Abstract
Purpose
Osteonectin/SPARC is a secreted protein that has been implicated in ocular disease. Deletion of osteonectin/SPARC causes age-onset cataract in mice and the cataractous human lens has increased expression of osteonectin/SPARC. In this study, the expression and localization of osteonectin/SPARC in the monkey retina were determined as was secretion by cultured human retinal pigment epithelial (RPE) cells.
Methods
Adult Rhesus monkey eyes (Macaca mulatta) were dissected, and 5-mm macula and peripheral retina punches were obtained. Supernatants were collected from cultured human RPE cells. Subcellular fractionation of whole monkey retina was also performed. Osteonectin/SPARC expression and/or secretion was monitored by Northern and Western blot analyses, and localization was determined by immunocytochemistry.
Results
Outside of the retina osteonectin/SPARC mRNA is broadly expressed in many human tissues. Northern blot analysis shows that in the retina osteonectin/SPARC is expressed almost exclusively by the macular RPE/choroid. Western blot analysis revealed osteonectin/SPARC in both the macula and the peripheral neural retina but only in trace amounts in the RPE/choroid. In subcellular fractions of the whole retina, osteonectin/SPARC was detected, mainly in the soluble fraction but also in the membrane and nuclear fractions. Immunohistochemical analysis localized osteonectin/SPARC specifically to the outer plexiform layer. Western blot analysis of conditioned medium from human RPE cells cultured on porous substrates indicated that osteonectin/SPARC is secreted in large amounts from both the apical and basal sides of the RPE.
Conclusions
Collectively these data provide evidence that osteonectin/SPARC is synthesized in the macular RPE, secreted, and subsequently transported to the outer plexiform layer. The expression pattern of osteonectin/SPARC in the subcellular retinal fractions is consistent with a soluble protein that is transported and internalized.
Osteonectin,1 also known as SPARC2 and BM-40,3 is a 43-kDa Ca2+ binding glycoprotein with complex biological functions.4 Osteonectin/SPARC is believed to regulate cell growth through interactions with the extracellular matrix.5 Increased secretion of osteonectin/SPARC is associated with endothelial cell injury in vitro6 and the inhibition of cell spreading on collagen. It can also induce cell rounding in cultured endothelial cells and fibroblasts.7 Osteonectin/SPARC will associate directly with platelet-derived growth factor (PDGF) and modulate its activity.8 This binding activity to PDGF seems to be Ca2+ independent, whereas its binding to collagen is Ca2+ dependent.9 Its Ca2+ binding domain is unique10 and may be involved in modulating cell shape and adhesion.11,12
Previous studies have implicated osteonectin/SPARC in ocular disease. Increased expression of osteonectin/SPARC mRNA13 and protein14 is associated with age-related human cataract, and several independent studies demonstrate that deletion of osteonectin/SPARC causes cataract in mice.15,16 Osteonectin/SPARC has previously been detected in quail,17 chicken,18 and monkey14 retinas.
In humans osteonectin/SPARC mRNA is found in two distinct messages, a predominant 2.2-kb message and a less abundant 3.0-kb message.19,20 These messages arise from different polyadenylation sites and have identical coding regions.19 The gene contains 10 exons and is located on chromosome 5q35.21 Presently, there are no genetic retinal diseases associated with that locus (http://www.sph.uth.tmc.edu/RetNet/disease.htm).
To establish a potential role for osteonectin/SPARC in retinal function, we examined the expression of osteonectin/SPARC protein and mRNA in the monkey retina. We also localized the protein by immunocytochemistry and examined secretion of osteonectin/SPARC protein by cultured human retinal pigment epithelial (RPE) cells. Collectively, these data provide evidence that osteonectin/SPARC is produced in the RPE and localized to the outer plexiform layer (OPL) of the retina.
Methods
Materials
Fresh eye tissue was obtained from Rhesus monkeys (Maccaca mulatta, 2–3 years old) through the courtesy of the Center for Biologics Research and Testing, US Food and Drug Administration (Bethesda, MD). Animal studies were conducted in accordance with the NIH guidelines and the ARVO statement on the care and use of animals in research. The monkeys were anesthetized, then exsanguinated, and the enucleation was performed within 3 to 4 minutes after death. The eyes were immediately placed on ice or in tissue fixative. Macular and peripheral retinal tissue was obtained using a 5-mm trephine. The neural retina was separated from the pigment epithelium–choroid and frozen at −75°C before further processing. Each retinal sample is from a pool of 10 eyes.
