Abstract
Previous research has shown that estradiol increases the anorexia associated with serotonin (5-HT) neurotransmission. To examine further the putative relationship between estradiol and 5-HT, we investigated whether estradiol increases the expression of Pet-1 and the 5-HT transporter (5-HTT), two genes implicated in the development and regulation of the 5-HT system. Ovariectomized (OVX) rats (n = 5-6/group) were treated with 0, 2, or 10 μg estradiol benzoate (EB) in sesame oil on 2 consecutive days. Food intake and body weight were recorded 2 days later when EB-treated rats typically display signs of behavioral estrus (e.g., reduced feeding). Following the collection of behavioral data, rats were perfused, brains were removed, and coronal sections were cut through the midbrain raphe nuclei. Pet-1 and 5-HTT mRNA levels were quantified throughout the dorsal and median raphe nuclei (DRN and MRN) by conducting in situ hybridization on free-floating tissue sections using 35S-labeled cDNA probes. As expected, EB treatment decreased food intake and body weight on the day that modeled estrus. At this same time, EB treatment increased Pet-1 and 5-HTT mRNA levels within the DRN and MRN. We conclude that a physiologically relevant regimen of estradiol treatment in OVX rats increases Pet-1 and 5-HTT mRNA levels in the midbrain raphe nuclei at a time when the anorexigenic effect of estradiol is apparent. Further studies are required to determine whether the increased expression of Pet-1 and 5-HTT mRNA play a causal role in the anorexigenic effect of estradiol.
Keywords: estrogen, anorexia, transcription factor, food intake
1. Introduction
Estradiol, the most common circulating form of estrogens, exerts a phasic inhibitory effect on food intake that is apparent during the estrous stage of the reproductive cycle in female rats and following estradiol treatment in ovariectomized (OVX) rats. This action of estradiol appears to be mediated, in part, by its ability to increase the anorexigenic effect of peptides that reduce meal size, including cholecystokinin (CCK) and glucagon (reviewed in Eckel, 2004). More recently, estradiol has also been shown to interact with 5-hydroxytryptamine (5-HT; serotonin), an anorexigenic neurotransmitter that, like estradiol, CCK, and glucagon, decreases food intake by selectively decreasing meal size (Eckel, 2004; Burton et al., 1981; Leibowitz and Alexander, 1998; Simansky, 1996).
There is considerable evidence that the anorexigenic effect of estradiol may be mediated, in part, by increased 5-HT neurotransmission. For example, the anorexigenic effect of fenfluramine, which increases synaptic levels of 5-HT, is greater in female than male rats (Eckel et al., 2005), is increased during estrus in cycling rats (Eckel et al., 2005), and is increased by estradiol treatment in OVX rats (Rivera and Eckel, 2005). It has also been shown that the orexigenic effect of 8-OH-DPAT, which decreases synaptic levels of 5-HT by stimulating 5-HT1A autoreceptors that regulate the firing rate of 5-HT neurons, is attenuated during proestrus and estrus in cycling rats (Uphouse et al, 1991) and by estradiol treatment in OVX rats (Salamanca and Uphouse, 1992). Finally, fluoxetine, a selective 5-HT reuptake inhibitor, produced a greater release of 5-HT in the mediobasal hypothalamus in estrous than diestrous cycling rats (Maswood et al, 1999).
Currently, little is known about the molecular mechanism underlying estradiol’s ability to interact with the 5-HT system. Because estradiol can alter gene expression after coupling with estrogen receptor (ER) proteins (Paech et al., 1997), and ERs are expressed within the midbrain raphe nuclei (Alves et al., 1998; Leranth et al., 1999; Sheng et al., 2004), it is possible that estradiol modulates the expression of serotonergic genes that regulate 5-HT neurotransmission. To investigate this hypothesis, we examined whether estradiol treatment increases mRNA levels of two serotonergic genes, pheochromocytoma 12 ETS domain transcription factor (Pet-1) and 5-hydroxytryptamine transporter (5-HTT), in the midbrain raphe nuclei of OVX rats. Pet-1, a transcription factor belonging to the E26 transformation-specific family, plays a critical role in the development and regulation of the 5-HT system (Hendricks et al., 1999). For instance, Pet-1 null mice have decreased levels of the serotonergic genes tryptophan hydroxylase (TPH) and 5-HTT in the dorsal raphe nucleus (DRN), and decreased levels of 5-HT in forebrain regions compared to wild-type littermates (Hendricks et al., 2003). These changes promote alterations in 5-HT-dependent behaviors since Pet-1 null mice show more aggression and anxiety than wild-type littermates (Hendricks et al., 2003).
