memory CD8+ T cells fail to form if this metabolic switch is prevented (Araki et al., 2009; Pearce et al., 2009). Regulating the molecule mTOR is key in this metabolic change, as illustrated by the capacity of the mTOR inhibitor rapamycin (or knockdown of components in the mTORC1 complex) favoring emergence of memory CD8+ T cells from the effector pool (Araki et al., 2009; Pearce et al., 2009), and a related pathway controlling fatty acid oxidation, is also implicated in the metabolic switch (Pearce et al., 2009). Once again, however, this regulation is a balancing act, as complete blockade of mTOR prevents initial T cell activation and expansion. Such results beg the question of why some cells in the normal response are successful at making the required metabolic switch to differentiate from the effector pool, whereas most cells are not. Via PI3K, mTOR is activated through activation of the TCR and costimulatory ligands and by various cytokine receptors which, as we have seen, can modulate the effector and memory cell differentiation. Further, in vitro assays show that culture of activated CD8+ T cells with IL-2 sustains an effector phenotype, while IL-15 promotes Tcm cell-like differentiation (Manjunath et al., 2001; Weninger et al., 2001), these differences being mediated, at least in part, by the intensity of PI3K and mTOR stimulation (Sinclair et al., 2008). Moderation of stimulatory cues may therefore be important for promoting the metabolic switch (as well as other effects) favoring development of the memory pool.
Whether the same rules apply to boosted memory populations is much less clear. Markers such as KLRG-1 and effector molecules such Granzyme B are strongly expressed by cells which seed the secondary (and tertiary) memory pools and these very stable populations very slowly, if at all, acquire a Tcm cell phenotype (Jabbari and Harty, 2006; Masopust et al., 2006a; Vezys et al., 2009). Because most vaccines are boosted (as, probably, most natural infections are reencountered) these data highlight an important gap in our knowledge of normal memory cell pools.
An unresolved issue is whether factors which alter the balance of effector versus memory cell generation do so by causing altered differentiation of a uniformly pliable precursor pool, or whether these stimuli promote expansion or survival of more committed precursors within the activated population (Fig 3). Resolving such questions, which have been carefully discussed in previous reviews (Harty and Badovinac, 2008; Kaech and Wherry, 2007; Williams and Bevan, 2007), will require further analysis of the differentiation potential of T cells generated early in the immune response.
The contraction phase: making life and death decisions
During contraction short-lived cells are eliminated from the antigen specific population. What is the basis for survival of some cells and death of others? It has become clear that the death pathway during contraction involves a prominent role for the pro-apoptotic factor Bim. Activity of Bim is normally restrained by Bcl-2 (and related molecules), but downregulation of Bcl-2 during activation leaves effector cells vulnerable. T cells lacking Bim undergo relatively normal expansion, but are more resistant to contraction than their wild-type counterparts (Hildeman et al., 2002; Hughes et al., 2008; Prlic and Bevan, 2008; Weant et al., 2008). The transcriptional regulator Id2 has an important role in preserving cells through the expansion phase, as Id2-deficient effector CD8+ T cells show increased cell death, accompanied by elevated Bim and reduced Bcl-2 expression (Cannarile et al., 2006)
But what makes short-lived cells susceptible to activating apoptotic pathways, whereas other cells emerge unscathed? An initially attractive candidate would be signals from homeostatic cytokines such as IL-7. Expression of IL-7Rα (CD127) is downregulated on activated T cells, but is re-expressed on a population of cells late in the expansion phase, which correlates with the KLRG-1lo pool (Joshi et al., 2007; Kaech et al., 2003). IL-7 is important for maintenance of the memory pool, and (as discussed above) cues that favor memory T cell generation drive re-expression of CD127. Furthermore, IL-7 (as well as other γc cytokines) induces expression of Bcl-2, which restrains Bim activity. However, several studies have shown that CD8+ T cell contraction occurs even in priming situations in which the majority of cells at the expansion peak express IL-7Rα (Lacombe et al., 2005), and also that enforced (transgenic) expression of IL-7Rα does not rescue activated cells into the long lived pool (Hand et al., 2007; Haring et al., 2008). Hence CD127 expression and reactivity to IL-7 is likely necessary but not sufficient to drive the effector to memory cell transition. Along similar lines, some priming conditions (e.g. DC vaccines) can very rapidly generate cells which exhibit memory properties (including KLRG-1loCD127hi phenotype and recall functional potential) – yet these cells contract to a similar extent as cells primed by conventional pathways (Badovinac et al., 2005).