Tissue Preparation
After enucleation, a small incision was made at the limbus, and the monkey eyes were submerged overnight in 4% paraformaldehyde buffered with 100 mM sodium phosphate, pH 7.4. The anterior chamber was removed, and the retinal cup was incubated for an additional 24 hours in the same buffer. The vitreous was removed carefully and the retinal cup was then trimmed, dehydrated, embedded in paraffin, and sectioned.
Subcellular Fractionation
Subcellular fractionation of the retina was performed as previously described.22 In brief, fresh Rhesus (Maccaca mulatta) monkey retinas were homogenized in 10 × volume of 10 mM HEPES buffer (pH 7.2) containing 5 mM MgCl2, 4% (wt/vol) sucrose and Complete Inhibitor (1 tablet/50 ml, Boehringer–Mannheim, Rockford, IL). The homogenate was subjected to a low speed (300g) centrifugation to separate the nuclei (P1), and the remaining supernatant was centrifuged at high speed (27,000g) to pellet the subcellular organelles (P2). The P2 pellet was subsequently subjected to a high salt wash and a detergent wash.
Northern Blot Analysis
Human RNAs were either purchased from Clontech (Palo Alto, CA) or purified directly from human and monkey tissues using the RNeasy Kit from Qiagen (Valencia, CA). The Northern blot analysis was probed with a 32P-labeled 304-bp PCR (polymerase chain reaction) product within the coding region of osteonectin/SPARC. The probe was generated with primers Ost-1F (CTGATGAGACAGAGGTGGTGGAAG) and Ost-1R (AAGAAGTGGCAGGAAGAGTCGAAG). The probe was labeled using the RTS RadPrime DNA labeling system (Life Technologies, Gaithersburg, MD) and Redivue α-32P dCTP (Amersham Pharmacia Biotech, Piscataway, NJ). The authenticity of the PCR probe was confirmed by sequencing. Human total RNAs (∼5 μg) were loaded in a 1% agarose/formaldehyde gel, separated by electrophoresis, and stained with SYBR Green II (Molecular Probes, Eugene, OR). The amount of osteonectin/SPARC mRNA was quantified relative to the 28S ribosomal RNA band using a Storm 860 phosphoimager (Molecular Dynamics, Sunnyvale, CA) as previously described.23
Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and Western Blot Analysis
Unless specified, all electrophoresis reagents and apparatus were purchased from NOVEX, San Diego, CA. Protein samples were mixed with sample buffer at a 1:1 volume ratio and heated at 100° degrees for 2 minutes. The samples were separated by electrophoresis in 10% to 20% Tricine–sodium dodecyl sulfate (SDS) gels at room temperature for 1 ½ hours at 150 volts. The gels were transferred onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH) using a Trans-Blot electrophoresis transfer cell apparatus (Bio–Rad, Richmond, CA). The transfers were performed overnight at 20 volts in 25 mM Tris, 192 mM glycine, 20% methanol at room temperature. The membrane was equilibrated in Tris-buffered saline (TBS), pH 7.4, 0.05% Tween-20 (Quality Biological, Gaithersburg, MD) for 15 minutes then blocked in TBS (pH 7.4), 5% Carnation nonfat milk, and 0.05% Tween-20, for 2 hours. The membrane was incubated with anti-osteonectin/SPARC antibody11 at 1:2500 dilution for 2 hours, followed by incubation for 1 hour with monoclonal anti-rabbit IgG alkaline phosphatase–conjugated secondary antibody (Sigma Chemical, St. Louis, MO). In between each incubation three successive washes of 10 minutes each were performed with TBS, 0.05% Tween-20. The blot was developed with BCIP/NBT (5-bromo-4chloro-3-indolyl phosphate/nitro blue tetrazolium) solution (Sigma Chemical) and scanned directly. Gels were also stained with Coomassie blue for half an hour and destained with 20% methanol and 10% acetic acid.
Immunocytochemistry
Paraffin-embedded monkey retina sections were deparaffinized with xylene and rehydrated with successive ethanol washes. The sections were blocked using diluted (1:200) goat serum for 1 hour. The tissue was then incubated with anti-osteonectin/SPARC (1:200) antibody for 2 to 4 hours and washed with TBS–Tween. Rabbit anti-osteonectin/SPARC was generated against a keyhole limpet hemocyanin (KLH)-conjugated synthetic peptide from the C-terminal region (amino acids 254–273) of mouse osteonectin/SPARC that contains the EF hand (Ca2+-binding motif). It has previously been shown to be specific in the mouse, humans, bovines, chickens, and rats.11 The secondary antibody (mouse anti-rabbit alkaline phosphatase conjugate, same as for westerns above) was applied at 1:500 dilutions for 2 hours. After the TBS–Tween washes, the sections were developed using Fast Red (Fast Red tablets; Boehringer–Mannheim, Indianapolis, IN) according to the manufacturer's instructions. The slides were photographed using a Nikon Eclipse E800 microscope with a SPOT II digital camera.