Our second gene of interest, 5-HTT, regulates synaptic levels of 5-HT (Mathews et al., 2004). We chose to examine the 5-HTT gene for two reasons. First, a Pet-1 binding site is located upstream of the 5-HTT gene and Pet-1 binding has been shown to increase transcription of the 5-HTT gene in vitro (Hendricks et al., 1999). Second, although there are species-specific differences in the effects of ovarian hormonal influence on 5-HTT levels in midbrain raphe nuclei (Bertrand et al., 2005; McQueen et al., 1997; Pecins-Thompson et al., 1998; Sumner et al., 1999), in rats, estradiol treatment increases 5-HTT mRNA levels in the DRN (McQueen et al., 1997; Sumner et al., 1999).
In the present study, we quantified mRNA levels of Pet-1 and 5-HTT in the midbrain raphe nuclei of OVX rats by in situ hybridization following injection of estradiol benzoate (EB; 0, 2 or 10 μg) in sesame oil vehicle. We focused our attention on the midbrain raphe nuclei (including the subnuclei of the DRN (dorsomedial (DRD), dorsolateral (DRL), ventromedial (DRV), and the median raphe nucleus (MRN)) because Pet-1 mRNA is restricted to this region (Hendricks et al., 1999) and this region has been implicated (by pharmacology and lesion studies) in the serotonergic control of food intake (Fletcher and Davies, 1990; Medeiros et al., 2005). The doses of EB were chosen because they produce reliable, anorexigenic effects in OVX rats, and the lower (2 μg) dose of EB models the pattern of plasma estradiol concentration observed in intact, cycling rats (Asarian and Geary, 2002; Geary and Asarian, 1999).
2. Results
2.1. Food intake and body weight
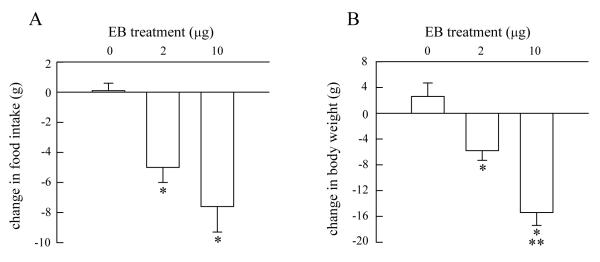
As expected, EB treatment reduced food intake and body weight, F(2, 16) = 9.43 and 25.63, respectively, Ps < 0.005 (Fig. 1). Both EB-treated groups displayed greater reductions in food intake and body weight than the oil-treated group, Ps < 0.05. The reduction in body weight was dose-related, such that the larger dose of EB promoted greater weight loss than the smaller dose of EB, P < 0.05.
Fig. 1.
EB treatment decreased 24-h food intake and body weight in OVX rats. Data are means ± SEM. OVX rats were treated with 0, 2, or 10 μg EB on two consecutive days (days 1 and 2). Food intake and body weight were measured two days after the second injection (i.e., on day 4, which models behavioral estrus). Reductions were calculated by subtracting the 24-h values obtained on day 4 from those obtained on day 1. (A) Both doses of EB produced similar decreases in food intake. (B) EB produced a dose-related decrease in body weight. *Less than the 0 μg EB-treated group, P < 0.05. **Less than the 2 μg EB-treated group, P < 0.05. Abbreviations: OVX, ovariectomized; EB, estradiol benzoate.
2.2. Pet-1 and 5-HTT mRNA levels in the midbrain raphe nuclei
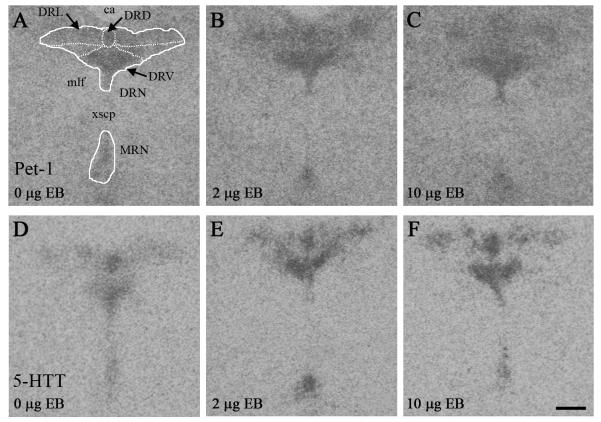
Pet-1 and 5-HTT in situ hybridization signals appeared stronger in the midbrain raphe nuclei of EB-treated groups, relative to the oil-treated group (Fig. 2). Semi-quantitative densitometric analysis revealed that EB treatment increased mRNA levels (the product of pixel number × pixel density) for Pet-1 in the whole tissue section containing the midbrain raphe nuclei, as well as in the DRN, DRV, and MRN (Table 1). Further analysis revealed that EB treatment increased mRNA levels for 5-HTT in the whole tissue section containing the midbrain raphe nuclei, as well as in the DRN and DRV (Table 2).