So, are there priming situations where contraction is averted or minimized? Very short term pathogen infection can lead to priming of a CD8+ T cell response which is modest in size but very effectively undergoes transition to a functional memory pool (Badovinac et al., 2004). The idea that this outcome is (at least in part) due to moderating inflammatory stimuli is further supported by the similar phenotype in IFN-γ deficient animals (Badovinac et al., 2000) (Harty and Badovinac, 2008). Furthermore, there is generally less contraction after secondary responses (Badovinac et al., 2003; Masopust et al., 2001a) and this effect is even more dramatic after tertiary stimulation (Masopust et al., 2006a; Vezys et al., 2009). Such findings might be related to more rapid elimination of antigen and reduced intensity or duration of inflammatory cytokines such as IFN-γ due to the high frequency of antigen reactive cells. Interestingly, the phenotype of the durable memory cells from boosting is similar to effector memory or even effector cells (Jabbari and Harty, 2006; Masopust et al., 2006a; Masopust et al., 2006b), which contrasts with models in which differentiation to Tcm cell phenotype predicts optimal longevity.
What about the opposite situation; priming situations which leads to more extensive contraction? As discussed earlier, insufficient duration or quality of TCR and other stimuli can yield effector cells which contract without leaving a memory pool. For the CD4+ T cell pool, this is also seen in situations of excessive competition with naïve cells of the same specificity (Blair and Lefrancois, 2007; Hataye et al., 2006; Whitmire et al., 2008). Strangely enough, for CD8+ T cells abnormally high naïve T cell precursor frequencies and short term antigen exposure seem to accelerate rather than block production of functional memory cells without augmenting contraction (Badovinac et al., 2007). The basis for these interesting differences between CD4+ and CD8+ T cells is unclear, but highlights the risks of interpreting studies using antigen specific naïve T-cells at non-physiologically high frequencies.
The issue of “not-so-short lived” effectors
Despite the substantial decline in antigen specific effector T cells during contraction, some cells with this phenotype are found a considerable time after clearance of antigen (many months in the spleen, and even longer in other tissue sites – see later). Further, adoptive transfer approaches show that effector-like cells actually have a fairly leisurely decay rate. For example, T cells isolated from the expansion phase as “effector” versus “memory precursor” pools (using markers such as IL-7Rα or KLRG-1) differ only slightly (∼3 fold) in their persistence at 4-6 weeks (Kaech et al., 2003; Sarkar et al., 2008), despite the fact that the effector pool exhibits minimal basal homeostatic proliferation. The continued survival of these effector-phenotype cells is not simply due to them differentiating into memory cells, because the majority of transferred KLRG-1hiIL-7Rαlo cells maintain this phenotype for many weeks after adoptive transfer (Sarkar et al., 2008). Likewise, studies on Sendai infected mice suggest CD8+ T cells with effector-like properties (including sustained expression of Granzyme B expression) persist for many months after infection (Hikono et al., 2007).
A more extreme situation is where repeated immunizations have been used to boost the memory T cell pool and for populations in non-lymphoid sites. Compared to primary immune responses, the boosted CD8+ T cell population sustains a population of cells with more effector-like properties (Granzyme B+KLRG-1hi and, in some cases, IL-7Rlo) suggesting such cells are not always short lived (Jabbari and Harty, 2006; Masopust et al., 2006a; Masopust et al., 2006b).
This finding leads to the important question of whether such “not-so-short-lived” effector cells have any functional relevance, or are purely detritus waiting to be cleared from tissues? We will return to this question under “function of the long-lived pool” later.
The persistence of memory
The typical view of a “useful” primed cell is one which is both long-lived (at the population level) and displays the functional traits of a memory cell (including the ability to undergo recall proliferation and differentiation into secondary effector and memory cells). A long-standing controversy regarded whether T cell memory maintenance depended on constitutive stimulation by cognate antigen. Resolution of this argument had implications for whether vaccines should provide persistent depots of antigen or require periodic boosting. In contrast to naïve T cells, memory CD8+ and CD4+ T cells are maintained in the absence of MHC molecules (Freitas and Rocha, 2000; Murali-Krishna et al., 1999; Swain et al., 1999). Memory CD8+ T cells appear to maintain both self-renewal and function in the complete absence of MHC I, (Leignadier et al., 2008; Murali-Krishna et al., 1999) however, optimum memory CD4+ T cell function may depend on exposure to MHC II (De Riva et al., 2007; Kassiotis et al., 2002). Most recently, it was elegantly demonstrated that ablation of TCR also had no effect on memory CD8+ T cell survival (Leignadier et al., 2008). However, chronic infections can markedly alter T cell survival requirements, resulting in cells that are “addicted” to antigen and wane upon transfer to naïve hosts (Shin et al., 2007).
After clearance of acute infections, memory CD4+ and CD8+ T cell maintenance depends on IL-7 and IL-15, which together support survival and basal homeostatic proliferation of the pool (for a recent comprehensive review of this interesting topic, see (Surh and Sprent, 2008)). Competition for these homeostatic cytokine resources may define a limited carrying capacity of the immune system for memory T cells. Such a mechanism may ensure that exposure to numerous infections throughout life does not result in an ever increasing expansion in the size of memory T cell compartment with age.