Cultured RPE Cells
Human fetal RPE (16–24 weeks' gestation) was collected in accordance with the tenets of the Declaration of Helsinki. Consent for research use of fetal tissue was obtained in compliance with federal and state laws and institutional regulations of the University of California. Institutional approval was obtained for the use of human eyes. The RPE was dissected from the choroid and cultured as described previously24 except that the substrate was 12 mm Millicell-PCF culture plate inserts (Millipore, Bedford, MA) with a pore diameter of 0.4 μm. After the cells had proliferated to confluence and resided in culture for at least 2 months, transepithelial resistances (TER) were measured. When the TER exceeded 700 Ohm × cm2, conditioned culture medium was separately collected from the apical and basolateral compartment of the culture chambers 3 days after each change of the culture medium. The volume in each compartment of the chamber was approximately 0.45 ml.
Results
Northern Blot Analysis of Osteonectin/SPARC in Macula versus Peripheral Retina and RPE
To determine the general location of osteonectin/SPARC mRNA in the retina, Northern blot analysis was performed on total RNA from monkey macula and peripheral retina (Fig. 1). Punches 5 mm in diameter were taken from fresh monkey retina and the tissue separated into neural retina and RPE/choroid. Each sample was from a pool of 10 retina punches. The blot was probed using a 304-bp PCR product from the coding region of osteonectin/SPARC. The levels of osteonectin/SPARC were quantified relative to the ribosomal 28S bands using only the 2.2-kb message as previously described.23 The probe also detects a 3.0-kb message that is produced by a downstream polyadenylation signal that increases the size of the 3′ untranslated region.19 The levels of osteonectin/SPARC mRNA in the macular RPE/choroid are approximately 10-fold greater than those detected in the neural macula and peripheral tissues. These results indicate that synthesis of osteonectin/SPARC is occurring mainly in the RPE–choroid region in the macula. Identical results were obtained using a different RNA preparation (also a pool of 10 retinas) and a 3′ untranslated probe (data not shown).
Figure 1.
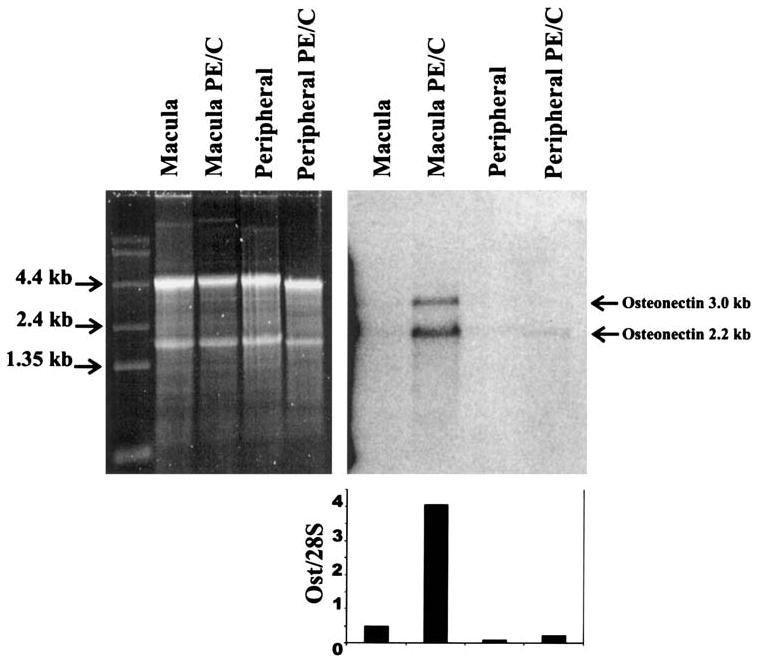
Expression of osteonectin/SPARC mRNA in monkey macula and peripheral retina by Northern blot analysis. Each lane was loaded with 5 μg of total RNA. Each sample represents a pool of 10 retinas. The left panel shows the SYBR Green II–stained gel, and the right panel is the blot probed with a 304-bp human osteonectin/SPARC probe generated with primers (CTGATGAGACAGAGGTGGTGGAAG and AAGAAGTGGCAGGAAGAGTCGAAG). The graph shows the relative levels of osteonectin/SPARC in the different tissues normalized to the 28S band as previously described.23 The 2.2-kb main osteonectin/SPARC band was used for quantification. Ost, osteonectin; PE/C, pigment epithelium/choroid.