Fig. 2.
EB treatment increased the strength of the Pet-1 and 5-HTT in situ hybridization signals in the midbrain raphe nuclei of OVX rats. Representative autoradiographic images of Pet-1 (A-C) and 5-HTT (D-F) in situ hybridization signals in the midbrain raphe nuclei of rats treated with 0 μg EB (A,D), 2 μg EB (B,E), or 10 μg EB (C,F). All images correspond to ~ 8.04 mm posterior to bregma (Paxinos and Watson, 1998). Scale bar = 400 μm. Abbreviations: ca, cerebral aqueduct; DRD, dorsomedial nucleus of the DRN; DRL, dorsolateral nucleus of the DRN; DRN, dorsal raphe nucleus; DRV, ventromedial nucleus of the DRN; mlf, medial longitudinal fasciculus; MRN, medial raphe nucleus; xscp, decussation of the superior cerebellar peduncle.
Table 1.
EB treatment increased Pet-1 mRNA levels in the midbrain raphe nuclei of OVX rats.
| EB treatment (μg) | ||||
|---|---|---|---|---|
| Brain area | 0 | 2 | 10 | F-values |
| tissue section | 55 ± 15 (100 ± 28 %) |
167 ± 29* (305 ± 54 %) |
151 ± 21* (276 ± 38 %) |
F(2,13) = 4.49, P < 0.05 |
| DRN | 433 ± 134 (100 ± 31 %) |
855 ± 114* (197 ± 26 %) |
802 ± 67* (185 ± 16 %) |
F(2,13) = 4.06, P < 0.05 |
| DRV | 162 ± 20 (100 ± 12 %) |
354 ± 43* (219 ± 26 %) |
374 ± 27* (231 ± 17 %) |
F(2,13) = 8.96, P < 0.005 |
| DRD | 119 ± 16 (100 ± 14 %) |
153 ± 16 (129 ± 14 %) |
178 ± 24 (149 ± 20 %) |
F(2,13) = 1.59, n.s. |
| DRL | 226 ± 164 (100 ± 72 %) |
272 ± 52 (120 ± 23 %) |
218 ± 39 (97 ± 17 %) |
F(2,12) = 0.27, n.s. |
| MRN | 64 ± 11 (100 ± 18 %) |
134 ± 20* (210 ± 32 %) |
146 ± 9* (229 ± 14 %) |
F(2,13) = 6.91, P < 0.05 |
Pet-1 mRNA levels were determined by the product of pixel density × pixel number (in thousands). Data are also expressed (in parentheses) as a percent of the mean of the 0 μg EB-treated (control) group.
Greater than 0 μg EB-treated (control) group, Ps < 0.05.
Table 2.
EB treatment increased 5-HTT mRNA levels in the midbrain raphe nuclei of OVX rats.
| EB treatment (μg) | ||||
|---|---|---|---|---|
| Brain areas | 0 | 2 | 10 | F-values |
| tissue section | 112 ± 14 (100 ± 13 %) |
302 ± 42* (271 ± 38 %) |
252 ± 23* (226 ± 20 %) |
F(2,14) = 9.58, P < 0.005 |
| DRN | 511 ± 63 (100 ± 12 %) |
1028 ± 151* (201 ± 30 %) |
943 ± 86* (184 ± 17 %) |
F(2,14) = 5.53, P < 0.05 |
| DRV | 223 ± 39 (100 ± 18 %) |
486 ± 78* (218 ± 35 %) |
484 ± 43* (218 ± 19 %) |
F(2,14) = 6.20, P < 0.05 |
| DRD | 124 ± 38 (100 ± 31 %) |
163 ± 31 (132 ± 24 %) |
164 ± 22 (132 ± 17 %) |
F(2,14) = 0.55, n.s. |
| DRL | 142 ± 52 (100 ± 37 %) |
264 ± 51 (186 ± 36 %) |
227 ± 23 (160 ± 16 %) |
F(2,14) = 2.06, n.s. |
| MRN | 132 ± 34 (100 ± 26 %) |
173 ± 37 (131 ± 28 %) |
149 ± 17 (113 ± 13 %) |
F(2,14) = 0.45, n.s. |
5-HTT mRNA levels were determined by the product of pixel density × pixel number (in thousands). Data are also expressed (in parentheses) as a percent of the mean of the 0 μg EB-treated (control) group.