Most studies have focused on memory populations sustained in secondary lymphoid organs (SLOs) and parenchymal sites, but recent evidence suggests memory CD4+ and CD8+ T cells may make a primary home in the bone marrow (Becker et al., 2005; Mazo et al., 2005; Tokoyoda et al., 2009). This notion creates an interesting anatomic separation issue in the response to non-systemic recall antigen, suggesting an obligation by antigen presenting cell to find the bone marrow resident memory cells, or egress of the memory T cell population to access SLOs or non-lymphoid sites.
It should be noted that Tem cells may express lower amounts of CD122 and undergo less homeostatic proliferation than Tcm cells, which may account for the relatively short life span of Tem cells within blood observed after certain primary infections (Jabbari and Harty, 2006; Wherry et al., 2003). However, heterologous prime-boost vaccination can result in a 4-fold expansion of the Tem cell pool outside of lymph nodes, with little evidence for a reciprocal reduction among the Tcm cell compartment (Vezys et al., 2009). In the absence of further infection, this compartment appears stable, suggesting the size of the Tem cell compartment is not subject to stringent regulation, and may depend on different factors for survival than those defined for Tcm cells. Indeed, in humans, CD45RA+ Tem cells gradually accumulate with age (Czesnikiewicz-Guzik et al., 2008). This process may occur in the absence of CMV infection, and may be distinct from the memory-like T cell clonal expansions observed among the very old (Clambey et al., 2008; Ely et al., 2007; Messaoudi et al., 2006).
The durability of T cell memory remains unclear. In mice, infection with LCMV results in a stable memory CD8+ T cell pool for >900 days, whereas memory CD4+ T cells underwent a 50-fold attrition over the same period (Homann et al., 2001). Although human memory T cell longevity studies are in their infancy (due to their greater logistical complications), a few important studies have examined this issue in situations where periodic pathogen re-exposure are unlikely. Fairly robust levels of memory CD8+ T cells were detected >15 years after infection with Puumala virus (PUUV) without evidence for viral persistence or re-infection (Van Epps et al., 2002). Up to 1% of CD8+ and CD4+ T cells remained specific for measles virus among adults that had a history of natural childhood infection (Nanan et al., 2000). The most comprehensive study to date recounts a non-randomized, cross-sectional analysis of CD4+ and CD8+ T cell memory performed between 1 month and 75 years after smallpox vaccination (Hammarlund et al., 2003). Virus-specific memory T cells were detectable up to 75 years after a single vaccination, with an estimated half-life between 8 and 15 years among both CD8+ and CD4+ T cells. However, it should be noted that long-lived CD8+ T cell immunity was not detected in all individuals, although the reasons for this are unclear.
Although these observations support the hypothesis that T cell memory may persist for the life of the host, it has been shown that certain heterologous infections can result in the ablation of pre-existing memory CD8+ T cells. This process is unlikely to be explained by direct competition between memory cells as the extent of attrition was severe (25-90%), and attrition was induced within only a few days of infection via a Type I or II interferon-dependent process (Dudani et al., 2008; Selin et al., 1999). It will be important to determine how natural infections, as well as immunization, impact the pre-existing memory T cell pool in humans. Although information remains limited, a recent study failed to detect an impact on the number of blood-born influenza and EBV specific CD8+ T cells upon primary HCMV infection (van Leeuwen et al., 2006). Although more studies are needed, available data suggest that T cell memory in humans is generally long-lived, but perhaps not as durable as humoral immunity, which has estimated half-lives that may exceed 50 years (Amanna et al., 2007; Crotty et al., 2003). However, it should be stressed that these data are derived from individuals whom were exposed to live-replicating agents. It is possible that certain immunizations may fail to produce truly long-lived memory T cells (see below).
Non-lymphoid tissues: Getting there
Activation within SLOs precipitates changes in homing molecule expression that results in the anatomic re-distribution of antigen-specific T-cells. Changes in migration potential are likely regulated both by the strength of stimulation, reflected by the intensity and duration of signaling via the TCR, co-stimulation, and the inflammatory milieu, and also by location specific cues that vary within different SLO environments.
In situations where strength of stimulation is likely reduced, either via high dose adoptive transfer of transgenic CD8+ T cells, or by spiking in naïve T cells a few days after the initiation of infection (artificially introducing “latecomers”), a much higher proportion of memory T cells express lymph node homing molecules (Catron et al., 2006; D'Souza and Hedrick, 2006; Sarkar et al., 2007; van Faassen et al., 2005). Similarly, CD4+ T-cells within lung were found to have undergone a larger number of divisions than their counterparts within lymph nodes (Roman et al., 2002). Interestingly, “strength of stimulation” may be cumulative, as boosting memory CD8+ T-cells via repeated infection results in the preferential accumulation of CD62L- cells that populate non-lymphoid organs (Jabbari and Harty, 2006; Masopust et al., 2006a).