Expression of Osteonectin/SPARC mRNA in Human Tissues
Northern blot analysis was also performed on 19 different human tissues to determine the tissue specificity of osteonectin/SPARC (Fig. 2). The same probe and quantification procedure described in Figure 1 was used in this blot. Osteonectin/SPARC was detectable, and the levels varied significantly among the human tissues examined. The lowest levels were detected in heart and skeletal muscle, and the highest levels were detected in testis, placenta, and uterus. In the latter tissues the levels of osteonectin/SPARC were four- to fivefold or greater than in all other tissues.
Figure 2.
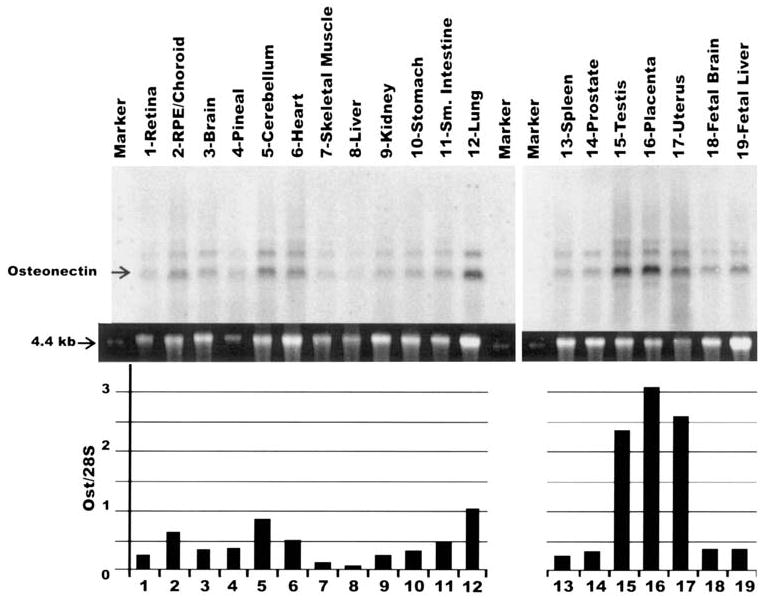
Northern blot analysis to measure the expression of osteonectin/SPARC mRNA in human tissues. The lanes were loaded with 5 μg of total RNA. The gel, probe, and quantification conditions were identical to those used in Figure 1. The top panel shows the probed and exposed blot. The middle panel shows the 28S SYBR green II–stained band. The bottom graph shows the relative levels of osteonectin/SPARC expression in the different human tissues normalized to the 28S band. Ost, osteonectin.
Western Blot Analysis of Osteonectin/SPARC in Monkey Retinas
To compare the location of the osteonectin/SPARC message (Fig. 1) with the location of the osteonectin/SPARC protein, a Western blot analysis of the monkey macula and peripheral retinal tissues was performed (Fig. 3A). Each lane contains approximately 20 μg of protein. The blot probed with a well-characterized anti-osteonectin/SPARC antibody.12 Abundant osteonectin/SPARC peptide was detected in the neural macula and peripheral retina but only in trace amounts in the RPE/choroid. This is opposite of what was observed for the mRNA (Fig. 1), suggesting that the macular RPE/choroid translates and secretes osteonectin/SPARC that is subsequently transported and widely distributed within the OPL of the neural retina.
Figure 3.