Greater than 0 μg EB-treated (control) group, Ps < 0.05.
3. Discussion
While previous research has shown that estradiol increases the anorexia induced by increased 5-HT neurotransmission (Eckel et al., 2005; Rivera and Eckel, 2005) and decreases the hyperphagia induced by decreased 5-HT neurotransmission (Salamanca and Uphouse, 1992, Uphouse et al, 1991), the molecular mechanism underlying these actions of estradiol are poorly understood. Here, we examined whether estradiol increases the expression of two genes, Pet-1 and 5-HTT, that regulate the development and activity of the 5-HT system. In support of our hypothesis, we found that a physiological regimen of EB treatment increased Pet-1 and 5-HTT mRNA levels in the midbrain raphe nuclei of OVX rats.
Currently, nothing is known about the influence of Pet-1 on food intake and only one study has reported that the body weights of male and female Pet-1 null mice, monitored at a single time point during adulthood, do not differ from their wild-type littermates (Hendricks et al., 2003). Because food intake, locomotor activity, and metabolism were not measured in this study, it remains possible that any change in food intake may have been offset by a change in locomotor activity or metabolism such that the overall energy homeostasis (body weight gain) was not affected. To date, hypothalamic-pituitary-ovarian function has not been examined in female Pet-1 null mice and it is not known whether female Pet-1 null mice are sensitive to estradiol in adulthood. Further studies examining Pet-1’s effects on food intake, locomotor activity, metabolism and reproductive function are needed to clarify the functional significance of estradiol’s ability to regulate transcription of the Pet-1 gene.
Given the relationship between Pet-1 and 5-HTT, it is interesting that EB treatment increased Pet-1 mRNA levels, but failed to alter 5-HTT mRNA levels, in the MRN. The failure of estradiol to increase MRN expression of 5-HTT is consistent with a previous study involving a similar regimen of EB treatment (McQueen et al., 1997). This differential effect of EB treatment may be related to the fact that the DRN and MRN project to non-overlapping sites in the forebrain. While the DRN provides fiber projections to lateral structures of the brain, the MRN provides fiber projections to medial structures of the brain (Vertes, 1991; Vertes et al., 1999). In addition, electrical stimulation of either the DRN or MRN increased 5-HT release in different forebrain regions (McQuade and Sharp, 1997). Although the current and previous findings suggest that EB acts differently on the DRN and MRN, we cannot discount the possibility that the methodology used in this study was not sensitive enough to detect subtle EB-mediated changes in 5-HTT mRNA levels in the MRN. For example, while in situ hybridization can detect between 20 and 200 copies of mRNA per cell, a more sensitive technique like in situ RT-PCR is able to detect single copies of mRNA per cell (Bartlett, 2002).
Previous studies have demonstrated that acute injection of EB increased 5-HTT mRNA levels in the DRN of OVX rats (McQueen et al., 1997; Sumner et al., 1999). Our current findings replicate and extend these studies by demonstrating that the EB-induced increase in 5-HTT mRNA, as well as the increase in Pet-1 mRNA, is localized within the DRV subnucleus of the DRN. The significance of this relates to the fact that the DRV has reciprocal connections with the hypothalamus and parabrachial nucleus (Petrov et al., 1992; Peyron et al., 1998). As a result, the DRV may be in a position to directly regulate a portion of the neural network controlling food intake.
While we and others (McQueen et al., 1997; Sumner et al., 1999) have found that acute estradiol treatment increases transcription of the 5-HTT gene in the DRN of OVX rats, this action of estradiol appears to be species specific. For example, estradiol treatment decreased 5-HTT mRNA levels in the DRN of spayed macaques (Pecins-Thompson et al., 1998), and ovariectomy failed to alter 5-HTT binding sites in the DRN and MRN of mice (Bertrand et al., 2005). Estradiol’s ability to regulate the transcription of a particular gene is dependent upon many factors, such as the presence of certain transcription factors in tissue (Beato and Klug, 2000), the ER subtypes that are present in tissue (Paech et al, 1997), and the DNA sequence within the promoter region of the gene (O’Lone et al, 2004). Specifies differences in the presence of any of these factors could lead to a species difference in the physiological actions of estradiol. It is noteworthy, therefore, that there are reports of species differences in the distribution of ER subtypes within the DRN. In rats, ERβ is co-localized in 5-HT neurons of the DRN, whereas ERα expression is limited to small interneurons in the DRN. In mice, both ERα and ERβ subtypes are co-localized in 5-HT neurons of the DRN. Only ERβ is co-localized in 5-HT neurons in the DRN of monkeys (reviewed in Bethea et al, 2002). These differences might contribute to the species-related differences in the effects of estradiol on 5-HTT mRNA levels in the DRN.
