Abstract
Objective
This fMRI study examined how working memory circuits are affected by face emotion processing in pediatric bipolar disorder (PBD) and attention-deficit hyperactivity disorder (ADHD).
Methods
Twenty-three patients with bipolar disorder, 14 patients with ADHD and 19 healthy controls (HC) (mean age = 13.36 ± 2.55) underwent an affective 2-back fMRI task with blocks of happy, angry and neutral faces.
Results
For angry vs neutral faces PBD patients, relative to ADHD patients, exhibited increased activation in subgenual anterior cingulate cortex (ACC) and orbitofrontal cortex, and reduced activation in dorsolateral prefrontal cortex (DLPFC) and premotor cortex. Relative to HC the PBD group showed no increased activation and reduced activation at the junction of DLPFC and ventrolateral prefrontal cortex (VLPFC). Relative to HC the ADHD patients exhibited greater activation in DLPFC and reduced activation in ventral and medial PFC, pregenual ACC, striatum and temporo-parietal regions. For happy vs neutral faces, relative to ADHD the PBD group exhibited greater activation in bilateral caudate, and relative to HC it showed increased activation in DLPFC, striatal and parietal regions, and no reduced activation. The ADHD group, compared to HC, showed no reduced activation and increased activation in regions that were under-active for the angry face condition.
Conclusions
Relative to the ADHD group the PBD group exhibited greater deployment of the emotion processing circuitry and reduced deployment of working memory circuitry. Commonalities across PBD and ADHD patients, relative to HC, entailed cortico-subcortical activity that is reduced under negative emotional challenge, and increased under positive emotional challenge.
Keywords: Functional magnetic resonance imaging (fMRI), pediatric bipolar disorder, ADHD, working memory, emotion
Introduction
This fMRI study examined the effects of emotion processing on working memory function in adolescents with pediatric bipolar disorder (PBD) and adolescents with attention deficit hyperactivity disorder (ADHD), two disorder models where emotion and cognitive systems are implicated. Overlapping symptoms and cognitive deficits in PBD and ADHD complicate diagnosis1, and there is need for a concerted effort in differentiating the pathophysiology and phenotypes underlying these two developmental disorders, both at a cognitive and neural level. Understanding mental disorders based on dimensions of behavior (e.g., executive functions, working memory, affect processing) and neuroscience-based criteria will not only pave the way for symptom-based classification, but also help decipher the neural operations that underlie the real-life interface between these two domains2. Building such knowledge will help accrue more informed methods on prevention and targeted early intervention for each of these two developmental disorders.
Emotional dysregulation and rapid mood cycling with mixed episodes are predominant in PBD3–5. However, in addition to the core deficits of inattention and impulsivity6 also ADHD seems to manifest symptoms of affect dysregulation that are persistent, though they are not episodic 7 as is the case for PBD. Moreover, manifestations of hyperactivity, distractibility, irritability and frustration are similar in ADHD and in hypomania and mania in PBD1,8.
Deficits in working memory, the ability to temporarily process and store information in short-term memory9, have been consistently found among patients with bipolar disorder in children10–11,12 and adults13–15. These deficits are present both in acutely ill and in euthymic PBD patients10. They also persist and lead to lower academic achievement in PBD, relative to HC, for example over a three year longitudinal study, and despite optimal treatment and euthymic phase10–11. Working memory deficits have also been consistently found among patients with ADHD both in children16–18 and adults6,19–21, and are associated with executive function deficits17, scholastic under achievement and poor interpersonal functions22–24. After a comprehensive qualitative review of studies on executive function in PBD and ADHD patients, mainly for each patient group relative to healthy controls (HC), Walshaw et al.8 concluded that there may be initial evidence of distinct phenotypic profiles for these two illnesses in terms of interference control, working memory, planning and cognitive flexibility. Of interest, the authors suggest that their review found evidence for impairment in verbal and spatial working memory as more specific to ADHD, whereas deficits in interference control, planning and cognitive flexibility would be more specific to PBD. Nevertheless, more research may be necessary to provide more support to the specific neurocognitive domains for these putative phenotypic differences, since other studies have found working memory and verbal memory deficits in PBD as well10–12.
Because we wanted to probe concurrently the cognitive and affective circuits that are often simultaneously engaged in thinking and feeling during activities at school and home among pediatric population, we decided to use an n-back task with emotional faces that requires both working memory processes and face emotion processing. The interaction between cognitive and affective functions results from synergistic neural mechanisms of the ventral affect processing system (i.e., amygdala, ventrolateral prefrontal cortex, rostral anterior cingulate cortex, orbitofrontal cortex, medial prefrontal cortex) and the dorsal executive control system (dorsolateral prefrontal cortex, dorsal anterior cingulate cortex, parietal regions) 25,26. Working memory functions, as examined using the popular n-back working memory paradigm, are carried out by an extended network of brain regions within in the dorsal executive control system27, including the dorsolateral prefrontal cortex (DLPFC) (BA 9/46) involved in online maintenance, monitoring and manipulation of information28, response selection29 and strategies implementation30, the mid- ventrolateral prefrontal cortex (mid-VLPFC) (BA 45, 47) implicated in selection, comparison and judgment of stimuli in short and long term memory28, the parietal cortex, involved in storage of working memory contents31 and response mapping32, and the dorsal anterior cingulate cortex (ACC), involved in attention processes, cognitive effort and error detection and correction33. Brain imaging research suggests that the fronto-striatal system may underlie the ‘central executive’ as defined in Baddeley’s working memory model9, while temporo-parietal regions are more involved in rehearsal and short-term memory processing9,27. In addition to involvement of the brain circuits discussed above in working memory function, a study by Braver et al.34, that used a n-back task with face stimuli, found increased activation in limbic and face processing regions in healthy adults, which suggests the appropriateness of this paradigm for examining effects of emotion processing on working memory function.
There is at present no published work on working memory in PBD or ADHD using the n-back task, but studies that used similar working memory paradigms found evidence that fronto-striatal-parietal dysfunction is implicated in adult bipolar disorder35–38 and in PBD39,40 as well as in children with ADHD16,41 and adults with ADHD20,42,43. Specifically, altered functioning of VLPFC, DLPFC, temporal and posterior parietal regions was found in euthymic adult patients with bipolar disorder (BD) relative to HC during a 2-back working memory task, which was ascribed to deficits in frontal executive functions35. Moreover, Haldane et al.37 found that, unlike HC, euthymic BD patients did not show increased DLPFC activation with increasing load during an n-back task, and Gruber et al.44 found increased right amygdala activation during a verbal working memory task in euthymic BD patients, even in the absence of emotional information and independently of task performance and medication. These findings suggest that working memory circuits may be impaired and may be persistently affected by emotion processing in PBD even during euthymic state. Furthermore, while affect dysregulation is prominent in PBD39,45–47,48, only a few studies have examined the interaction of cognitive and affective systems in bipolar disorder relative to HC, and have reported an overactive limbic system together with reduced activation in a prefrontal region at the intersection of DLPFC and VLPFC, during affective stroop tasks both in adolescents39,40 and adults44, 49,50 with bipolar disorder.
With regard to ADHD, findings from imaging studies with children with ADHD16,19,41, together with our previous findings of decreased VLPFC activation in children with ADHD relative to HC during an affective stroop task40 and a response inhibition task51, suggest that both inhibitory and working memory deficits in ADHD may stem from neuropathology of VLPFC52,53. Abnormalities in ACC functioning have been also implicated in response inhibition and working memory deficits in patients with ADHD52,53. Finally, it is noteworthy that deficits in self regulation of emotions54, in facial affect recognition55 and in the ability to separate emotion from cognitive processes56 have also been documented in ADHD.
Most neurocognitive or fMRI studies do not compare ADHD and PBD directly, and it is still unclear whether these two illnesses present different or similar pathophysiological phenotypes for cognitive and emotion processing. Importantly, a recent fMRI study by Brotman et al.57 found that children with ADHD exhibited hyperactive amygdala activation while rating subjective fear of neutral faces compared not only to HC but also to pediatric patients with bipolar disorder and with severe mood dysregulation, in spite of overlapping behavioral deficits and clinical symptoms. This novel finding suggests the importance of further investigating differing neural correlates of face emotion processing, and also of cognitive processing at the interface with emotion processing, in ADHD relative not only to HC but also to other pediatric groups with mood dysregulation.
While there are not at present published studies examining the interface of working memory and affect directly in PBD and ADHD, when comparing children with PBD and with ADHD using a response inhibition task51 and an affective color word matching task40 we illustrated under-activity in prefrontal cortex (PFC) and anterior cingulate cortex (ACC) in ADHD relative to PBD. Building on these earlier studies, and given the importance of probing concurrently the cognitive and affective circuits that are often simultaneously engaged in daily activities, we aimed to probe the interface of these two circuits by administering a 2-back working memory task that typically engages fronto-striato-parietal circuits27,58.