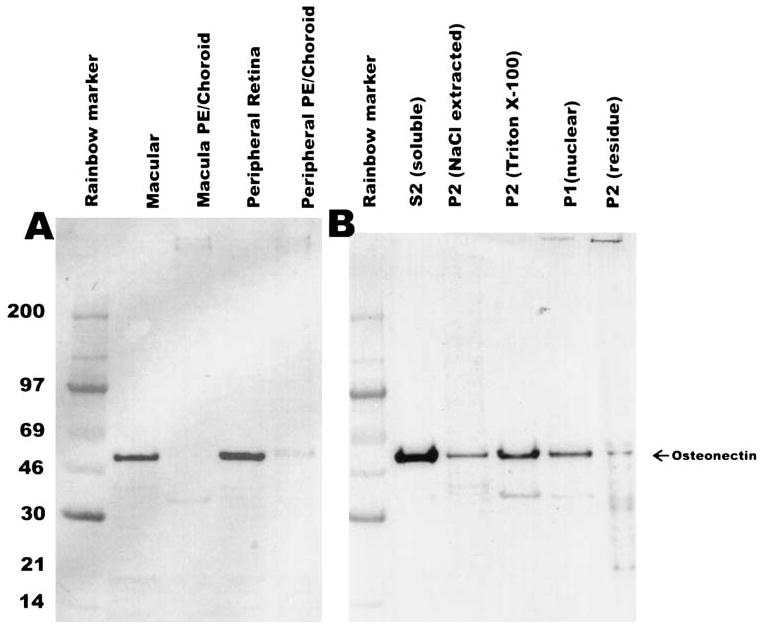
Western blot analyses of osteonectin/SPARC in monkey retinal tissues. Each lane was loaded with approximately 20 μg total protein. (A) Results of Western blot analysis of the macular and peripheral retina punches (same as Fig. 1) probed with rabbit anti-osteonectin/SPARC antibody (1:3000). (B) Results of Western blot analysis of the subcellularly fractionated whole monkey retina. The blots were processed as described in the Methods section. PE, pigment epithelium.
To further localize osteonectin/SPARC, whole monkey retina was fractionated as previously described.22 The different subcellular fractions were run on SDS–polyacrylamide gel electrophoresis (SDS–PAGE), and osteonectin/SPARC was detected by Western blot (Fig. 3B). As expected, osteonectin/SPARC was detected mostly in the soluble S2 fraction. However, osteonectin/SPARC was also detected in the other fractions, indicating that it is interacting with a number of cellular components and possibly entering the nucleus as previously described.25
Expression of Osteonectin/SPARC by Cultured RPE Cells
Conditioned media from cultured fetal RPE cells were collected from both the apical and basal sides and the protein precipitated in 2% trichloroacetic acid (TCA). The proteins were dissolved in 0.25 volume of sample buffer. Each lane was loaded with an equivalent of 160 μl of conditioned media. The presence of osteonectin/SPARC was determined by Western blot analysis (Fig. 4). Osteonectin/SPARC was detected in both the apical and basal media in two different culture preparations.
Figure 4.
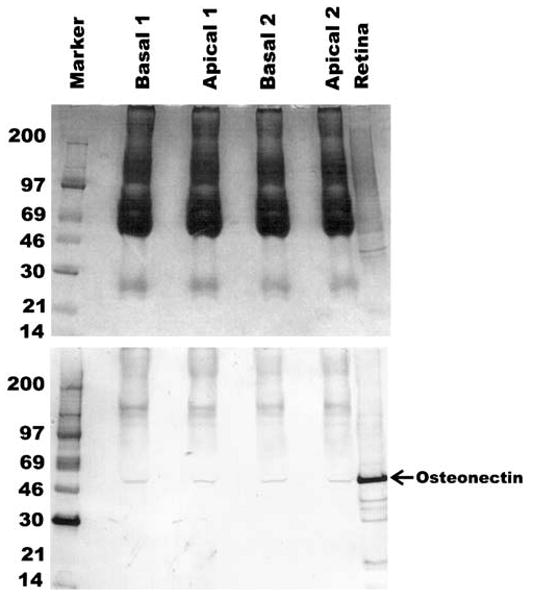
Expression of osteonectin/SPARC in conditioned media from cultured fetal RPE cells. Conditioned media from the apical and basal sides of the RPE were collected and the proteins concentrated by TCA precipitation. An equivalent of 160 μl of conditioned media was loaded per lane. Top panel shows Coomassie blue–stained gel, and bottom panel shows the blot probed with anti-osteonectin/SPARC at 1:3000 dilution. Two different preparations were tested. The development of the Western blot was performed as described in the Methods section. A monkey retina sample was run as a positive control.
Immunocytochemical Localization of Osteonectin/SPARC in Monkey Retinas
Paraformaldehyde-fixed monkey retina embedded in paraffin were used to localize osteonectin/SPARC by immunocytochemistry (Fig. 5). Sections were cut across the macular region to see if there were differences between the two areas. Osteonectin/SPARC was localized to the OPL, and less intense staining was observed in the macula (Fig. 5A) versus the peripheral retina (Fig. 5B). Punctate labeling was observed throughout the retina in both the macular and peripheral areas. Müller cell processes around the OPL may also be labeled, but no generalized labeling of the Müller cells was observed. No significant labeling was observed in the macular RPE (site of mRNA synthesis), although the dark pigment may mask intracellular labeling (Fig. 5A).