In the present study, EB treatment reduced food intake and body weight in OVX rats. While changes in body weight can influence the expression of feeding-related genes, there is some evidence to suggest that the increased expression of 5-HTT mRNA levels observed here was not secondary to decreased body weight. First, weight loss following either 24-h food deprivation in male and female mice (Jahng et al., 1998) or 3-days of reduced feeding in male rats (Choi et al., 2003) did not alter 5-HTT mRNA levels in the DRN. Second, chronic food restriction for 5 weeks promoted a decrease in 5-HTT mRNA levels in the DRN of male rats (Jahng et al., 2007). Thus, it appears unlikely that a decrease in body weight contributed to the increases in 5-HTT mRNA levels in the midbrain raphe nuclei of EB-treated OVX rats. Rather, there is reason to believe that this change in gene expression resulted either directly or indirectly from our estradiol manipulation. That is, the increase in 5-HTT mRNA levels may have been mediated by a direct action of estradiol, since a consensus estrogen-response element (ERE) is located 1.2 kb upstream of the transcription start site of the human 5-HTT gene (Flattem and Blakely, 2000). It is also possible that the increase in 5-HTT mRNA expression was secondary to the EB-mediated increase in Pet-1 expression since Pet-1 binding sites are located on the promoter of the 5-HTT gene and Pet-1 binding increases transcription of the 5-HTT gene in vitro (Hendricks et al., 1999). Additional studies are required to elucidate the mechanism underlying the observed increases in 5-HTT mRNA levels in EB-treated OVX rats.
In conclusion, EB treatment increased Pet-1 and 5-HTT mRNA levels in the midbrain raphe nuclei of OVX rats at a time of reduced food intake and body weight. Further studies are required to determine whether the increased expression of Pet-1 and 5-HTT mRNA promote increased serotonergic tone in brain regions implicated in the control of food intake and, thereby, play a causal role in the anorexigenic effect of estradiol. Moreover, the apparent estradiol/5-HT interaction evident in this study does not only speak to the regulation of feeding behavior but it may also be involved in the regulation of other behaviors where estradiol/5-HT interactions have been observed, such as reproductive behavior (Uphouse, 2000), depression (Estrada-Camarena et al, 2004), and anxiety (Andrade et al, 2005; Olivier et al., 2008). With respect to human research, our findings may also have clinical relevance given that polymorphisms in the human 5-HTT promoter (Heils et al., 1996) are associated with anorexia nervosa (Fumeron et al., 2001), an eating disorder that affects primarily women.
4. Experimental procedures
4.1. Animals, housing, and OVX surgery
Seventeen female Long-Evans rats (Charles River Breeding Laboratories, Raleigh, NC), weighing 390 ± 10 g at study onset, were housed individually in custom-designed Plexiglas shoe-box cages. The cages were equipped with specialized feeding niches that provided free access to spill-resistant food cups containing powdered chow (Purina 5001) and drinking tubes containing tap water. The room was maintained at 20 ± 2 °C with a 12:12 h light:dark cycle (dark onset = 1200 h).
At study onset, rats were anesthetized by intraperitoneal (i.p.) injections of a mixture of 70 mg/kg ketamine (Ketaset, Fort Dodge, Fort Dodge, IA) and 4.5 mg/kg xylazine (Rompun, Mobay, Shawnee, KS). They were then bilaterally OVX using an intra-abdominal approach. Following surgery, rats were given a 7-day postoperative recovery period. Animal usage and all animal procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
4.2. Protocol
Following postoperative recovery, OVX rats received subcutaneous (s.c.) injections of either 0, 2, or 10 μg EB (Sigma Chemical, St. Louis, MO; n = 5-6 per group) dissolved in 100 μl of sesame oil vehicle (Sigma). Injections were administered at 1100 h on two consecutive days. This acute regimen of EB treatment was used for two reasons. First, acute administration of 2 μg EB produces a pattern of plasma estradiol concentration that is similar to that observed in intact, cycling rats (Asarian and Geary, 2002; Geary and Asarian, 1999). That is, plasma estradiol is low on day 1, begins to rise on day 2, peaks on day 3, and then falls to basal levels on day 4, the day that models estrus (Becker et al., 2005). Second, acute administration of EB in OVX rats can reinstate the estrous-related decrease in food intake observed in intact cycling rats (Asarian and Geary, 2002; Geary and Asarian, 1999).