To our knowledge this is the first study to examine the effects of emotion on working memory processing concurrently in children with PBD and children with ADHD. While it is also very important to study comorbidity aspects in these two illnesses, for the present study we decided to include in our PBD and ADHD samples only individuals without comorbidity in either illness, in order to maximize potential differences in brain activation between the two groups that could help identify phenotypic differences for the effects of emotion on cognition. Our main comparison of interest was between neural activity during the working memory task for angry versus neutral faces and for happy vs neutral faces. We hypothesized that the ADHD and PBD groups would show worse performance accuracy relative to HC, as found in previous studies on working memory performance in these groups10,11,16,18, and that functioning of working memory circuits would be altered in ADHD and PBD relative to HC, as also previously found12,16,36, 40. Moreover, our recent work 40,51 together with findings from the study by Brotman et al.57 guided our basic hypothesis that there would be phenotypic differences in the way working memory and emotion circuits work together, both when comparing PBD and ADHD, and when comparing each patient group to HC. We predicted that with this affective n-back task both patient groups would show reduced activation in cognitive and emotion regulation regions like the VLPFC and ACC, relative to HC, because emotional information may challenge the already dysfunctional affect regulation systems in PBD5,40,47 and ADHD21,54,55. But we also predicted that the ADHD group, relative to the other two groups, would show the most severe impairment in working memory and regulatory regions in prefrontal cortex, whereas the PBD group would show greater effects of emotion processing on neural functioning relative to the other two groups.
Finally, we expected that within- and between-group differences in working memory function would be modulated by face emotion valence (i.e., angry or happy faces). More specifically, we hypothesized that there would be more dramatic group differences for the angry vs neutral faces comparison than for the happy vs neutral faces comparison, because relative to neutral valence stimuli negative valence stimuli capture attention to a greater degree than positive valence or non-emotional stimuli59,60, and more so in individuals with mood dysregulation40,61.
Method
Participants
Patient participants were recruited from the child psychiatry clinics at the University of Illinois at Chicago (UIC) and healthy controls were recruited from the neighboring community through word-of-mouth and advertisement. This study was approved by the UIC Institutional Review Board. We obtained assent for children younger than 15 years, and informed consent for children aged 15 or older. Consent from at least one parent or legal guardian was also obtained.
We matched our groups based on age, sex, SES, handedness, race, and IQ as estimated with the Wechsler Abbreviated Scale of Intelligence62. Inclusion criteria were as follows: Age of 10 to 18 years for all subjects; for patient groups, Axis I diagnosis of bipolar disorder, type I and II, for the PBD group, and a diagnosis of ADHD, combined subtype, for the ADHD group, based on the DSM-IV criteria63; consent to be scanned in a medication-free state. Patients were already medication-free, not requiring a wash-out at study entry, or were sufficiently unstable on medications to justify discontinuation of an ineffective treatment regimen prior to the beginning of the study with the consent of parents and the assent or consent of patients. The washout period consisted of tapering previous medications over one week, allowing a medication-free period of at least 4–7 days prior to scanning. None of the patients were on fluoxetine that would have required a longer wash-out period. With regard to Exclusion criteria, subjects with comorbid DSM-IV diagnosis of bipolar disorder in the ADHD group or with DSM-IV diagnosis of ADHD in the PBD group were excluded from the study. Conversely, for the purposes of answering the central question of the study, that is to differentiate the phenotype of ADHD from that of PBD, patients who had a prior diagnosis or history of ADHD before the onset of PBD were excluded from the PBD group. The purpose of this selection criterion was to ensure that we would not be studying ADHD pathology in a PBD patient by virtue of ADHD being present without PBD before 7 years of age. However, presence of cross sectional symptoms of criteria A in ADHD (i.e., inattention, hyperactivity and impulsivity) among PBD patients was acceptable for inclusion. Further, severe mental illnesses such as schizophrenia or autism, psychosis not otherwise specified, pervasive developmental disorder, compulsive disorder, obsessive-compulsive disorder, and any other disorder requiring pharmacotherapy were excluded to avoid major confounds of pathophysiology and medication effects. Also, patients and HC were excluded from the study if they had a history of non febrile seizures, head trauma with loss of consciousness for more than 10 minutes, neurological symptoms, speech or hearing difficulties, an IQ score of less than 70, a history of substance abuse, and any contraindications to MRI scans (i.e., metal implants, retractors, braces, established or possible pregnancy, and claustrophobia). Based on these criteria our sample included twenty-three un-medicated PBD patients (19 manic/mixed, 4 hypomanic), 14 un-medicated ADHD patients, and 19 HC (mean age = 13.36 ± 2.55, tot N=56).
Clinical Assessment
The subject and a parent or legal guardian were interviewed using the Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) 3 to determine the DSM IV63 diagnoses of PBD or ADHD, and the absence of these and other psychiatric diagnoses in the HC group. Additionally, a Parent ADHD Rating Scale IV-Revised64 and a handedness questionnaire65 were also administered to all participants. Clinicians rated the Young Mania Rating Scale66 and the Child Depression Rating Scale-Revised67.
fMRI session and n-back task with emotional faces
After a brief training session in a mock scanner, participants underwent an fMRI scanning session when they were administered a block design 2-back working memory task with emotional faces for approximately 7 min. The paradigm involved two runs with one condition each. The first run consisted of blocks of angry and neutral faces and the second run consisted of blocks of happy and neutral faces. On each trial a face stimulus with a certain emotion (i.e., happy, angry) or a neutral expression was presented for 3 sec and subjects responded by pressing a response key if they saw the same face as the one presented two trials earlier (Figure 1). In this task a 2-back match trial always involved a match both in face identity and face emotion, so that subjects needed to attend to both aspects while performing the task. We did not have trials in which there was a match based on face identity or face emotion alone in order to avoid confounds due to selective attention processes in this working memory task that may complicate data interpretation.
Figure 1.
Illustration of match trials in the 2-back working memory task, with happy, neutral and angry faces.
For our task stimuli we employed 160 Gur faces68 with neutral, angry or happy expressions. Face stimuli were balanced by gender, race and facial expression. Each face emotion condition (angry, happy, neutral) consisted of four 30 second trial blocks, with angry, neutral and happy face blocks presented in a counter-balanced pseudo-random sequence. There were 10 trials in each block. As done in a previous study we included a 20 sec fixation in-between blocks to allow for emotional arousal to return to baseline levels4 (Figure 1). A color high-resolution LCD projector projected visual stimuli onto a rear projection screen that was viewed via an angled double mirror system mounted on a standard GE head coil. A camera monitored subjects’ right eye during the scan to ensure that subjects were looking at the visual stimuli.
MRI Protocol
Gradient-echo echo-planar functional imaging and structural acquisitions were performed with a 3.0 Tesla whole body scanner (Signa, General Electric Medical System, Milwaukee, WI). We acquired twenty-five slices in the axial plane (TE=25 ms; flip angle=90°; field of view=20 × 20 cm2; acquisition matrix=64 × 64; TR=2.5s; slice thickness=5 mm with 1 mm gap). Anatomical images were also acquired in the axial plane (three-dimensional spoiled gradient recalled [SPGR], 1.5 mm thick contiguous axial slices) and were later co-registered with the functional data.
Image Processing and Data Analysis
For head motion correction in our functional images we used FIASCO software (Functional Imaging Analysis Software-Computational Olio)69. FIASCO implements both 3D motion estimation and correction, removal of slow signal drift, and identification of images with artifacts such as high shot noise or displacement that cannot be readily corrected by motion correction algorithms68. We excluded from the analyses individual volumes from the time series if head displacement from the median head position in the time series was greater than 1.5 mm, or if head rotation from the median head position was greater than 0.5 degrees. There were no significant group differences in the number of volumes retained after discarding those with motion artifact.
For each subject, voxel-wise effect size (r) maps were obtained by contrasting activation for angry versus neutral as well as for happy versus neutral face emotions. A Fisher z transform was also applied, voxel by voxel, to normalize the effect size maps (zr) 70. The zr-maps and SPGR anatomical images were imported in AFNI (Analysis of Functional Neuroimages) 71 and warped into Talairach space using AFNI’s auto-talairaching procedures72. Lastly, we re-sampled each individual Talairached functional map (3.125 × 3.125 × 6 mm grid) to an isotropic 3 × 3 × 3 mm grid.
