Abstract
Purpose
The Emory mouse is a well-characterized model for age-onset cataract. The purpose of the present study was to identify differentially expressed genes between pre- and post-cataract Emory mouse lenses.
Methods
Eyes were extracted from Emory mice at 3 weeks (precataract) and 7.5 months (postcataract) of age, and lenses were dissected. Lens RNA was compared for gene expression differences by RT-PCR differential display, and transcripts exhibiting altered levels of gene expression were cloned and identified by sequencing. The levels of two transcripts were further evaluated by RT-PCR in 3-week- and 7.5-month-old lenses and the remainder of the eye. The same transcripts were also measured in lenses from three non–Emory mouse strains (FVB/N, 129Sv, and CD1) ages 4 weeks to 11.5 months.
Results
Three transcripts were identified as exhibiting altered levels of gene expression between 3-week- and 7.5-month-old Emory mouse lenses. These encoded αA-crystallin (decreased), βA3/A1-crystallin (decreased), and adhesion-related kinase (ARK) receptor tyrosine kinase (increased). Decreased αA-crystallin and increased ARK expression were not detected in lenses isolated from three non–Emory mouse strains of similar age. Increased expression of ARK was not detected between 3-week- and 7.5-month-old Emory mouse eye nonlens tissues.
Conclusions
The present data confirm that expression of the αA-crystallin gene is decreased in cataract in the Emory mouse lens relative to age-matched control lenses and they provide evidence for cataract- and lens-specific upregulation of the ARK receptor tyrosine kinase in the Emory mouse.
The Emory mouse is a well-characterized model for age-onset cataract.1 Two substrains of Emory mice in which cataracts develop at 5 to 6 months (early-cataract strain) and 6 to 8 months (late-cataract strain) are known.1 Emory mouse cataracts increase in severity with age and first develop in the anterior superficial cortex region of the lens.2 They eventually progress into the anterior deep cortex region and ultimately result in complete lens opacification.2
Emory mouse cataracts are also associated with numerous lens changes that appear to mimic accelerated aging. These include abnormal lens growth; decreased protein accumulation; conversion of soluble to insoluble protein; decreased glutathione and protein sulfhydryl levels1; increased sodium and water content3; decreased superoxide dismutase, catalase, glutathione peroxidase,4 and γ-glutamylcysteine synthetase5 activities; and accelerated conversion of MIP-26 to MIP-24.6
The Emory mouse is associated with changes in crystallin composition, including covalent changes in α-crystallin7 and decreased levels of β- and γ-crystallin proteins.1 Levels of mRNA encoding αA-crystallin, βB1-crystallin, γ-crystallin, al-dose reductase, and especially MIP-26 are decreased8 in Emory mouse cataract in comparison with control lenses, indicating that altered expression of genes is associated with aging and cataract in the Emory mouse.
The present study sought to further identify specific genes with expression levels that are altered between pre- and post-cataract Emory mouse lenses. Reverse transcription-PCR differential display was used, as previously described for human lenses.9 Using this approach, we confirmed results in a previous study demonstrating decreased expression of αA-crystallin8 and identified two new genes exhibiting altered gene expression in Emory mouse cataract, including βA3/A1-crystallin (decreased) and ARK receptor tyrosine kinase (increased).
Crystallins make up more than 90% of the water-soluble protein of the mammalian eye lens, where they are critical for lens transparency and refraction. In the vertebrate eye lens, three major classes of ubiquitous crystallins are found: α-, β-, and γ-crystallins.10,11 The β- and γ-crystallins have a common polypeptide chain fold, share conserved sequences, and together form a superfamily of βγ-crystallins.12 In contrast, the α-crystallins form a separate family of proteins related to small heat-shock proteins.13 αA-crystallin is a molecular chaperone,14 with properties consistent with its having an important role in the maintenance of lens transparency.
Adhesion-related kinase (ARK) is associated with cell adhesion,15,16 and its activating ligand has been identified as the growth arrest–specific gene Gas6, which is upregulated in serum-starved fibroblast cell lines.17,18 Expression of ARK has been previously localized to nervous tissues.16 The human homologue of ARK, AXL-UFO, has been identified.19,20
The present data demonstrate lens- and aging-specific changes in the gene expression levels of ARK receptor tyrosine kinase, αA-crystallin, and βA3/A1-crystallin in the Emory mouse. The results support the hypothesis that specific gene expression changes are associated with Emory mouse cataract and associate the functions of the identified genes with the maintenance of lens transparency.