At 0930 h on day 4, food intake and body weight data were recorded and rats were then anesthetized with i.p. injections of 65 mg/kg sodium pentobarbital (Butler, Columbus, OH). When completely unresponsive, rats were transcardially perfused with isotonic, heparinized saline containing 0.5% NaNO2 followed by a fixative solution, 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2. Brains were rapidly dissected, blocked, post-fixed in fixative solution for 2 h at 4°C and then transferred to 30% sucrose for 3 days at 4°C for cryoprotection.
Blocks of tissue containing the midbrain raphe nuclei were mounted on a freezing, sliding microtome (Microm HM440E, Walldorf, Germany), and two series of coronal sections, spanning ~8.04 mm through 7.56 mm posterior to bregma (Paxinos and Watson, 1998), were cut at 40 μm intervals. This region of the midbrain raphe was chosen because levels of mRNA for serotonergic genes, such as TPH-2, 5-HTT, 5-HT1A, and 5-HT1B, appear highest within this range (Clark et al., 2006) and because it has been previously reported that chronic crystalline 17-β-estradiol treatment increased mRNA levels for TPH-2 within this range (Hiroi et al., 2006). Alternate tissue sections were processed for Pet-1 and 5-HTT in situ hybridization. In the whole tissue section, whole DRN, whole MRN, DRV, and DRD, there was an average of 6 tissue sections quantified from each rat for each probe. In the DRL, there was an average of 4 tissue sections quantified from each rat for each probe.
4.3. In situ hybridization and semi-quantitative analysis of Pet-1 and 5-HTT mRNA
Free-floating tissue sections containing midbrain raphe nuclei were collected into 20 ml glass scintillation vials containing ice-cold 2 × SSC (0.3M NaCl, 0.03M sodium citrate) for in situ hybridization. The SSC in each vial was pipetted off, and sections were then suspended in 1 ml of warm prehybridization buffer (50% formamide, 10% dextran sulfate, 2 × SSC, 1× Denhardt’s solution, 50mM dithiothreitol, 0.5 mg/mL denatured salmon sperm DNA) and placed in a 48°C water bath. Two h later, 35S-dCTP-labeled cDNA probes (10 × 106 CPM/vial) of either Pet-1 (prepared from a 1.0 kb restriction fragment (Fyodorov et al., 1998)) or 5-HTT (prepared from a 0.8 kb restriction fragment (Jahng et al., 1998)) were added to the vials and hybridized overnight at 48°C.
After overnight hybridization, the sections were washed at 15-min intervals in decreasing concentrations of SSC (2×, 2×, 1×, 0.5×, 0.25×, 0.125×, 0.125×) at 48°C. After washes, the tissue sections were stored in 0.1M phosphate buffer at 4°C and then mounted on gelatin-subbed slides, air-dried, and apposed to Kodak Biomax autoradiographic film (Eastman Kodak Co., NY, USA). 5-HTT and Pet-1 film exposure times were 24 h and 9 days, respectively, to obtain autoradiographic images within a linear range of optical density. Following development, images on the autoradiographic films were digitized with a Zeiss Stemi-2000 stereoscope attached to a Dage-MTI CCD 72 camera and Macintosh image analysis system (MindsEye 1.26b, T. Houpt). Tissue sections from rats from each treatment condition (0, 2, and 10 μg EB) were hybridized within the same vial and apposed to the same film. Thus, in situ hybridization was carried out on representative members of each experimental group at the same time and under identical conditions allowing for direct comparison of mRNA levels.