Because we had specific hypotheses that group differences in working memory function would be modulated by the valence of the face emotion, the primary analysis for this study was a whole-brain voxel-wise 3 × 2 ANOVA with Group (PBD, ADHD, HC) as the between-subjects factor and Face emotion (angry vs neutral, happy vs neutral) as the within-subjects factor. A significant two-way interaction was followed by pair-wise comparisons for the significant clusters emerging from the interaction, to clarify within- and between-group differences in activation for the face emotion conditions. Moreover, to correct Type I error rates for multiple group comparisons, we adopted an adjusted voxel-wise probability threshold for significance of p<.016. Then, to correct for voxel-wise multiple comparisons in the fMRI analyses we adopted AFNI’s AlphaSim Monte Carlo simulations73 at the whole-brain level, which were restricted to in-brain voxels, to identify clusters of voxels with significant group difference using a contiguity threshold (minimum volume threshold = 351 cubic mm; minimum clustering radius: 3.1 mm; uncorrected p=.016) that ensured an experiment-wise Type 1 error rate of p < 0.02 (corrected p).
Finally, we performed exploratory Spearman correlation analyses to further explore the relationship between brain activation in anatomical ROIs for which we had predictions and that resulted significant in the ANOVA interaction (i.e., left and right DLPFC, VLPFC, medial PFC, ACC, amygdala, precuneus), and behavioral performance (RT, accuracy) as well as clinical measures (YMRS, CDRS-R and ADHD rating scale) for the two conditions in each group. Anatomical ROIs were defined in standard Talairach space using AFNI tools. These regions in AFNI format, as well as the rationale for anatomical ROI definition, are available at our UIC Center for Cognitive Medicine (CCM) web site74.
Demographic, Clinical and Behavioral Data Analyses
For demographic and clinical data, separate ANOVAs were carried out for each demographic or clinical measure (Age, Estimated IQ, SES, YMRS, CDRS-R, ADHD-IV-R), which was the within-subjects factor, while group (PBD, ADHD, HC) was the between-subjects factor. Planned comparisons between groups were then carried out for significant effects by using a corrected p<.002 to correct for multiple comparisons. Fisher’s p tests (two-tailed) were carried out for categorical variables (sex, handedness, race). With regard to behavioral performance analyses, repeated measures ANOVAs with group (PBD, ADHD, HC) as a between-subjects factor, and face emotion (negative, positive, neutral) as a within-subjects factor, were carried out on mean response time (RT) and Accuracy data.
Results
Demographic and Clinical Data
Clinical and demographic data for the three study groups are illustrated in Table 1. Separate ANOVAs for each demographic measure revealed no group differences for age, [F(2,53)=.23, p=.79], and estimated IQ [F(2,53)=2.44, p=.10]. For SES there were no significant group differences, although there was a non-significant trend [F(2,53)=2.79, p=.07] suggesting somewhat lower SES scores in the PBD group compared to the ADHD and the HC groups. We also found no significant group differences for handedness, gender, and racial composition using Fisher’s p tests (two-tailed). The three groups differed for mean YMRS [F(2,5)=38.35, p=.000001] and CDRS-R [F(2,53)=38.85, p=.000001] scores. The PBD group had significantly higher ratings than the other two groups for both scales, while the ADHD group differed from HC only on the YMRS scores. Finally, there were group differences on the ADHD Rating Scale IV-R [F(2,53)=76.64, p=.000001] in that PBD and ADHD scores did not differ from each other but were significantly higher than HC scores.
Table 1.
Demographic and clinical characteristics for the Pediatric Bipolar Disorder (PBD), Attention-Deficit Hyperactivity Disorder (ADHD) and Healthy Control (HC) groups.
| PBD (N=23) | ADHD (N=14) | HC (N=19) | ||
|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | (F), p |
| Age (years) | 13.55 (2.48) | 13.00 (2.35) | 13.53 (3.16) | (.23), p<.79 |
| Estimated IQ a | 96.74 (12.99) | 94.93 (14.05) | 105.58 (13.16) | (2.44), p<.10 |
| SES | 1.49 (.30) | 1.92 (.83) | 1.84 (.65) | (2.79), p<.07 |
| YMRS | 18.86 (8.57) | 8.91 (6.72) | 1.32 (1.50) | (38.35), p<.000001 |
| PBD>ADHD (20.39), p<.00004 PBD>HC (75.69), p<.000001 ADHD>HC (10.97), p<.002 |
||||
| CDRS-R | 50.36 (18.24) | 21.78 (3.00) | 19.53 (6.83) | (38.85), p<.000001 |
| PBD>ADHD (45.53), p<.000001 PBD>HC (63.30), p<.000001 ADHD=HC (.26), p<.61 |
||||
| ADHD Rating Scale IV- R | 23.45 (6.71) | 26.31 (8.22) | 2.93 (3.57) | (76.64), p<.000001 |
| PBD=ADHD (1.85), p<.18 PBD>HC (113.23), p<.000001 ADHD>HC (113.93), p<.000001 |
||||
| Variable | N (%) | N (%) | N (%) | Fisher’s p (two-tailed) |
| Sex | ||||
| Male | 10 (43%) | 9 (64%) | 9 (47%) | ADHD vs PBD: p=.31 ADHD vs HC: p=.48 PBD vs HC: p=1.00 |
| Female | 13 (57%) | 5 (36%) | 10 (53%) | |
| Handedness | ||||
| Right | 22 (96%) | 14 (100%) | 19 (100%) | ADHD vs PBD: p=1.00 ADHD vs HC: p=1.00 PBD vs HC: p=1.00 |
| Left | 1 (4%) | 0 (0%) | 0 (0%) | |
| Race Composition | ||||
| Caucasian | 13 (57%) | 3 (21%) | 10 (53%) | ADHD vs PBD: p=.06 ADHD vs HC: p=.09 PBD vs HC: p=1.00 |
| Other | 10 (43%) | 11 (79%) | 9 (47%) | |
Note: ADHD = Attention Deficit Hyperactivity Disorder; CDRS-R = Child Depression Rating Scale-Revised; HC = Healthy Control; PBD = Pediatric Bipolar Disorder; SES= Socioeconomic status; YMRS = Young Mania Rating Scale.
Estimated with Wechsler Abbreviated Scale of Intelligence (WASI; Matrix Reasoning and Vocabulary Subtests);
Behavioral Performance Results
Table 2 illustrates mean response time (RT) and accuracy for the two study conditions in each group. Behavioral data from one ADHD subject were not available due to technical difficulties and therefore the behavioral analyses were conducted on 13 ADHD subjects. A repeated-measures ANOVA with group (PBD, ADHD, HC) as a between-subjects factor, and face emotion (negative, positive, neutral) as a within-subjects factor, was carried out on mean RT and accuracy data. For RT, there was only a significant effect of face emotion [F(2,106)=3.37, p=.04]. Planned comparisons revealed that across groups RT for angry faces was significantly slower than RT for neutral faces [F(1,53)=7.25, p=.009]. No other significant results were found. For accuracy, planned comparisons on the significant group effect [F(2,53)=5.59, p=.006] revealed that the PBD group had significantly lower accuracy than HC [F(1,53)=12.16, p=.001], whereas there was only a non-significant trend for the ADHD group to be less accurate than HC [F(1,53)=3.61, p=.06]. The PBD and ADHD groups did not differ from each other (p=.23) for performance accuracy. No other significant results were found.
Table 2.
Response Time in ms and Accuracy for the 2-back working memory task with angry, happy and neutral face emotions in patients with Pediatric Bipolar Disorder (PBD), Attention Deficit Hyperactivity Disorder (ADHD), and in Healthy Controls (HC).
| PBD | ADHD | HC | |
|---|---|---|---|
| RT (in ms) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) |
| Angry Face Emotion | 1143 (329) | 1082 (188) | 961 (211) |
| Happy Face Emotion | 987 (265) | 1052 (245) | 946 (258) |
| Neutral Face Emotion | 996 (186) | 1017 (209) | 932 (199) |
| Total Average | 1042 (260) | 1050 (214) | 946 (223) |
| Accuracy (% correct) | % (S.D.) | % (S.D.) | % (S.D.) |
| Angry Face Emotion | 85 (20) | 92 (8) | 97 (4) |
| Happy Face Emotion | 91 (10) | 92 (10) | 97 (4) |
| Neutral Face Emotion | 90 (08) | 92 (7) | 96 (5) |
| Total Averagea | 89 (13) | 92 (8) | 97 (4) |
Note: The PBD group exhibited lower accuracy rates than HC across emotion conditions (p=.001). The ADHD group did not differ from the PBD group (p=.23) and showed a non-significant trend (p=.06) for lower accuracy relative to HC. There were no significant group differences for response time (RT).