Location specific cues have been best defined within SLOs associated with the intestinal mucosa and the skin (Agace, 2006; Mora et al., 2008; Sigmundsdottir and Butcher, 2008). After activation within Peyer's patches and mesenteric lymph nodes, activated T cells preferentially up-regulate receptors involved in homing to the small intestine. This instructional program is delineated in part by CD103+ DCs and the local availability of retinoic acid. In contrast, T-cells primed within the skin-associated inguinal lymph nodes preferentially up-regulate skin homing receptors, a process intriguingly promoted by local higher concentrations of Vitamin D3 metabolites. Although priming within local SLO environments thus favors T cell trafficking to regionally-associated non-lymphoid tissues, it should be stressed that there are several examples in which local immunization routes result in widely disseminated T cell responses within all tissues examined (Kaufman et al., 2008; Liu et al., 2006; Masopust et al., 2004). It remains possible that promiscuous seeding of numerous non-lymphoid compartments is dependent on immunization regimens that induce strong “strength of signal” priming, as induced by live replicating agents, and that subunit vaccines may result in responses that remain preferentially distributed within lymph nodes and local tissues.
Non-lymphoid tissues: Upon arrival
After clearance of infection, large numbers of T cells remain distributed within non-lymphoid tissues (Masopust et al., 2001b; Reinhardt et al., 2001). Confirming predictions based on analyses of human blood T cells that lacked lymph node homing receptors (Sallusto et al., 1999), T cells isolated from tissues differed from “classic” memory T cells in many important functional respects. This precipitated the paradigm that long-lived (i.e. “memory”) T-cells should be divided into two subsets; Tcm cells, which exhibit classic memory T cell properties and traffic through SLOs, and Tem cells, which re-circulate through non-lymphoid tissues (Sallusto et al., 2004).
The generic term “non-lymphoid tissue” accounts for all cells outside of the major inductive sites of immune responses (e.g. lymph nodes and the white pulp of spleen) and does not discriminate between the varied anatomic compartmentalization outside of SLOs. For instance, some cells are likely confined to the vasculature, even among solid organs that have been perfused to remove the majority of red blood cells. One example includes the liver, in which the vast majority of lymphocytes are likely confined to the sinusoids, rather than being located within the parenchyma (Geissmann et al., 2005). Some of these cells may be just quickly passing through, and others may be actively retained on endothelium as has been recently described for a subset of monocytes (Auffray et al., 2007). In contrast, memory T cells are also present within the parenchyma of non-lymphoid tissues, such as the skin and mucosal tissues. Moreover, there are populations of non-lymphoid T cells, such as those within the lung airways, that are localized neither in blood vessels nor in the tissue parenchyma (Woodland and Kohlmeier, 2009). To add to this complexity, there are regional specializations in environments with respect to the cytokine milieu, density of lymphocytes and other hematopoietic cells, tissue architecture, and environment. These tissue-specific differences may reflect exposure to microbial products (for example, the commensal flora in the intestinal mucosa), and the ability of each tissue to tolerate inflammation while maintaining organ function (for instance, inflammation in the lower lung airways inhibits gas exchange).
Therefore, it is perhaps not surprising that T cell properties vary considerably among different anatomic locations, and recent data suggest that the tissue environment plays a direct role in shaping this process independent of variables associated with T cell priming within SLOs. For instance, the environment of the peritoneum in mice induces expression of CD49d among memory CD4+ T cells (Kassiotis and Stockinger, 2004). The lung environment also directly modulates memory CD8+ T cell phenotype (Marzo et al., 2007). For instance, transfer of Tcm cells into the lung airways via the trachea results in the rapid adoption of a unique tissue-specific signature phenotype characterized by down-regulation of CD27 and CD127 (Kohlmeier et al., 2007). Cells in the lung airways also down-regulate Ly6C and CD11a, which are typically highly expressed among antigen-experienced T cells in other tissues. These cells are not long-lived, but must be continually maintained by an influx of memory T cells from the periphery (Ely et al., 2006). A quite distinct tissue-specific phenotype is adopted among CD8+ T-cells after entry within the epithelium of the small intestine. These cells adopt several cardinal features of recently activated T-cells, including up-regulation of CD69 and the maintenance of lytic activity indefinitely (Masopust et al., 2006b). Interestingly, these cells express only low amounts CD122 and undergo relatively little homeostatic division, yet the population can remain stable without evidence for continued recruitment, suggesting that they may have unique maintenance requirements (Ma et al., 2009; Masopust et al., 2006b). Intravenous transfer of memory T-cells from the intestinal epithelium, followed by re-stimulation, resulted in a secondary response that distributed to numerous tissues. Those daughter cells that were recovered from spleen eight months later had adopted the cardinal features of classic Tcm cells, further supporting the hypothesis that tissue environments directly influence T-cell differentiation state, and also indicating that a very “effector-like” phenotype is not terminal (Masopust et al., 2006b). Although we only cited a few examples, it appears likely that T-cells in many tissues, including skin and the central nervous system, may adopt tissue-specific signature phenotypes (Gebhardt et al., 2009; van der Most et al., 2003). Non-lymphoid memory T-cells that are essentially occupying blood vessels or are rapidly re-circulating, such as those in the liver or low pressure alveolar capillary beds of the lung, may be less subject to tissue derived environmental cues.