For each section, Pet-1 and 5-HTT mRNA levels across the whole tissue section containing the midbrain raphe nuclei were determined automatically (using MindsEye) by quantifying the product of the pixel number (area of hybridization signal) and pixel density (relative optical density of pixels with densities of at least 2 standard deviations above the density of the image background). In addition, specific subregions of the midbrain raphe nuclei were outlined by hand on each section image, following the boundaries of Paxinos and Watson (Paxinos and Watson, 1998). Outlined areas included the DRN, subnuclei of the DRN (i.e., DRV, DRD, and DRL) and MRN. Within delineated regions of each tissue section, the pixel number and pixel density were calculated by MindsEye. The pixel density of each delineated region was determined by subtracting the background pixel density of each section from the pixel density of the region of interest. Estimates of mRNA level within areas of interest were calculated by multiplying pixel number and pixel density. Such estimates were averaged across all tissue sections to obtain mean mRNA levels for individual rats.
4.4. Data analyses
Data are presented as means ± SEM across rats within each treatment group. Reductions in food intake and body weight were determined by calculating the change in food intake and body weight observed on day 4 (the day that models behavioral estrus) relative to that observed just prior to hormone treatment on day 1. One-way ANOVAs were used to analyze the effect of hormone treatment (0, 2, or 10 μg EB) on the reduction in food intake and body weight, as well as on changes in Pet-1 and 5-HTT mRNA levels in the following regions: whole tissue section containing the midbrain raphe nuclei, DRN, DRV, DRD, DRL, and MRN. Newman-Keuls posthoc tests were used to investigate differences between means following significant (P<0.05) ANOVA effects. Due to problems with processing of tissue, two rats (one oil-treated and one treated with 2 μg EB) were not included in the final analysis of mRNA levels (when these 2 rats were excluded from the analysis of the reduction in food intake and body weight, the outcome of the analysis was not changed). Also, insufficient tissue was collected to analyze Pet-1 mRNA in one additional oil-treated rat. Thus, the final numbers of rats included in the mRNA analysis were oil-treated, n = 4 (n = 3 for Pet-1 mRNA), 2 μg EB-treated, n = 5, and 10 μg EB-treated, n = 6.
Acknowledgments
This work was supported by grants from the NIH: DK-073936 (LAE), DC-03198 (TAH), and T32 DC-00044 (HMR).
Abbreviations
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tertraline)
- ca
cerebral aqueduct
- CCK
cholecystokinin
- cDNA
complementary deoxyribonucleic acid
- CPM
counts per million
- dCTP
deoxycytidine triphosphate
- DRD
dorsomedial nucleus of the DRN
- DRL
dorsolateral nucleus of the DRN
- DRN
dorsal raphe nucleus
- DRV
ventromedial nucleus of the DRN
- EB
17-β-estradiol-3-benzoate
- ER
estrogen receptor
- ERE
estrogen-response element
- 5-HT
5-hydroxytryptamine
- 5-HT1A
5-hydroxytryptamine autoreceptor
- 5-HTT
5-hydroxytryptamine transporter
- i.p.
intraperitoneal
- MRN
medial raphe nucleus
- mRNA
messenger ribonucleic acid
- OVX
ovariectomized
- Pet-1
Pheochromocytoma 12 ETS (E26 transformation-specific) domain transcription factor
- NaCl
sodium chloride
- NaNO2
sodium nitrite
- SSC
NaCl-sodium citrate
- s.c.
subcutaneous
- TPH
tryptophan hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: 6. Regulatory Systems
REFERENCES
- Alves SE, Weiland NG, Hayashi S, McEwan B. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J. Comp. Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- Andrade TGCS, Nakamuta JS, Avanzi V, Graeff FG. Anxiolytic effect of estradiol in the median raphe nucleus mediated by 5-HT1A receptors. Behav. Brain Res. 2005;1:18–25. doi: 10.1016/j.bbr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Bartlett JMS. Approaches to the analysis of gene expression using mRNA: a technical review. Mol. Biotechnol. 2002;21:149–160. doi: 10.1385/MB:21:2:149. [DOI] [PubMed] [Google Scholar]
- Beato M, Klugg J. Steroid hormone receptors: an update. Hum. Reprod. Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KB, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Paranavitane UT, Chavez C, Gogos A, Jones M, van den Buuse M. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: a behavioral and electrochemical study. Brain Res. 2005;1064:10–20. doi: 10.1016/j.brainres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of steroids in the serotonin neural system. Front. Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Burton MJ, Cooper SJ, Popplewell DA. The effect of fenfluramine on the microstructure of feeding and drinking in the rat. Br. J. Pharmacol. 1981;72:621–633. doi: 10.1111/j.1476-5381.1981.tb09142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kwon BS, Lee S, Houpt TA, Lee HT, Kim DG, Jahng JW. Systemic 5-hydroxy-L-tryptophan down-regulates the arcuate CART mRNA level in rats. Regul. Pept. 2003;115:73–80. doi: 10.1016/s0167-0115(03)00126-5. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J. Comp. Neurol. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: an indirect control of meal size. Physiol. Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Rivera HM, Atchley DPD. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1486–R1491. doi: 10.1152/ajpregu.00779.2004. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharm. 2004;173:139–145. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- Flattem NL, Blakely RD. Modified structure of the human serotonin transporter promoter. Mol. Psychiatry. 2000;5:110–115. doi: 10.1038/sj.mp.4000585. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Davies M. Dorsal raphe microinjection of 5-HT and indirect 5-HT agonists induces feeding in rats. Eur. J. Pharmacol. 1990;184:265–271. doi: 10.1016/0014-2999(90)90618-g. [DOI] [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Aubert R, Herbeth B, Siest G, Rigaud D. Association of a functional 5-HT transporter gene polymorphism with anorexia nervosa and food intake. Mol. Psychiatry. 2001;6:9–10. doi: 10.1038/sj.mp.4000824. [DOI] [PubMed] [Google Scholar]
- Fyodorov D, Nelson T, Deneris E. Pet-1, a novel ETS domain factor that can activate neuronal nAchR gene transcription. J. Neurobiol. 1998;34:151–163. [PubMed] [Google Scholar]
- Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol. Behav. 1999;67:141–147. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heils A, Wichems C, Mossner R, Petri S, Glatz K, Bengel D, Murphy DL, Lesch KP. Functional characterization characterization of the murine serotonin transporter gene promoter in serotonergic raphe neurons. J. Neurochem. 1998;70:932–939. doi: 10.1046/j.1471-4159.1998.70030932.x. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol. Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Houpt TA, Joh TH, Son JH. Differential expression of monoamine oxidase A, serotonin transporter, tyrosine hydroxylase and norepinephrine transporter mRNA by anorexia mutation and food deprivation. Dev. Brain Res. 1998;107:241–246. doi: 10.1016/s0165-3806(98)00013-3. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Kim JG, Kim HJ, Kim BT, Kang DW, Lee JH. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res. 2007;1150:100–107. doi: 10.1016/j.brainres.2007.02.080. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Alexander JT. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiatry. 1998;44:851–864. doi: 10.1016/s0006-3223(98)00186-3. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Estrogen receptor-alpha in the raphe serotonergic and supramammillary area calretinin-containing neurons of the female rat. Exp. Brain Res. 1999;128:417–420. doi: 10.1007/s002210050863. [DOI] [PubMed] [Google Scholar]
- Maswood S, Truitt W, Hotema M, Caldarola-Pastuszka M, Uphouse L. Estrous cycle modulation of extracellular serotonin in mediobasal hypothalamus: role of the serotonin transporter and terminal autoreceptors. Brain Res. 1999;831:146–154. doi: 10.1016/s0006-8993(99)01439-0. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J. Neurosci. Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J. Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17B increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Mol. Brain Res. 1997;45:13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- Medeiros MA, Costa-e-Sousa RH, Olivares EL, Côrtes WS, Reis LC. A reassessment of the role of serotonergic system in the control of feeding behavior. An. Acad. Bras. Cienc. 2005;77:103–111. doi: 10.1590/s0001-37652005000100008. [DOI] [PubMed] [Google Scholar]
- Olivier JDA, Van Der Hart MGC, Van Swelm RPL, Dederen PJ, Homberg JR, Cremers T, Deen PMT, Cuppen E, Cools AR, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol. Endocrinology. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Scienc. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Mol. Brain Res. 1998;53:120–129. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J. Comp. Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol. Behav. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Salamanca S, Uphouse L. Estradiol modulation of the hyperphagia induced by the 5-HT1A agonist, 8-OH-DPAT. Pharm. Biochem. Behav. 1992;43:953–955. doi: 10.1016/0091-3057(92)90431-e. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (α,β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species effects. Neurosci. Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav. Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Mol. Brain Res. 1999;73:119–128. doi: 10.1016/s0169-328x(99)00243-0. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Female gonadal hormones, serotonin, and sexual receptivity. Brain Res. Rev. 2000;33:242–257. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Salamanca S, Caldarola-Pastuszka M. Gender and estrous cycle differences in the response to the 5-HT1A agonist 8-OH-DPAT. Pharm. Biochem. Behav. 1991;40:901–906. doi: 10.1016/0091-3057(91)90104-a. [DOI] [PubMed] [Google Scholar]
- Vertes R. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. [PubMed] [Google Scholar]