Significant group effect (p=.006) for accuracy.
fMRI Results
The two-way interaction of group by face emotion [F(2,53)= 3.93, p= .025] was significant. The clusters for which we found the significant group by face condition interaction are as follows: left medial orbitofrontal cortex, bilateral orbitofrontal cortex, bilateral superior frontal gyrus, bilateral DLPFC, bilateral IFG, bilateral VLPFC, bilateral medial prefrontal cortex, left dorsal and subgenual ACC, bilateral pregenual ACC, bilateral amygdala, bilateral posterior cingulate gyrus, bilateral thalamus, bilateral putamen, bilateral caudate, bilateral middle temporal gyrus, bilateral precuneus and inferior parietal lobule, bilateral fusiform gyrus, and bilateral parahippocampal gyrus.
We conducted pair-wise t-test comparisons on these significant clusters from the two-way interaction in order to further explore the directionality of within-group and between-group differences due to face emotion condition. Below and in Table 3 we report our findings for the angry or happy faces vs neutral faces conditions, which are the primary contrasts of interest in this study.
Table 3. Between-Group Comparisons for Angry or Happy vs Neutral Faces.
Talairach coordinates and t values for clusters for which there is a significant two-way interaction (p<0.020 with contiguity threshold) in the whole-brain analysis of variance (ANOVA) for the Angry vs Neutral faces and the Happy vs Neutral faces contrasts.
| Talairach Coordinates for peak values | Area | BA | Volume (mm3) | t value for peak activation | Group Difference | |
|---|---|---|---|---|---|---|
| ANGRY vs NEUTRAL | ||||||
| −10, 26, 56 | L superior FG | BA 6 | 405 | 3.51 | HC > PBD | |
| −58, 5, 26 | L inferior FG | BA 9/45 | 297 | 3.11 | HC > PBD | |
| −25, −7, 2 | L putamen | 378 | 3.14 | HC > PBD | ||
| −7, 23, 2 | L subgenual ACC | BA 24/25 | 324 | 2.59 | PBD > ADHD | |
| −4, 53, −4 | L OFC/medial FG | BA 10 | 324 | 3.17 | PBD > ADHD | |
| −10, 14, 62 | L superior FG | BA 6 | 945 | 3.29 | ADHD > PBD | |
| −49, 20, 32 | L middle FG | BA 9 | 378 | 3.08 | ADHD > PBD | |
| 38, 17, 53 | R middle FG | BA 9 | 405 | 4.62 | ADHD > HC | |
| 17, 38, −16 | R OFC | BA 11 | 1242 | 3.77 | HC > ADHD | |
| 41, 29, 2 | R inferior FG | BA 45/47 | 945 | 3.64 | HC > ADHD | |
| −40, 17, −4 | L inferior FG | BA 47 | 621 | 2.97 | HC > ADHD | |
| −28, 35, −4 | L inferior FG | BA 47 | 999 | 3.14 | HC > ADHD | |
| 14, 44, 5 | R medial FG | BA 10/32 | 1053 | 3.72 | HC > ADHD | |
| −7, 50, −1 | L medial FG | BA 10/24 | 1890 | 3.98 | HC > ADHD | |
| −58, −49, 5 | L middle TG | BA 22 | 432 | 3.31 | HC > ADHD | |
| −4, 20, 4 | L caudate | 3213 | 3.8 | HC > ADHD | ||
| 8, 32, 11 | R pregenual ACC | BA 24/32 | 324 | 3.16 | HC > ADHD | |
| −7, 35, 11 | L pregenual ACC | BA 32/24 | 621 | 4.46 | HC > ADHD | |
| −22, 11, 32 | L posterior CG | BA 32 | 405 | 3.76 | HC > ADHD | |
| 23, −3, 8 | R putamen | 1782 | 2.99 | HC > ADHD | ||
| −24, −7, 6 | L putamen | 1242 | 2.17 | HC > ADHD | ||
| 55, −30, 37 | R inferior PL | BA 40 | 783 | 3.58 | HC > ADHD | |
| HAPPY vs NEUTRAL | ||||||
| 2, 65, 17 | R medial FG | BA 10 | 2781 | 3.43 | PBD > HC | |
| −1, 53, 35 | L middle FG | BA 9 | 2349 | 3.43 | PBD > HC | |
| 2, −55, 14 | R posterior CG | BA 23 | 4455 | 3.46 | PBD > HC | |
| −1, −25, 17 | L posterior CG | BA 23 | 567 | 3.04 | PBD > HC | |
| −’7, 11, 14 | L caudate | 1296 | 3.1 | PBD > HC | ||
| 23, −1, −7 | R putamen | 486 | 3.32 | PBD > HC | ||
| 11, −49, 56 | R precuneus | BA 7 | 297 | 3.66 | PBD > HC | |
| −1, −61, 59 | L precuneus | BA 7 | 1242 | 3.54 | PBD > HC | |
| 5, −34, 8 | R thalamus | 432 | 3.77 | PBD > HC | ||
| −1, −7, 14 | L thalamus | 486 | 3.57 | PBD > HC | ||
| 44, −31, 38 | R inferior PL | BA 40 | 297 | 3.00 | PBD > HC | |
| −46, −34, 44 | L inferior PL | BA 40 | 297 | 3.08 | PBD > HC | |
| 45, −58, −19 | R fusiform gyrus | BA 37 | 1350 | 3.06 | PBD > HC | |
| −10, 23, −1 | L caudate | 1053 | 3.26 | PBD > ADHD | ||
| 8, 29, 2 | R caudate | 432 | 3.34 | PBD > ADHD | ||
| 23, 56, 32 | R superior FG | BA 9 | 351 | 2.91 | ADHD > HC | |
| −13, 59, 29 | L superior FG | BA 9 | 783 | 3.04 | ADHD > HC | |
| −46, 29, 5 | L inferior FG | BA 45 | 729 | 2.99 | ADHD > HC | |
| −49, 11, 29 | L middle FG | BA 9 | 459 | 3.95 | ADHD > HC | |
| 53, −61, −10 | R fusiform gyrus | BA 37 | 2700 | 3.87 | ADHD > HC | |
| 14, 2, 23 | R caudate | 1620 | 3.11 | ADHD > HC | ||
| −1, 29, 32 | L dorsal ACC | BA 32 | 648 | 3.08 | ADHD > HC | |
| 11, −49, 26 | R posterior CG | BA 31 | 297 | 2.72 | ADHD > HC | |
| 2, −16, 38 | R mid CG | BA 24 | 378 | 2.93 | ADHD > HC | |
| −22, −25, −22 | L parahippocampal gyrus | BA 35 | 378 | 3.91 | ADHD > HC | |
| −49, −34, 35 | L inferior PL | BA 40 | 405 | 2.76 | ADHD > HC | |
| 41, −55, 53 | R inferior/superior PL | BA 7, 40 | 297 | 3.14 | ADHD > HC | |
Note: The table lists the significant group differences obtained from post-hoc decomposition of the significant two-way interaction. ACC = anterior cingulate cortex; ADHD = Attention-Deficit/Hyperactivity Disorder group; BA = Brodmann’s Area; CG = cingulate gyrus; DLPC = dorsolateral prefrontal cortex; FG = frontal gyrus; HC = Healthy Control group; L = Left;; OFC = orbitofrontal cortex; PBD = Pediatric Bipolar Disorder group; PL = parietal lobule; R = Right; TG = temporal gyrus; VLPFC = ventrolateral prefrontal cortex.
Group Differences for the Negative Emotion Condition (Angry versus Neutral Faces) (Table 3, Fig. 2a)
Figure 2.