In this light, conceptually lumping together all non-lymphoid sites is not likely to account for the complexity of T-cell differentiation states. Experimentally, cells isolated from a single non-lymphoid tissue are unlikely to represent the properties of Tem cells in other tissues. This issue is but one reflection of the difficulty in defining Tem cells as a homogenous entity. In fact, in its current usage, Tem cells reflect a broad array of T cell differentiation states that differ from the classical definition of memory T cells (Figure 2). These differentiation states are regulated both by the strength of stimulation during priming, but also by largely unknown tissue-specific factors. An important issue of continuing study is whether memory cell survival is equivalent in all tissue locations (Woodland and Kohlmeier, 2009), although the challenge will be to assess this in situ. It must also be appreciated that properties of memory T cells may be affected transiently or permanently by local cues. These observations are not an attempt to muddy the waters, but rather to confront the true diversity of T-cell qualities and to acknowledge the rather imprecise meaning of the term Tem cells. One current challenge is to determine more completely how T cell differentiation states are regulated, how they are coupled with T cell trafficking, and how these issues relate to protective immunity (see below).
T cell protective Immunity: a matter of quality, quantity, time and location
This review has recounted numerous studies that have attempted to penetrate the processes by which memory T-cells are made, and the factors that govern their maintenance, anatomic distribution, and functional potential. An overarching raison d'être of all these studies is to determine how to exploit this knowledge for the development of safe, effective vaccines. Achieving this ultimate goal will require a thorough understanding of the parameters that relate to protection.
For a memory T cell to participate in protection, it must still be present upon re-infection. As discussed above, certain subsets of antigen specific T cells wane at a leisurely rate after clearance of infection. However, does this mean that these short-lived memories (or long-lived effectors) are useless by-products of an immune response, or do they confer a transient state of heightened host protection? In a mouse model of LCMV infection, CD62L- CD8+ T cells gradually decay or convert to central memory T cells (Wherry et al., 2003). Although these cells were somewhat impaired in their ability to undergo proliferation upon re-stimulation, they were able to confer protection upon transfer to secondary recipients. A more natural, albeit anecdotal, example comes from a mouse model of heterosubtypic immunity (Liang et al., 1994). Mice were infected with influenza virus, resulting in long-lived memory in most tissues. As described above, short-lived T-cells persist in the lung airways, but maintenance of this population depends on continued recruitment from the periphery. For reasons unknown, this recruitment abates over time, resulting in the presence of a very small population of antigen-specific CD8+ T-cells by six months after infection. Gerhard and colleagues found that protective immunity against a serotypically distinct influenza virus (against which T-cells mediate protection) waned with a kinetics similar to the attrition of the memory population in the lung airways (Liang et al., 1994).
This finding suggests that short-lived T-cells within the lung airways provide short-lived protection, and also supports the appealing notion that T-cells positioned at the point of pathogen entry may play an important role in protective immunity against certain infections. Woodland and colleagues re-visited this issue more directly by transferring Sendai virus specific T-cells from the lung airways of immune mice into the airways of naïve mice (Hogan et al., 2001). In this model, they showed that CD4+ T-cells conferred some degree of protection upon homologous challenge. Similar findings were recently reported in an elegant model of herpes simplex viral challenge, demonstrating a role for skin resident CD8+ T cells in mediating rapid protection at the site of infection (Gebhardt et al., 2009). These studies support concerted efforts to establish site-specific T-cell immunity through vaccination in the hopes of contributing to rapid containment of pathogens such as HIV. However, defining the role that T-cells positioned outside of SLOs play in protective immunity remains a major challenge.
Delineation of memory T-cells into Tcm and Tem cell subsets has attracted substantial interest among vaccinologists, in the hopes that creation of the right “kind” of memory T-cells might provide the key to protection. In turn, so-called Tcm and Tem cells have been compared in various protection models, with conflicting results, depending on the experimental system. It should be noted that Tcm cells are often generated by different methods among different studies (for example, live viral infections vs. heat killed Listeria monocytogenes, LM), and delineated solely by the expression of markers such as CD127 and CD62L. Such a readout does not account for variables in the function of Tcm cells after different priming regimens (e.g. whether they rapidly produce IFN-γ upon re-stimulation). Similar concerns regard comparisons of Tem cells among different studies, highlighting the challenges that imprecise definitions of T-cell subsets pose to the field. With these substantial caveats in mind, it appears that Tem cells are most effective at controlling vaccinia virus and LM infections, as measured by evaluating infectious burden 3-4 days after infection (Bachmann et al., 2005; Huster et al., 2006). In contrast, Tcm cells may be more effective at controlling a systemic challenge with a strain of LCMV that causes a chronic infection in naïve mice (Wherry et al., 2003). In the latter study, it should be noted that Tcm cells did not contribute to protection until more than a week after viral challenge. In this instance, it seems likely that substantial expansion of the antigen-specific CD8+ T cell population, as well as differentiation into effector cells, was required before it was able to contribute to viral control. A tentative conclusion that could be drawn from these studies is that Tem cells might be more effective at controlling infections very early, especially outside of SLOs. However, if the infectious challenge exceeds the capacity of pre-existing Ag-specific cells to control infection, then the greater proliferation potential of “classic” memory CD8+ T-cells wins the day. Thus, the importance of different subsets may vary depending on the pathogen in question, the route and dose of infection, and the quantity of antigen-specific T cells present prior to infection.