Figure 5.
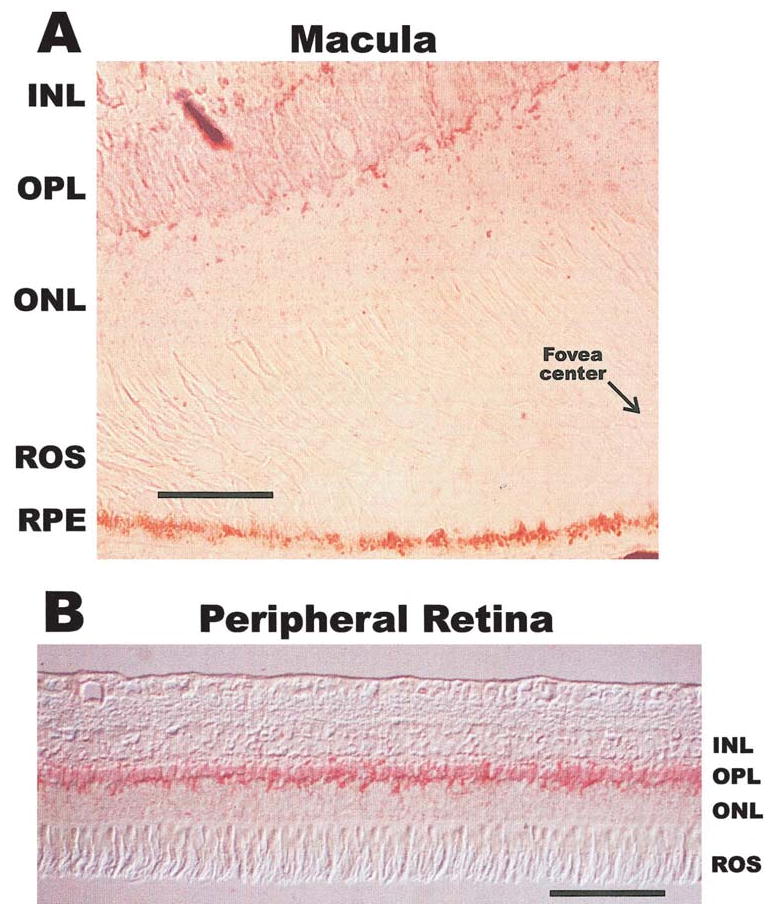
Immunocytochemical localization of osteonectin/SPARC in the OPL of the monkey retina. (A) shows the anti-osteonectin/SPARC labeling of the monkey macula including a portion of the fovea. (B) shows the more intense labeling observed in the peripheral retina. The immunoreactivity is seen in red. ROS, rod outer segments; ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar, 200 μm.
Discussion
The most important conclusions from our data are that osteonectin/SPARC is mainly synthesized by the macular pigment epithelium but that the peptide is widely distributed throughout the OPL of the retina. The results of Northern blot analysis shown in Figure 1 indicate that osteonectin/SPARC mRNA expression was approximately 10-fold greater in the macular RPE/choroid than in the peripheral retina. Although the Northern blot analysis barely detected osteonectin/SPARC in the neural retina, we were able to detect message there by reverse transcription–PCR using cDNA reverse-transcribed on magnetic beads as template (see Ref. 26; data not shown). This suggests that there may be additional source(s) of osteonectin/SPARC in the neural retina. Although the macular RPE/choroid seems to be the most active site of mRNA synthesis, the peripheral RPE is also synthesizing osteonectin/SPARC mRNA, and the differences in amounts seen between the two tissues may be due to differential rates of protein turnover. The differential turnover rate could explain both the differences in message synthesis observed in Figure 1 and the differences in intensities seen in the immunocytochemistry in Figure 5, because the macula is noticeably more metabolically active than the peripheral retina. The clear detection of osteonectin/SPARC in only 160 μl of RPE conditioned media (Fig. 4) suggests that the RPE is manufacturing this protein in significant amounts. Western blot analyses of macula and peripheral retinas (Fig. 3) and the immunocytochemical localization (Fig. 5) indicated that osteonectin/SPARC is distributed throughout the OPL of the retina. We also observed punctate labeling throughout the photoreceptor layer and the inner and outer nuclear layers (Fig. 5A). This is in agreement with the observations from the subcellular fractionation of whole retina (Fig. 3B), where osteonectin/SPARC is observed mostly in the soluble fraction but is also associated with membranes and the nucleus. The nuclear association of osteonectin/SPARC has been previously observed,25 but its function as a nuclear protein is not well understood. Because the OPL is comprised mainly of synaptic terminals from the photoreceptor cells, horizontal cells, and the receptive arbors of the bipolar cells, the possible function of osteonectin/SPARC in this area of the retina is very interesting. Osteonectin/SPARC may also be interacting with specific areas in the Müller cells because their processes traverse the OPL in numerous places.