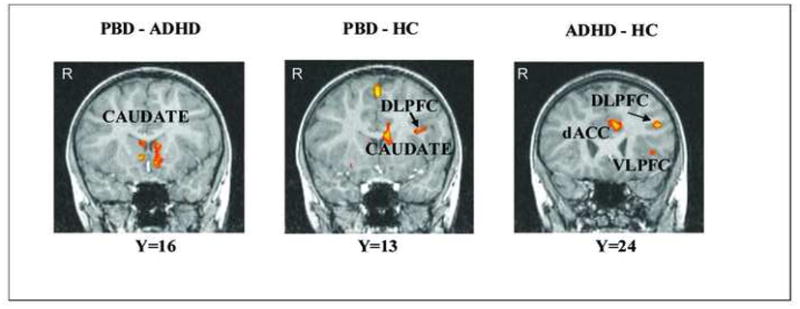
a) Between-group differences in significant clusters of brain activation for the Angry vs. Neutral face contrast. Note: Red indicates greater activation in the first group compared to the second group. Blue indicates greater activation in the second group compared to the first group. PBD vs ADHD: DLPFC= 378 mm3 p<.02; Subgen ACC=324 mm3 p<.02. PBD vs HC: IFG=297 mm3 p<.02. ADHD vs HC: left VLPFC=621 mm3 p<.02; right VLPFC=945 mm3 p<.02; left dACC=621 mm3 p<.02; right dACC=324 mm3 p<.02. b) Between-group differences in significant clusters of brain activation for the Happy vs. Neutral face contrast. Red indicates greater activation in the first group compared to the second group. Blue indicates greater activation in the second group compared to the first group. PBD vs ADHD: left caudate= 1053 mm3 p<.02; right caudate= 432 mm3 p<.02. PBD vs HC: left DLPFC=2349 mm3 p<.02; left caudate=1296 mm3 p<.02. ADHD vs HC: left DLPFC=783 mm3 p<.02; right DLPFC=351 mm3 p<.02; left dACC=648 mm3 p<.02; left VLPFC=729 mm3 p<.02. Note that the right side of the brain image corresponds to the left hemisphere (i.e., Right=Left). ADHD = Attention Deficit Hyperactivity Disorder group; dACC = dorsal anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; HC=Healthy Controls; IFG = inferior frontal gyrus; PBD = Pediatric Bipolar Disorder group; Subgen ACC=subgenual ACC VLPFC = ventrolateral prefrontal cortex.
PBD vs. ADHD
Relative to the ADHD group, the PBD group exhibited greater activation in left medial orbitofrontal cortex (OFC) (BA 10) and left subgenual ACC, and reduced activation in left DLPFC (BA 9), and premotor regions (BA 6).
PBD vs HC
Compared to HC the PBD group showed no greater activation and reduced activation in left superior frontal gyrus (BA 6), left putamen and in left IFG at the junction of BA 9 and BA 45.
ADHD vs HC
The ADHD group, relative to the HC group, exhibited greater activation in right DLPFC (BA 9), and reduced activation in bilateral VLPFC (inferior frontal gyrus, BA 45/47), bilateral medial prefrontal cortex (BA 10), right OFC (BA 11), cingulate regions (bilateral pregenual ACC, and left posterior cingulate gyrus), bilateral putamen, left caudate, left middle temporal gyrus (BA 22) and right inferior parietal lobule (BA 40).
Group Differences for the Positive Emotion Condition (Happy versus Neutral Faces) (Table 3, Fig. 2b)
PBD vs ADHD
The PBD, relative to the ADHD group, exhibited greater activation in bilateral caudate and no reduced activation.
PBD vs HC
The PBD group, compared to the HC group, showed greater activation in right medial PFC (BA 10), left DLPFC (BA 9), left posterior cingulate, striatal regions (left caudate, right putamen), bilateral thalamus, bilateral parietal regions (precuneus, inferior parietal lobule), and right fusiform gyrus. There were no regions that showed reduced activation in PBD relative to HC.
ADHD vs HC
The ADHD group, relative to HC, exhibited no reduced activation, and greater activation in bilateral DLPFC (BA 9), left VLPFC (BA 45), cingulate gyrus (left dorsal ACC, right mid- and posterior cingulate gyrus), right fusiform gyrus, right caudate, left parahippocampal gyrus, and bilateral inferior parietal lobule (BA 40).
Within-Group Differences for the Negative or Positive Emotion Condition
Table S4, illustrating within-group activation for the angry vs neutral and happy vs neutral face emotion condition is available on our CCM web site as Appendix A75. Below we summarize the main results for this comparison.
PBD Group
The PBD group exhibited greater activation for angry than neutral faces in right inferior frontal gyrus (BA 46), at the junction of DLPFC and VLPFC, and in right fusiform, parahippocampal and middle temporal gyrus, and no reduced activation. Similarly, the PBD group showed increased activation for happy than for neutral faces in right VLPFC (BA 45, 47), bilateral amygdala, fusiform gyrus, caudate, temporal regions and right posterior cingulate gyrus. No reduced activation was found for this comparison.
ADHD Group
The ADHD group exhibited greater activation for angry than for neutral faces in right premotor regions, and reduced activation in a network of regions comprising left medial frontal gyrus and pregenual ACC, left orbitofrontal cortex (OFC) (BA 11), right DLPFC, and left caudate. This group showed increased activation for happy than neutral faces in right DLPFC and fusiform gyrus, and no reduced activation.
HC Group
The HC group exhibited greater activation for angry than for neutral faces in bilateral VLPFC (BA 45, 47), right medial frontal gyrus, and bilateral temporal, parietal and striatal regions. Decreased activation for angry relative to neutral faces was found in right DLPFC. For happy relative to neutral faces the HC group showed increased activation in right medial frontal gyrus and decreased activation in right VLPFC (BA 47), left inferior frontal gyrus (BA 46/10), at the junction of DLPFC and VLPFC, right DLPFC, parietal regions (right precuneus, bilateral inferior parietal lobule) posterior cingulate gyrus, left middle temporal gyrus, bilateral thalamus and left caudate.
Neural Correlates of Behavioral Performance and Clinical Measures
Based on significant findings from the whole-brain analyses for the primary contrasts of interest (i.e., angry or happy vs neutral faces), Spearman correlation analyses were performed between behavioral measures (i.e., RT and accuracy) or our clinical measures, and the anatomical ROIs corresponding to clusters that resulted significant in the ANOVA interaction, for each group and each condition. Although some significant results (e.g., p<.05) were found, they did not survive correction for multiple comparisons (corrected p<.002), possibly because of the small sample size. We briefly report our findings with uncorrected p values for multiple comparisons, for exploratory purposes, as they may inform future studies with larger samples.
Behavioral performance
For the PBD group there was a significant negative correlation between accuracy for the working memory task with angry faces and left DLPFC activation (r= −.45; p=.03). Across all groups, there was a negative correlation between right VLPFC activation and accuracy on the task with angry faces (r=−.34, p=.01).
Clinical Measures
For the PBD group there was a significant correlation between CDRS-R scores and activation in right medial frontal gyrus for angry faces (r=−.45, p=.037) suggesting that for angry face stimulus processing the higher the CDRS scores, the lower activation was in this region. Furthermore, the ADHD total score in the ADHD group correlated significantly with activation in right caudate (r=−.54, p=.04) and left caudate (r=−.56, =.03). The ADHD scale total score across groups correlated significantly with activation in right DLPFC (r=.30, p=.031) and left precuneus (r=.28, p=.041).
Discussion
The central finding of the present fMRI study is that the neural substrate of working memory is differentially influenced by face emotion processing in PBD and ADHD, in that relative to the ADHD group the PBD group exhibited greater deployment of the emotion processing circuitry and relative to the PBD group the ADHD group showed greater deployment of prefrontal working memory circuitry. While preliminary, this finding possibly suggests different neural phenotypes for the interface of cognition and affect in these two illnesses. In fact, relative to HC, both the PBD and ADHD groups showed reduced activity in cortico-subcortical circuitry in response to angry face stimuli, and increased activity in these circuits in response to happy face stimuli. However, relative to the ADHD group the PBD group showed increased activation in emotion regulation regions, such as the left medial PFC and subgenual ACC, whereas the ADHD group, relative to the PBD group, exhibited greater activation of working memory and pre-motor regions in the left hemisphere. In line with previous behavioral findings from studies using stimuli with emotional valence such as the emotional stroop task 59,61, for the present behavioral data all the groups exhibited significantly slower RT for trials with angry faces relative to neutral faces, which underscores the overall impact of emotions on cognitive processes at the performance level in both patients with mood dysregulation61 and HC59,60.
In terms of neural activation, it is noteworthy that neural engagement in response to the affective n-back task was different for each group and contributed to the between-group differences that we discuss in detail below. Specifically, the HC group showed increased activation in emotion regulation (i.e., bilateral VLPFC), and working memory regions (i.e., medial PFC, bilateral temporal, parietal and striatal regions) for the angry vs neutral face emotion condition, and reduced activation in the same circuitry for the happy vs neutral face condition. This is in agreement with fMRI findings from studies using the emotional stroop task, suggesting that stimuli with negative valence draw attention and increase neural activation more than stimuli with positive valence59–61. On the contrary, when examining data from our manic/mixed and hypomanic PBD patients it appears that both for the angry and happy face vs neutral condition the PBD patients showed increased activation in face emotion and emotion regulation circuits, which is in line with previous findings with emotional stimuli in manic adults with bipolar disorder76. This possibly suggests overall increase in perceived salience of emotional stimuli and neural overreactivity to emotional content in bipolar disorder because of the underlying dysfunction in mood regulation in PBD that is independent of mood state 4,5,44. Finally, opposite to the pattern found in HC, for angry vs neutral faces, the ADHD group showed reduced activation in emotion regulation and working memory regions (i.e., dorsal and pregenual ACC, DLPFC, and caudate), and for happy vs neutral faces the ADHD group showed increased activation in right DLPFC. While further replications of this differential neural response to differing emotional valences in ADHD are needed, this pattern of results suggests perhaps a more severe reduction in attention or DLPFC engagement in response to negative relative to positive emotions in children with ADHD.