Methods
Isolation of Emory Mouse Lenses
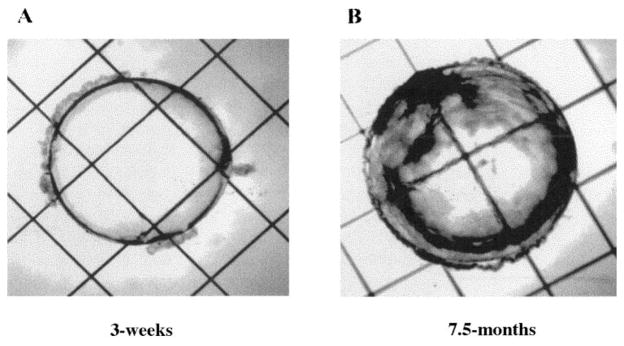
The study was conducted in accordance with the provisions of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Five pairs of late-cataract strain (6 – 8 month) Emory mice were obtained from Jackson Laboratories (Bar Harbor, ME) and raised in accordance with the Albert Einstein College of Medicine institutional protocol. The mice were bred and raised to 3 weeks and 7.5 months of age. Eyes were removed, and lenses were dissected. No adverse health effects were noted in the mice. Lenses were evaluated under a microscope and were confirmed to be clear at 3 weeks of age and opaque at 7.5 months of age, as previously demonstrated.1,2 Lens photographs were taken with a microscope (model IX 70; Olympus, Melville, NY) with a 1.25×, numeric aperture 0.035 plan neofluar infinity-corrected objective (Carl Zeiss, Thornwood, NY). Images were collected with a camera (Photometrics Sensys CCD; Tucson, AZ). Representative 3-week- and 7.5-month-old Emory mouse lenses are shown in Figure 1.
Figure 1.
Photographs of 3-week-and 7.5-month-old Emory mouse lenses. (A) An Emory mouse lens at 3 weeks, showing no evidence of cataract. (B) An Emory mouse lens at 7.5 months, showing obvious signs of opacity.
Isolation of RNA from Emory Mouse Lenses
RNA was isolated from 3-week- and 7.5-month-old whole Emory mouse lenses and from the remainder of the eye after lens removal. Total RNA was prepared from all samples with a kit, as specified by the manufacturer (Totally RNA; Ambion, Woodlands, TX). For RT-PCR-differential display, RNA samples were treated with RNase-free DNaseI to remove possible DNA contamination.
Reverse Transcription–Polymerase Chain Reaction Differential Display
Differential RT-PCR display reactions were performed in duplicate to reduce the potential for artifacts, as previously described.9
First-Strand cDNA Synthesis
Samples containing 200 ng each of 3-week- or 7.5-month-old lens RNAs were subjected to reverse transcription using 0.2 μM of an anchored primer (AP1) of sequence 5′-ACGACTCACTATAGGGCTTTTTTTTTTTTAA-3′ containing the T7 promoter sequence (italic), a T12 anchoring sequence, and two anchoring bases (AA). First-strand synthesis was performed by incubation at 25°C for 10 minutes, 42°C for 60 minutes, and 70°C for 15 minutes, in the presence of 25 μM of each deoxyribonucleoside triphosphate, 10 mM dithiothreitol (DTT), 1 U RNasin (Promega, Madison, WI), and 40 U reverse transcriptase (Superscript II; Gibco-BRL, Gaithersburg, MD) in a volume of 20 μL reverse transcription buffer (50 mM Tris [pH 8.3], 6 mM MgCl2, 10 mM KCl).
Amplification of Double-Stranded cDNA Fragments
Double-stranded cDNAs were generated by PCR using one primer set. The reactions used 0.2 μM of the anchored first-strand synthesis primer and also 0.2 μM of arbitrary annealing primer 1 (AR1; 5′-ACAATTTCA- CACAGGACGACTCCAAG-3′). The arbitrary annealing primer contains the M13 promoter reverse sequence (italic). PCR was performed with 1 U Taq polymerase (AmpliTaq; Perkin Elmer, Norwalk, CT) in the presence of 2.5 μCi [α-33P]-deoxyadenosine triphosphate (1000 –3000 Ci/mmol; New England Nuclear-DuPont, Boston, MA), 1.5 mM MgCl2, and 100 μM deoxynucleoside triphosphates, in a total reaction volume of 20 μL. PCR cycles were as follows: 1 cycle at 95°C for 2 minutes; 4 cycles at 92°C for 15 seconds, 46°C for 30 seconds, and 72°C for 2 minutes; 25 cycles at 92°C for 15 seconds, 60°C for 30 seconds, and 72°C for 2 minutes; and 1 cycle at 72°C for 7 minutes. After amplification, [α-33P]-labeled cDNA fragments were separated by electrophoresis on 5% polyacrylamide, 8-M urea gels and visualized by autoradiography.
Reamplification of Differentially Displayed Bands
Bands of differing intensity between the 3-week- and 7.5-month-old samples were excised from the gel and the resultant gel slices were directly subjected to PCR. cDNAs were bidirectionally amplified with 0.2 μM of each full-length T7 primer (5′-GTAATACGACTCACTAT-AGGGC-3′) and M13 reverse-sequencing (−48) primers (5′-AGCG-GATAACAATTTCACACAGGA-3′). The PCR conditions and cycles used in these procedures were as follows: 1 cycle at 95°C for 2 minutes; 4 cycles at 92°C for 15 seconds, 50°C for 30 seconds, and 72°C for 2 minutes; 25 cycles at 92°C for 15 seconds, 60°C for 30 seconds, and 72°C for 2 minutes; and 1 cycle at 72°C for 7 minutes. [α-33P]-deoxyadenosine triphosphate was omitted from the reaction mixture. Products were separated by electrophoresis on 1.2% agarose gels and visualized by ethidium bromide staining.