Osteonectin/SPARC is widely expressed in human tissues,19,20 but interestingly the reproduction-related tissues such as testis, placenta, and uterus (Fig. 2) show a 5- to 10-fold greater level of expression than most other tissues. The exception is the prostate gland, which seems to have mRNA expression levels similar to most of the other human tissues. In our experience, having done dozens of Northern blot analyses on dozens of individual human samples (data not shown), mRNA levels will vary mostly in the 2- to 3-fold range. These variations are likely due to the complexities in obtaining and processing human tissues samples. However, variations in the 5- to 10-fold range especially in a group of related tissues are probably significant and warrant further investigation. Other investigators have shown that osteonectin/SPARC mRNA is abundant in mouse reproductive tissues27 and in tissues with high rate of turnover.28
Osteonectin/SPARC is preferentially associated with highly proliferative and migrating tissues,4 but because the retina is composed of terminally differentiated cells that are neither migrating nor proliferating, the function of osteonectin/SPARC in the OPL may be related to its extracellular Ca2+-modulating properties.4 Its counter-adhesive function may also be important in maintaining the integrity of the synaptic junctions in the OPL.
In summary, our results strongly suggest that osteonectin/SPARC is performing an important function in the retina. Before more specific functional questions can be asked, some basic properties of the protein need to be determined, such as its turnover in the macula versus peripheral retina, possible feedback mechanisms that affect regulation of transcription, its calcium binding activity, and which retinal cell type(s) other than the RPE may be synthesizing it. This preferential expression and secretion by the macular RPE is rather unique and may be of particular interest to macular degenerations.
Acknowledgments
Supported by NIH Grants EY00444 (DB), EY00331 (DB), and 1R01EY13022 (MK); The Foundation Fighting Blindness (DB); the UCLA Dolly Green Endowed Chair at UCLA (DB); and the National Eye Institute Intramural Research Program (IRR and EFM).
This work would not have been possible without the generous gift of anti-SPARC antibody from Helene Sage. We also thank Sue Gentleman for the subcellularly fractionated whole monkey retina, Xian Jie Yang for help with the photography, Jane Hu for RPE cell culture and collection of conditioned medium, and Quingling Huang for help with the Western blot analyses. Marc Kantorow would like to thank Joseph Howitz for his support and guidance during the course of this work.
Footnotes
Commercial relationships policy: N.
References
- 1.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 2.Mason IJ, Murphy D, Munke M, Francke U, Elliott RW, Hogan BL. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. Embo J. 1986;5:1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dziadek M, Paulsson M, Aumailley M, Timpl R. Purification and tissue distribution of a small protein (BM-40) extracted from a basement membrane tumor. Eur J Biochem. 1986;161:455–464. doi: 10.1111/j.1432-1033.1986.tb10466.x. [DOI] [PubMed] [Google Scholar]
- 4.Yan Q, Sage EH. SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem. 1999;47:1495–1506. doi: 10.1177/002215549904701201. [DOI] [PubMed] [Google Scholar]
- 5.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. [PubMed] [Google Scholar]
- 6.Sage H, Decker J, Funk S, Chow M. SPARC: a Ca++-binding extracellular protein associated with endothelial cell injury and proliferation. J Mol Cell Cardiol. 1989;21(suppl 1):13–22. doi: 10.1016/0022-2828(89)90833-x. [DOI] [PubMed] [Google Scholar]
- 7.Sage EH. Secretion of SPARC by endothelial cells transformed by polyoma middle T oncogene inhibits the growth of normal endothelial cells in vitro. Biochem Cell Biol. 1992;70:579–592. doi: 10.1139/o92-089. [DOI] [PubMed] [Google Scholar]