Differential Involvement of Affective and Cognitive Circuitry in PBD and ADHD
The present study, together with recent studies from our laboratory40,51, and a study by Brotman et al.57 is one of the first to show potential neural differences between PBD and ADHD during emotion and cognitive processing that point at different neurofunctional phenotypes in these two illnesses. Though much more research is needed in this regard, this is a promising initial approach towards the identification of specific bio-markers for different mental illnesses and ultimately for brain-based classification of disorders2. By testing working memory processing under affective challenge, we confirmed our main prediction that affective circuitry would be relatively more impaired in PBD, whereas cognitive circuitry would be relatively more impaired in ADHD. In fact, the two groups showed similar behavioral performance and severity scores on the ADHD rating scale, which agrees with neuropsychological findings of attention and working memory deficits both in PBD9,12 and ADHD11,16. Nevertheless, the two patient groups differed with regard to engagement of PFC, ACC and striatal regions.
When examining group differences between PBD and ADHD, planned contrasts on clusters of activation that were significant in the ANOVA two-way interaction showed that for angry vs neutral faces the PBD group exhibited greater activation than the ADHD group in left medial orbitofrontal cortex (OFC), a region involved in emotion processing and decision-making27,77, and in left subgenual ACC, a region involved in emotion regulation processes78. A previous study using a n-back visuo-spatial working memory task showed increased ACC activation in PBD relative to HC45, and also Pavuluri et al.4 found increased pregenual ACC activation in euthymic PBD patients relative to HC for both angry and happy face stimuli. Based on the existing literature, the functional significance of the present group difference is possibly that of increased deployment of emotional regulation regions in PBD relative to ADHD in response to negative emotions, because of the generalized over-sensitivity to emotions in PBD. The ADHD group showed greater activation than the PBD group in DLPFC and supplementary motor area (SMA). We may interpret this difference between PBD and ADHD as potentially due to increased working memory and attentional engagement with negative emotion in children with ADHD relative to those with PBD. Finally, for the happy vs neutral face condition the two patient groups did not differ, with the exception that the PBD group showed greater bilateral caudate activation compared to the ADHD group. While we do not have a clear explanation for this group difference in caudate activation, a tentative explanation is that it may be indicative of differences in attentional gating mechanisms during working memory processes79.
The present study did not find group differences in amygdala activation, possibly because for our analyses we contrasted emotional and neutral faces, and amygdala activation may have been present for both types of face stimuli in our groups47. Of relevance, a recent study by Brotman et al. 57, which examined amygdala activation during subjective fear ratings of neutral faces in ADHD patients, PBD patients, patients with severe mood dysregulation and HC, did find amygdala hyperactivity in ADHD relative to PBD and the other groups. Interpretation of this interesting finding may be complicated by the fact that in that study ADHD patients were not on medication, whereas the two mood disorder patient groups were medicated and euthymic. Therefore it is possible that the un-medicated ADHD group may have exhibited greater mood dysregulation than the other two medicated groups, who typically exhibit more severe affect regulation deficits relative to ADHD3–8, because of medication issues. Nevertheless, this new finding further emphasizes the need to investigate in more depth the differing neural correlates of emotion processing in ADHD relative to other pediatric groups with mood dysregulation, both with regard to limbic circuits and cognitive and regulatory PFC systems.
In summary, the group differences in our study, while preliminary, point at phenotypic differences between PBD and ADHD neuropathology in terms of how they deploy the affective and cognitive circuits while they perform the same task, with more robust group differences for the negative emotion condition (i.e., angry vs neutral face condition). These results will need to be replicated with greater group samples and with emotional stimuli other than faces in order to obtain more conclusive evidence.
Specific Dysfunction of the Neural Interface of Affective and Cognitive Circuitry in PBD relative to HC
Previous studies found affect circuitry dysregulation in PBD relative to HC during face emotion processing4,46,47,48 but the differential effects of positive or negative emotional valence stimuli on cognition still need to be further documented in PBD. We examined group differences between PBD and HC by performing planned contrasts on the significant clusters of activation from the significant group × condition interaction. Our main finding was that for the angry vs neutral face condition the PBD group, relative to HC, exhibited reduced activation in a left IFG region at the junction of DLPFC and VLPFC (BA 9/45), which integrates cognitive and affective processes 39,80. Several studies have shown that negative emotional information can hinder cognitive processes59–61. This finding with the n-back task agrees with previous studies that found reduced functioning of the DLPFC/VLPFC interface while using similar tasks involving both cognitive and emotion processes, like the affective stroop task, both in adolescents39,40 and adults49,50 with bipolar disorder relative to HC.
In contrast, for the happy vs neutral face condition relative to HC the PBD group exhibited greater neural activity in parietal working memory regions, which are associated with rehearsal27, in medial PFC, which may be due to cognitive processes 27,81, generated by the happy face, and in posterior cingulate cortex, which could be due to greater attentional allocation while relaying information between PFC, limbic structures and precuneus60. Note that the two groups did not differ in activation of the VLPFC/DLPFC junction for happy vs neutral faces. The group differences with positive emotion valence may be driven by the fact that the HC group showed decreased activation for happy relative to neutral faces, whereas the PBD group showed increased activation for this comparison as well as for the angry vs neutral face comparison. The PBD patients in this study were manic/mixed and hypomanic. Some literature is emerging showing that different from depressed bipolar patients, manic bipolar patients have an attentional bias towards both positive and negative valence stimuli76. This may indicate a greater perceived salience for emotional stimuli overall that may contribute to the underlying mood dysfunction in bipolar disorder (Lawrence et al., 2004). There is also growing evidence that neural dysfunction for emotion processing in bipolar disorder differs from that of other illnesses with overlapping symptoms. For example, Lawrence et al. 82 found that relative to adult HC and patients with major unipolar depression, euthymic and depressed adults with bipolar disorder showed greater dysfunction in sub-cortical and VLPFC regions for both positive and negative face emotions, whereas the depressed patient group revealed overall a state of emotional blunting.
In summary, our present fMRI results, together with the existing literature, further our understanding on the mechanisms underlying affect dysregulation in PBD, by suggesting that it is important to consider neural response to stimuli with different emotional valence, and how it may be affected by illness state76. Moreover, the present data showing patterns of brain activation in PBD relative to HC suggest that a break-down in functioning of the neural interface of the dorsal working memory system and the ventral affective system in PBD is more evident with negative valence stimuli, such as angry faces, relative to those with a positive valence, such as happy faces.
VLPFC and ACC Dysfunction in ADHD relative to HC
Group differences between ADHD and HC were revealed by planned contrasts on the significant clusters of activation from the two-way interaction. Similar to findings from the comparison between PBD and HC, in response to the angry vs neutral face condition, relative to HC the ADHD group showed reduced activation in emotion and behavior regulation regions such as the VLPFC (BA 45/47), pregenual ACC, OFC and in the medial prefrontal cortex, involved in performance monitoring83. These findings parallel those of previous imaging studies with children with ADHD16,19,41 and our previous finding of decreased VLPFC activation in children with ADHD relative to HC during an affective color word matching task40 and a response inhibition task with no emotional information51. Indeed, the VLPFC is crucial for emotional modulation78, performance monitoring, behavior control and working memory functions27, and it has been suggested that both inhibitory and working memory deficits in ADHD stem from neuropathology of VLPFC52. Furthermore, abnormal functioning of dorsal, or perigenual ACC (BA 24/32), has also been implicated in response inhibition and working memory deficits in patients with ADHD52. Therefore, the dual involvement of VLPFC and rostral ACC, the regions of the cognitive and affective interface, may be the reason why there is both emotional and working memory involvement in ADHD. A few studies are starting to show VLPFC or limbic dysfunction in ADHD not only relative to HC but also to other pathologies involving similar deficits in behavior control and emotion regulation. For example, a study by Rubia et al. 84 found decreased activation in right inferior prefrontal cortex in boys with ADHD relative to HC but also relative to boys with obsessive compulsive disorder during response inhibition and cognitive flexibility tasks. Under-activation of the inferior prefrontal cortex is consistently found in ADHD with tasks involving cognitive control, attention and cognitive flexibility40,41,85. This further validates the view that this region could be a neuro-functional marker for ADHD that may underlie the cognitive deficits described above. Moreover, findings from the Brotman’s study57 that we described above also suggest that limbic circuits may be compromised in ADHD. Since VLPFC modulates amygdala reactivity through the rostral ACC80 it will be important that future studies further examine the relation between limbic, VLPFC and ACC dysfunction in ADHD relative to HC, but also relative to PBD and other syndromes with overlapping symptoms and deficits.