The above discussion raises another important question. How important is T cell quantity? This question takes on additional prescience in light of the recent and highly publicized failure of the Phase 2b efficacy STEP trial, which represented an ambitious test of CD8 T cell vaccination against HIV in humans. Although this vaccine generated 100-500 HIV-specific memory CD8+ T cells per 106 PBMC, it afforded no protection. Was this a failure of CD8+ T cell quantity, quality and/or anatomic distribution, or rather a revelation that CD8+ T cells cannot protect against HIV (Masopust, 2009)? There is little information to guide informed speculation. However, recent studies found that higher frequencies of CD8+ T cells (∼2000 per 106 PBMC) generated by prime-boost vaccination or immunization with a persistent vector, afforded some degree of protection against fairly stringent SIV challenges in non-human primates (Hansen et al., 2009; Liu et al., 2008). Although this bolsters hope in the CD8 T cell vaccine concept, more information is needed regarding the relationship between CD8+ T-cell quantity, quality, and protective immunity. In this light, a recent study from Harty and colleagues carries both good and bad news. Using a creative model of prime-boost vaccination, they modulated the number of memory CD8+ T cells specific for Plasmodium (Schmidt et al., 2008). They found that CTL immunity was sufficient to prevent blood-stage parasitemia, but only if the quantity of antigen specific CD8+ T-cells exceeded a very large minimum threshold (>1% of blood lymphocytes). In comparison, 100-1000 fold fewer CD8+ T cells were sufficient for protection against LM or LCMV. Although this study did not rule out changes in quality induced by the different immunization schemes that may also have contributed to protection, it illustrates that the requirements for CD8+ T-cell-mediated protective immunity against some pathogens may greatly exceed that generated by current human vaccine strategies that rely heavily on replication-deficient vectors.
Bad Memories
Just as there are many ways to define a “good” memory cell, there are also ways these cells can fail to contribute to the recall response. A well studied case is CD8+ T memory cells primed in the absence of CD4+ T cell help. In many acute infection models, the production of effector and memory CD8+ T cell populations follows the normal pattern despite CD4+ T cell deficiency. These “unhelped” CD8+ T memory cells show reduced maintenance, but more dramatically show impaired function (including protection) and an almost complete loss of recall proliferative capacity (Janssen et al., 2003; Sun and Bevan, 2003) (Janssen et al., 2003; Shedlock and Shen, 2003). Although the exact basis for these defects is currently unclear, an unexpected twist is that T-bet deficiency partially rescues the “unhelped” CD8+ T memory cell phenotype (Intlekofer et al., 2007). Interestingly, analogous defects in recall proliferative potential are observed for CD8+ T cells which lack expression of IL-2Ra (CD25) (Williams et al., 2006), raising the possibility that CD4+ T cell-produced IL-2 may be an critical element in CD4+ T cell “help” for CD8+ T cell memory. Thus, despite the fact that both unhelped and CD25-deficient (Il2ra-/-) CD8+ T memory cells resemble Tcm cells, they show defective homeostasis and/or reactivity (Fig 3).
Functional deficiency may also arise due to the suboptimal priming, and this may be difficult to observe by simply monitoring T cell numbers or individual functional readouts. Testing various vaccination approaches for priming CD4+ T cell responses to Leishmainia antigens revealed that protective immunity correlated with the cells capacity for multiple functional responses (Darrah et al., 2007).
Alternative ways to make memory cells: Heterologous memory
Are the specificities of T cell memories always restricted to the pathogens that induced them? Perhaps not. One important example is the case of heterosubtypic immunity, by which memory CD8+ T cells mount secondary responses upon challenge with serologically distinct, but phylogenetically related viruses. Such observations have been made in several models, including infection of mice with vesicular stomatitis viruses of the New Jersey and Indiana strains, and influenza viruses, which also confer a degree of protective immunity against the second challenge (Christensen et al., 2000). In addition, situations have also been documented in which immunity to one virus, LCMV, confers protection to an unrelated virus, vaccinia. Interestingly, the specificity of cross-reactive CD8+ T cell clones between LCMV and vaccinia virus varies among individual inbred mice, suggesting that cross-reactivity between different infectious agents may not be uncommon (Kim et al., 2005). Indeed, cross-reactive human CD8+ T cell clones have been detected that recognize epitopes from both influenza A virus and Epstein-Barr virus (Clute et al., 2005). These observations suggest that primary responses among individuals with a diverse repertoire of memory CD8+ T cells may contain re-activated secondary clonal expansions of rare cross-reactive pre-existing memory T cells. Such events may influence the quality and immunodominance hierarchy of the response, and may have implications for protective immunity.