- 8.Raines EW, Lane TF, Iruela–Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci USA. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gohring W, Sasaki T, Heldin CH, Timpl R. Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur J Biochem. 1998;255:60–66. doi: 10.1046/j.1432-1327.1998.2550060.x. [DOI] [PubMed] [Google Scholar]
- 10.Hohenester E, Maurer P, Hohenadl C, Timpl R, Jansonius JN, Engel J. Structure of a novel extracellular Ca(2+)-binding module in BM-40. Nat Struct Biol. 1996;3:67–73. doi: 10.1038/nsb0196-67. [DOI] [PubMed] [Google Scholar]
- 11.Engel J, Taylor W, Paulsson M, Sage H, Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987;26:6958–6965. doi: 10.1021/bi00396a015. [DOI] [PubMed] [Google Scholar]
- 12.Lane TF, Sage EH. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990;111:3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantorow M, Horwitz J, Carper D. Up-regulation of osteonectin/SPARC in age-related cataractous human lens epithelia. Mol Vis. 1998;4:17. [PubMed] [Google Scholar]
- 14.Kantorow M, Sage EH, Moreira E, Rodriguez IR. Increased expression of SPARC in cataractous human lenses and monkey macular RPE [ARVO Abstract] Invest Ophthalmol Vis Sci. 1999;40(4):S580. Abstract nr 3052. [Google Scholar]
- 15.Gilmour DT, Lyon GJ, Carlton MB, et al. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. EMBO J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norose K, Clark JI, Syed NA, et al. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- 17.Guermah M, Crisanti P, Laugier D, et al. Transcription of a quail gene expressed in embryonic retinal cells is shut off sharply at hatching. Proc Natl Acad Sci USA. 1991;88:4503–4507. doi: 10.1073/pnas.88.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Ondhia N, Vidgen D, Malaval L, Ringuette M, Kalnins VI. Spatiotemporal distribution of SPARC/osteonectin in developing and mature chicken retina. Exp Eye Res. 1997;65:681–689. doi: 10.1006/exer.1997.0377. [DOI] [PubMed] [Google Scholar]
- 19.Swaroop A, Hogan BL, Francke U. Molecular analysis of the cDNA for human SPARC/osteonectin/BM-40: sequence, expression, and localization of the gene to chromosome 5q31–q33. Genomics. 1988;2:37–47. doi: 10.1016/0888-7543(88)90107-3. [DOI] [PubMed] [Google Scholar]
- 20.Young MF, Day AA, Dominquez P, McQuillan CI, Fisher LW, Termine JD. Structure and expression of osteonectin mRNA in human tissue. Connect Tissue Res. 1990;24:17–28. doi: 10.3109/03008209009152419. [DOI] [PubMed] [Google Scholar]
- 21.Villarreal XC, Mann KG, Long GL. Structure of human osteonectin based upon analysis of cDNA and genomic sequences. Biochemistry. 1989;28:6483–6491. doi: 10.1021/bi00441a049. [DOI] [PubMed] [Google Scholar]
- 22.Putilina T, Wong P, Gentleman S. The DHHC domain: a new highly conserved cysteine-rich motif. Mol Cell Biochem. 1999;195:219–226. doi: 10.1023/a:1006932522197. [DOI] [PubMed] [Google Scholar]
- 23.Spiess AN, Ivell R. Normalization of RNA hybridization signals by means of SYBR green II-stained 28S or 18S ribosomal RNA and a phosphor imager. BioTechniques. 1999;26:46–48. doi: 10.2144/99261bm06. [DOI] [PubMed] [Google Scholar]
- 24.Hu JG, Gallemore RP, Bok D, Frambach DA. Chloride transport in cultured fetal human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1996;62:443–448. doi: 10.1006/exer.1996.0049. [DOI] [PubMed] [Google Scholar]
- 25.Gooden MD, Vernon RB, Bassuk JA, Sage EH. Cell cycle-dependent nuclear location of the matricellular protein SPARC: association with the nuclear matrix. J Cell Biochem. 1999;74:152–167. [PubMed] [Google Scholar]
- 26.Rodriguez IR, Mazuruk K, Schoen TJ, Chader GJ. Structural analysis of the human hydroxyindole-O-methyltransferase gene: presence of two distinct promoters. J Biol Chem. 1994;269:31969–31977. [PubMed] [Google Scholar]
- 27.Howe CC, Overton GC, Sawicki J, Solter D, Stein P, Strickland S. Expression of SPARC/osteonectin transcript in murine embryos and gonads. Differentiation. 1988;37:20–25. doi: 10.1111/j.1432-0436.1988.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 28.Sage H, Vernon RB, Decker J, Funk S, Iruela–Arispe ML. Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem. 1989;37:819–829. doi: 10.1177/37.6.2723400. [DOI] [PubMed] [Google Scholar]