For the happy vs neutral face condition the ADHD group, relative to the HC group, showed increased activation in the same key regions that were showing reduced activation for the angry vs neutral face condition, suggesting that engagement of these regions is affected by the valence of emotional information. It is difficult to explain the increased brain activation in the emotion regulation regions, including VLPFC and ACC, with happy and neutral faces in ADHD relative to HC. As we pointed out for the PBD group, this result may be partially driven by the fact that the HC group showed decreased activation for happy relative to neutral faces whereas the ADHD group, similar to the PBD group, showed increased activation for this condition. A tangible explanation may be that the ADHD patients, relative to HC, experience increased neural and behavioral excitability while performing the working memory task with happy face stimuli as compared to angry face stimuli, which may be more challenging for PFC regulatory regions because of their negative connotation. Somewhat in line with findings from the Brotman et al study57 we also found that the ADHD group showed increased activation for neutral as compared to angry faces, although this pattern was not present for neutral relative to happy faces. Future research is needed to explicate the effects of different emotions on neural engagement during cognitive processes in ADHD and PBD relative to other pediatric disorder groups with overlapping symptoms.
Some limitations of the present study need to be noted. First, we adopted a block design because it offers the benefit of greater statistical power and signal stability relative to an event-related design, which is especially important with clinical population presenting highly variable neural activation. Nevertheless, a block design also presents with several limitations that will need to be addressed in future studies. For example, it did not allow us to differentiate between brain activation for correct and incorrect trials, and this may be limiting given that we found between group differences in accuracy. Therefore we cannot exclude the possibility that differences in brain activation between patients and HC may be associated with differences in performance accuracy, possibly due to different levels of cognitive effort or different strategies adopted to perform the task. Future event-related design studies will need to better examine the neural circuits underlying correct vs. incorrect responses during working memory tasks in patients relative to HC. In addition, for this initial study our main comparison of interest to test our hypotheses was between angry vs neutral and happy vs neutral faces, but this method of analysis did not enable us to directly compare angry vs happy faces within or between groups. We also kept the working memory load constant (i.e., 2-back) to enable all subjects to have high accuracy levels, and also so that there would be no variations in task difficulty that may affect more the patient groups relative to HC. But it will be important to parametrically vary working memory load in future studies, to better elucidate the nature of working memory deficits in relation to cognitive effort, emotion processing and brain function in PBD and ADHD.
Moreover, while the focus of the present study was to examine effects of emotion processing on working memory function, we were unable to assess brain activation for working memory function in the absence of any emotional informational as a baseline against which to compare findings for emotion effects, because we did not have in our design a true non-emotional condition. Previous studies showed that neutral faces may be perceived as threatening by subjects with bipolar disorder47 and with ADHD57. Therefore future studies may need to include a non-face condition to assess baseline working memory function.
Finally, given the growing evidence of specific dysfunction in VLPFC, ACC, DLPFC and limbic regions in PBD and ADHD57, new functional connectivity investigations that follow seminal connectivity studies in PBD86 and ADHD19,87 will also enable us to better understand dysfunctional interactions between cognitive and affective circuits in these two illnesses.
The PBD patients, relative to the ADHD patients and HC, demonstrated affective circuitry impairment regardless of the emotion valence of face stimuli. Patients with ADHD demonstrated more severe impairment in working memory circuitry relative to patients with PBD and HC. In both disorder groups, relative to HC, there was a similar response of decreased activity with negative emotional challenge and increased activity with positive emotional challenge in cortico-subcortical circuitry. Our findings provide preliminary evidence that PBD and ADHD may exhibit phenotypic variation in neural response while performing a task that requires concurrently working memory and emotional processing. While we are only at the beginning, the scientific advance towards domain-based classification will help differentiate the phenotypes of PBD and ADHD, leading the way to functionally informed disorder classification and treatment2.
Acknowledgments
This work is supported by NIH K23 RR18638-01, the Dana Foundation and NARSAD.
We thank the children and families who participated in this study. Thanks also to Ms. Erin Harral of the University of Illinois at Chicago for her help with subject testing and data processing.
Footnotes
Disclosure: Dr. Pavuluri has received research support from the National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award, the National Institute of Child Health and Human Development, GlaxoSmithKline – NeuroHealth, Abbott Pharmaceuticals, and Janssen Research Foundation. Dr. Sweeney has received research support from the National Institutes of Health, GlaxoSmithKline, AstraZeneca, Janssen, and Eli Lilly and Co. Dr. Passarotti reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galanter CA, Leibenluft E. Frontiers between attention deficit hyperactivity disorder and bipolar disorder. Child and Adolescent Psychiatric Clinics of North America. 2008;17:325–346. doi: 10.1016/j.chc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biological Psychiatry. 2009;66(11):988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. Journal of Affect Disorder. 1998;51(2):93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 4.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Pavuluri MN, Passarotti AM. Neural bases of emotional processing in pediatric bipolar disorder. Expert Review of Neurotherapeutics. 2008;8(9):1381–1387. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Skirrow C, McLoughlin G, Kuntsi J, Asherson P. Behavioral, neurocognitive and treatment overlap between attention-deficit/hyperactivity-disorder and mood instability. Expert Review of Neurotherapeutics. 2009;9:489–503. doi: 10.1586/ern.09.2. [DOI] [PubMed] [Google Scholar]
- 8.Walshaw PD, Alloy LB, Sabb FW. Executive function in pediatric bipolar disorder and attention-deficit hyperactivity disorder: in search of distinct phenotypic profiles. Neuropsychology Review. 2010;20(1):103–120. doi: 10.1007/s11065-009-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baddeley A. Working memory: Looking back and looking forward. Nature Reviews. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 10.Pavuluri MN, Shenkel LS, Aryal S, Harral E, Hill K, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163(2):286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 11.Pavuluri M, West A, Hill S, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-ups show cognitive development lagging behind health youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:235–236. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickstein DP, Garvey M, Pradella AG, Greenstein DK, Sharp WS, et al. Neurologic examination abnormalities in children with bipolar disorder or attention deficit/hyperactivity disorder. Biological Psychiatry. 2005;58:517–524. doi: 10.1016/j.biopsych.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological Psychiatry. 2000;48(7):674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 14.Ferrier IN, Thompson JM. Cognitive impairment in bipolar affective disorder: implications for the bipolar diathesis. The British Journal of Psychiatry. 2002;180(4):293–295. doi: 10.1192/bjp.180.4.293. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JM, Gallagher P, Hughes J, Watson S, Gray JM, Ferrier IN, Young AH. Neurocognitive impairment in euthymic patients with bipolar affective disorder. The British Journal of Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- 16.Rubia K, Taylor E, Smith H, Oksannen H, Overmeyer S Neworking memoryan, S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal of Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- 17.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A Meta-Analysis of Working Memory Impairments in Children with Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005 doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 18.Alloway TP, Rajendran G, Archibald LMD. Working memory in children with developmental disorders. Journal of Learning Disabilities. 2009;42:372–382. doi: 10.1177/0022219409335214. [DOI] [PubMed] [Google Scholar]
- 19.Castellanos F, Tannock R. Neuroscience of attention-deficit/hyperactivity-disorder: The search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 20.Seidman LJ, Valera EM, Bush G. Brain function and structure in adults with attention-deficit/hyperactivity disorder. Psychiatry Clinics of North America. 2004;27(2):323–47. doi: 10.1016/j.psc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Rapport LJ, Friedman S, Tzelepis A, VanVoorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactitiy disorder. Neuropsychology. 2002;16:102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- 22.Fergusson D, Lynsky M, Horwood L. Attentional difficulties in middle childhood and psychosocial outcomes in young adulthood. Journal of Child Psychology and Psychiatry. 1997;38:633–644. doi: 10.1111/j.1469-7610.1997.tb01690.x. [DOI] [PubMed] [Google Scholar]
- 23.Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcome of hyperactive children: adaptive functioning in major life activities. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(2):192–202. doi: 10.1097/01.chi.0000189134.97436.e2. [DOI] [PubMed] [Google Scholar]