“Homeostatic” memory
Over the last 10 years it has become clear that the scheme shown in Fig 1 is not the only means to generate memory T cells. During studies on T cell homeostasis, it was found that naïve cells respond to T cell lymphopenia by undergoing a slow proliferation (variously called “homeostatic proliferation” or, less ambiguously “lymphopenia induced proliferation”, or LIP), during which time the naïve cells change in their phenotype and function to resemble memory cells (Surh and Sprent, 2008). This process involves recognition of self peptide-MHC molecules and the cytokines IL-7 and IL-15 (Surh and Sprent, 2008), but cells responding to lymphopenia appear not to transition through a detectable effector stage. This process may therefore resemble the low intensity stimuli which promote memory T cell differentiation discussed earlier. LIP generates memory cells which exhibit functional properties similar to “true” (i.e. antigen primed) memory cells (Surh and Sprent, 2008), and are capable of undergoing a strong proliferative response when stimulated by foreign antigen and mediating protective immunity against infection (Hamilton et al., 2006). Although typically studied in artificial situations of lymphopenia, similar process may occur in normal physiological stages, such as during the lymphopenia associated with neonatal mice (Ichii et al., 2002; Min et al., 2003; Schuler et al., 2004). This raises the possibility that the endogenous T cell pool may contain memory-like cells produced without conventional priming
While an “endogenous” memory T cell pool has long been documented in mice (including those maintained in SPF or even germ free conditions (Haluszczak et al., 2009; Huang et al., 2005), the specificity of this pool was largely unexplored. Recent studies suggest that the endogenous pool of memory phenotype cells includes cells with reactivity toward unencountered foreign antigens (Haluszczak et al., 2009). This normal pool, which has hallmarks of deriving from LIP, exhibits at least some of the functional traits of memory cells (Haluszczak et al., 2009). However, this population is extremely rare, representing only a fraction of the naïve antigen specific precursor pool, and how these cells contribute to physiological immune responses, and whether their contribution differs from that of their naïve counterparts, awaits further studies. However, these findings do suggest unprimed endogenous memory cells may be active participants in “primary” immune responses.
Concluding remarks
The chief objectives in this review were not only to discuss new developments in our understanding of the generation and function of memory T cells, but to highlight the fact that memory T cell populations are tremendously diverse in terms of phenotype, function, developmental plasticity, distribution, longevity and protective capacity. A single, or even a handful, of markers is unlikely to reliably predict either the developmental or protective potential of “subsets” that transcends single experimental systems. Ignoring this complexity, and splitting T cells into poorly defined effector and memory subsets, may create disagreements that are more grounded in semantics than biology. However, a range of innovative techniques, including analyses of single cells, cellular barcoding, and genome wide transcriptional profiling, seem poised to push our insight even further into T-cell fate decisions. When such information is coupled to bona fide studies of protective immunity, the field has the potential to make tremendous and much needed contributions to the development of protective T-cell vaccines.
References
- Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol. 2006;6:682–692. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009 doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buentke E, Mathiot A, Tolaini M, Di Santo J, Zamoyska R, Seddon B. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood. 2006;108:1949–1956. doi: 10.1182/blood-2006-04-016857. [DOI] [PubMed] [Google Scholar]
- Calame K. Transcription factors that regulate memory in humoral responses. Immunological reviews. 2006;211:269–279. doi: 10.1111/j.0105-2896.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. The Journal of experimental medicine. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Christensen JP, Doherty PC, Branum KC, Riberdy JM. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J Virol. 2000;74:11690–11696. doi: 10.1128/jvi.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci U S A. 2008;105:12997–13002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, Welsh RM, Selin LK. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest. 2005;115:3602–3612. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- De Riva A, Bourgeois C, Kassiotis G, Stockinger B. Noncognate interaction with MHC class II molecules is essential for maintenance of T cell metabolism to establish optimal memory CD4 T cell function. J Immunol. 2007;178:5488–5495. doi: 10.4049/jimmunol.178.9.5488. [DOI] [PubMed] [Google Scholar]
- Dudani R, Murali-Krishna K, Krishnan L, Sad S. IFN-gamma induces the erosion of preexisting CD8 T cell memory during infection with a heterologous intracellular bacterium. J Immunol. 2008;181:1700–1709. doi: 10.4049/jimmunol.181.3.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely KH, Ahmed M, Kohlmeier JE, Roberts AD, Wittmer ST, Blackman MA, Woodland DL. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]
- Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- Ely KH, Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol. 2003;171:3338–3342. doi: 10.4049/jimmunol.171.7.3338. [DOI] [PubMed] [Google Scholar]
- Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- Glimcher LH. Trawling for treasure: tales of T-bet. Nat Immunol. 2007;8:448–450. doi: 10.1038/ni0507-448. [DOI] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. The Journal of experimental medicine. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. The Journal of experimental medicine. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. The Journal of experimental medicine. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. The Journal of experimental medicine. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Huang T, Wei B, Velazquez P, Borneman J, Braun J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clin Immunol. 2005;117:221–230. doi: 10.1016/j.clim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur J Immunol. 2006;36:1453–1464. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. The Journal of experimental medicine. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Wherry EJ, Reiner SL. Not-so-great expectations: re-assessing the essence of T-cell memory. Immunological reviews. 2006;211:203–213. doi: 10.1111/j.0105-2896.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. The Journal of experimental medicine. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp1 is required for the differentiation of protective effector CD8+ T cells and memory responses. Immunity. 2009 doi: 10.1016/j.immuni.2009.06.021. In Press. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Stockinger B. Anatomical heterogeneity of memory CD4+ T cells due to reversible adaptation to the microenvironment. J Immunol. 2004;173:7292–7298. doi: 10.4049/jimmunol.173.12.7292. [DOI] [PubMed] [Google Scholar]
- Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. 2008;181:4188–4198. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. The Journal of experimental medicine. 2005;201:523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: Antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Leignadier J, Hardy MP, Cloutier M, Rooney J, Labrecque N. Memory T-lymphocyte survival does not require T-cell receptor expression. Proc Natl Acad Sci U S A. 2008;105:20440–20445. doi: 10.1073/pnas.0806289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2008 doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T, Radbruch A, Zinkernagel RM, Hengartner H. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. The Journal of experimental medicine. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, Acero LF, Zal T, Schluns KS. Trans-Presentation of IL-15 by Intestinal Epithelial Cells Drives Development of CD8{alpha}{alpha} IELs. J Immunol. 2009 doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris CH, Miller JD, Altman JD, Jacob J. A transgenic mouse model genetically tags all activated CD8 T cells. J Immunol. 2003;171:2393–2401. doi: 10.4049/jimmunol.171.5.2393. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265:125–137. doi: 10.1111/j.1365-2796.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006a;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Masopust D, Jiang J, Shen H, Lefrancois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J Immunol. 2001a;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001b;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006b;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunological reviews. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- Nanan R, Rauch A, Kampgen E, Niewiesk S, Kreth HW. A novel sensitive approach for frequency analysis of measles virus-specific memory T-lymphocytes in healthy adults with a childhood history of natural measles. J Gen Virol. 2000;81:1313–1319. doi: 10.1099/0022-1317-81-5-1313. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009 doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc Natl Acad Sci U S A. 2008;105:16689–16694. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. The Journal of experimental medicine. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. The Journal of experimental medicine. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Blimp-1 promotes terminal differentiation of virus-specific CD8 T cells and represses the acquisition of central memory T cell properties. Immunity. 2009 doi: 10.1016/j.immuni.2009.05.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of experimental medicine. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- Schepers K, Swart E, van Heijst JW, Gerlach C, Castrucci M, Sie D, Heimerikx M, Velds A, Kerkhoven RM, Arens R, Schumacher TN. Dissecting T cell lineage relationships by cellular barcoding. The Journal of experimental medicine. 2008;205:2309–2318. doi: 10.1084/jem.20072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Schuler T, Hammerling GJ, Arnold B. Cutting edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells. J Immunol. 2004;172:15–19. doi: 10.4049/jimmunol.172.1.15. [DOI] [PubMed] [Google Scholar]
- Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- Shaulov A, Murali-Krishna K. CD8 T cell expansion and memory differentiation are facilitated by simultaneous and sustained exposure to antigenic and inflammatory milieu. J Immunol. 2008;180:1131–1138. doi: 10.4049/jimmunol.180.2.1131. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. The Journal of experimental medicine. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- van der Most RG, Murali-Krishna K, Ahmed R. Prolonged presence of effector-memory CD8 T cells in the central nervous system after dengue virus encephalitis. Int Immunol. 2003;15:119–125. doi: 10.1093/intimm/dxg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps HL, Terajima M, Mustonen J, Arstila TP, Corey EA, Vaheri A, Ennis FA. Long-lived memory T lymphocyte responses after hantavirus infection. The Journal of experimental medicine. 2002;196:579–588. doi: 10.1084/jem.20011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- van Leeuwen EM, Koning JJ, Remmerswaal EB, van Baarle D, van Lier RA, ten Berge IJ. Differential usage of cellular niches by cytomegalovirus versus EBV- and influenza virus-specific CD8+ T cells. J Immunol. 2006;177:4998–5005. doi: 10.4049/jimmunol.177.8.4998. [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. The Journal of experimental medicine. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Benning N, Eam B, Whitton JL. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J Immunol. 2008;180:6777–6785. doi: 10.4049/jimmunol.180.10.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]