- 24.Barnett R, Maruff P, Vance A. Neurocognitive function in attention-deficit-hyperactivity disorder with and without comorbid disruptive behaviour disorders. Australian and New Zeland Journal of Psychiatry. 2009;43(8):722–730. doi: 10.1080/00048670903001927. [DOI] [PubMed] [Google Scholar]
- 25.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart SJ, Green SR, Casp M, Belger A. Emotional priming during Stroop task performance. Neuroimage. 2010;49:2662–2670. doi: 10.1016/j.neuroimage.2009.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional Neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrides M. Frontal lobe and behavior. Current Opinions in Neurobiology. 1994;4:207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 29.Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- 30.Bor D, Cumming N, Scott CEM, Owen Prefrontal cortical involvement in encoding strategies, independent of stimulus modality. European Journal of Neuroscience. 2004;19:3365–3370. doi: 10.1111/j.1460-9568.2004.03438.x. [DOI] [PubMed] [Google Scholar]
- 31.Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 32.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 33.Rama P, Martinkauppi S, Linnankoski I, Koivisto J, Aronen H, Carlson S. Working memory of identification of emotional vocal expressions: an fMRI study. Neuroimage. 2001;13:1090–1101. doi: 10.1006/nimg.2001.0777. [DOI] [PubMed] [Google Scholar]
- 34.Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14(1 Pt 1):48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- 35.Monks P, Thompson J, Bullmore E, Suckling J, Brammer M, Williams S, Simmons A, Giles N, Lloyd A, Harrison C, Seal M, Murray R, Ferrier I, Young A, Curtis V. A functional MRI study of working memory task in euthymic bipolar disorder: Evidence for task-specific dysfunction. Bipolar Disorders. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 36.Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler L. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Research. 2010;182(1):22–9. doi: 10.1016/j.pscychresns.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haldane M, Frangou S. Functional neuroimaging studies in mood disorders. Acta Neuropsychiatrica. 2006;18:88–99. doi: 10.1111/j.1601-5215.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 38.Drapier D, Surguladze S, Marshall N, Schulze K, Fern A, Hall MH, Walshe M, Murray RM, McDonald C. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biological Psychiatry. 2008;64:513–520. doi: 10.1016/j.biopsych.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 39.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Research. 2008;162(3):244–5. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passarotti AM, Sweeney JA, Pavuluri MN. Differential Engagement of Cognitive and Affective Neural Systems in Pediatric Bipolar Disorder and Attention Deficit Hyperactivity Disorder. Journal of the International Neuropsychological Society. 2010;16(1):106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 42.Bush G, Frazier JA, Rauch SL, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 43.Schweitzer JB, Faber TL, Grafton ST, Tune LE, Hoffman JM, Kilts CS. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. American Journal of Psychiatry. 2000;157:278–280. doi: 10.1176/appi.ajp.157.2.278. [DOI] [PubMed] [Google Scholar]
- 44.Gruber O, Tost H, Henseler I, Schmael C, Scherk H, Ende G, Ruf M, Falkai P, Rietschel M. Pathological amygdala activation during working memory performance: evidence for a pathophysiological trait marker in bipolar affective disorder. Human Brain Mapping. 2010;31(1):115–125. doi: 10.1002/hbm.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang K, Adleman NE, Dienes K, Simeonova DI, Memon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 46.McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Deficits in social cognition and response flexibility in pediatric bipolar disorder. J of Child Adolesc Psychopharmacol. 2005;15(4):563–570. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 47.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder (2006) Proc Natl Acad Sci U S A. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavuluri M, Passarotti A, Harral E, Sweeny JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disorder. 2005;7(Supplement 5):58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 50.Lagopoulos J, Malhi GS. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. NeuroReport. 2007;18(15):1583–1587. doi: 10.1097/WNR.0b013e3282efa07a. [DOI] [PubMed] [Google Scholar]
- 51.Passarotti AM, Sweeney JA, Pavuluri MN. Neural Correlates of Response Inhibition Deficits in Pediatric Bipolar Disorder and Attention Deficit Hyperactivity Disorder. Psychiatry Research: Neuroimaging. 2010;181(1):36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, Sahakian BJ. Between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology. Biological Psychiatry. 2007;61:1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Durston S, Mulder M, Casey BJ, Ziermans T, Van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60(10):1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 54.Braaten EB, Rosen LA. Self-regulation of affect in attention-deficit hyperactivity disorder (ADHD) and non-ADHD boys: Differences in empathic responding. Journal of Consulting and Clinical Psychology. 2000;68:313–321. doi: 10.1037/0022-006X.68.2.313. [DOI] [PubMed] [Google Scholar]
- 55.Casey RJ. Emotional competence in children with externalizing and internalizing disorders. In: Lewis M, Sullivan M, editors. Emotional development in atypical children. Hillsdale, NJ: Erlbaum; 1996. pp. 161–183. [Google Scholar]
- 56.Friedman SR, Rapport LJ, Lumley M, Tzelepis A, VanVoorhis A, Stettner L, Kakaati L. Aspects of Social and Emotional Competence in Adult Attention-Deficit/Hyperactivity Disorder. Neuropsychology. 2003;17(1):50–58. [PubMed] [Google Scholar]
- 57.Brotman MA, Rich BA, Guyer AE, Lunsfoird JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K. Amygdala activation during emotion processing of neutral faces in childre nwith severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2009;AiA:1–9. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith EE, Jonides J. Neuroimaging analyses of working memory. PNAS. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Scalf PE, Webb A, Heller W. Paying attention to emotion: an fMRI investigation of cognitive and emotional stroop task. Cognitive Affective and Behavioral Neuroscience. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- 60.Posner J, Russel JA, Gerber A, Gorman D, Colibazzi T, Yu S, Wang Z, Kangarlu A, Zhu H, Peterson BS. The neurophysiological bases of emotion: An fMRI study of affective circumplex using emotion-denoting words. Human Brain Mapping. 2009;30(3):883–895. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams JMG, Matthews A, McLead C. The emotional stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 62.Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Brace & Company; 1999. [Google Scholar]
- 63.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 64.DuPaul GJ, Power TJ, Anastopulous AD, Reid R. ADHD Rating Scale, Vol IV. New York: Guilford Press; 1998. [Google Scholar]
- 65.Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 66.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 67.Poznanski E, Grossman J, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child and Adolescent Psychiatry. 1984;23(2):191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 68.Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 69.Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Heidelberg: Physica-Verlag; 1996. pp. 39–49. [Google Scholar]
- 70.Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 71.Cox RW. AFNI. Software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 72.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Stuttgart New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 73.Ward B. ALPHASIM. Natl. Inst. Of Health; Bethesda: 2000. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. [Google Scholar]
- 74. [accession date: 1/10/2010]; http://ccm.psych.uic.edu/Research/NormalBrain/ROI_rules.htm.
- 75. [accession date: 1/25/2010]; http://ccm.psych.uic.edu/Research/ResearchProgram/MoodDisorder/AdditionalResults.aspx.
- 76.Murphy FC, Sahakian BJ, Rubinsztein JS, Michaele A, Rogers RD, Robbins TW, et al. Emotional bias and inhibitory contro process in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 77.Bechara A, Damasio A, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 78.Fountoulakis KN, Giannakopoulos P, Kovari E, Bouras C. Assessing therole of cingulate cortex in bipolar disorder: neuropathological, structural and functional imaging data. Brain Research Reviews. 2008;59:9–21. doi: 10.1016/j.brainresrev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 79.McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: An fMRI, within-subjects investigation. Neuropsychologia. 2008;46:2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 80.Petrides M, Pandya D. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. The European Journal of Neuroscience. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 81.Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- 82.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips M. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 83.Rubia K, Halari R, Cubillo A, Mohammad A-M, Brammer M, Taylor E. Metilphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 84.Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer M. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Human Brain Mapping. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silk T, Vance A, Rinehart N, Egan G, O’Boyle M, Bradshaw JL, Cunnington R. Decreased fronto-parietal activation in Attention Deficit Hyperactivity Disorder, combined type (ADHD-CT): an fMRI study. British Journal of Psychiatry. 2005;187(3):282–283. doi: 10.1192/bjp.187.3.282. [DOI] [PubMed] [Google Scholar]
- 86.Almeida JRC, Mechelli A, Hassel S, Versace A, Kupfer DJ, Phillips ML. Abnormally increased effective connectivity between parahippocampal gyrus and ventromedial prefrontal regions during emotion labeling in bipolar disorder. Psychiatry research: Neuroimaging. 2009;174:195–201. doi: 10.1016/j.pscychresns.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murias M, Swanson JM, Srinivasan R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cerebral Cortex. 2007;17(8):1788–1799. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]