Cloning and Sequence Analysis of Differentially Displayed cDNAs
Reamplified differentially displayed bands were analyzed by electrophoresis on 1.2% agarose gels. The products were cloned into the TOPO TA cloning vector (Invitrogen, San Diego, CA), according to the manufacturer’s instructions. Cloned differentially displayed products were sequenced by fluorescent dye terminator cycle sequencing, as specified by the manufacturer (PE Biosystems, Warrington, UK), using a sequencing primer (5′-GCTCGGATCCACTAGTAACGG-3′) complementary to the vector (TOPO TA) SP6 sequence. Sequences were further analyzed using the BLAST algorithm with GenBank data (Gen-Bank is provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD, and is available at http://www.ncbi.nlm.nih.gov/Genbank), and sequence alignments were performed using the sequence alignment program (MegAlign) contained in a commercial software package (Lasergene; DNASTAR, Madison, WI).
Reverse Transcription–Polymerase Chain Reaction
RT-PCR was performed using the one-step system according to the manufacturer (Gibco-BRL, Gaithersburg, MD). Primers were designed to cross intron–exon boundaries. The primer concentration of 200 nM used in these experiments was chosen to ensure that the amount of primers would not be limiting. PCR cycling parameters were chosen to ensure linear product formation over the amounts of RNA and other reagents described. The sequences of gene-specific primers used in this study, along with their corresponding GenBank accession numbers, annealing temperatures, and product sizes, are shown in Table 1. Products were separated on 1.2% agarose gels and visualized by ethidium bromide staining. Reaction products were sequenced to ensure they represented authentic transcripts.
Table 1.
Sequences of Gene-Specific RT-PCR Primers
| Primer | Accession No. | Sequence | Product Size (bp) | Annealing Temperature |
|---|---|---|---|---|
| Ark1-forward | X63535 | TTAAATGCCCAAGAATACA | 332 | 42 |
| Ark1-reverse | X63535 | GAGGCACCAGAGTCCAC | 42 | |
| Ark2-forward | X63535 | CTCGGGGACCAGCCAGTGTTC | 473 | 57 |
| Ark2-reverse | X63535 | GGCCGGTCTCGAGGGTTCAGT | 57 | |
| αA-crystallin-forward | J00376 | GACCCAGGCTCAACACCAG | 310 | 51 |
| αA-crystallin-reverse | J00376 | CCAGGGGCTCCATTCTCA | 51 |
Results
Differential Display of Transcript Levels between 3-Week- and 7.5-Month-Old Emory Mouse Lenses
Differential display was performed on RNAs isolated from 20 pooled 3-week-old (precataract) and 24 pooled 7.5-month-old (postcataract) Emory mouse lenses. Representative lenses are shown in Figure 1. Twelve differentially displayed transcripts were detected (Fig. 2), and four bands (Fig. 2, bands 1– 4) were cloned and identified by sequencing. Three bands (Fig. 2, bands 1–3) exhibited decreased expression in 7.5-month- relative to 3-week-old lenses and were identified as encoding βA3/A1-crystallin (GenBank accession no. V00728; Fig. 2, bands 1, 2) and αA-crystallin (GenBank accession no. J00376; Fig. 2, band 3). One band exhibited increased expression in 7.5-month-old relative to 3-week-old lenses (Fig. 2, band 4) and was identified as having 100% sequence identity with the ARK (UFO/AXL; GenBank accession no. X63535) receptor tyrosine kinase.
Figure 2.
RT-PCR differential display profile of 7.5-month-old (7.5 M) versus 3-week-old (3 Wk) Emory mouse lenses. The autoradiogram was obtained by differential display of 7.5-month-old (24 pooled) versus 3-week-old (20 pooled) Emory mouse lenses. Lanes 1 to 3: 400, 200, and 150 ng of 7.5-month-old Emory mouse RNA, respectively; lanes 4 to 6: 100 ng each of 3-week-old Emory mouse RNA. Arrows: bands chosen for further analysis. Cloning and sequencing of these bands identified them as βA3/A1-crystallin (bands 1, 2), αA-crystallin (band 3), and ARK (band 4). Approximate sizes are indicated.
Confirmation of αA-Crystallin and ARK Transcript Levels between 3-Week- and 7.5-Month-Old Emory Mouse Lenses
Because very low amounts of RNA were obtained from 3-week-old Emory mouse lenses, the relative levels of αA-crystallin and ARK transcripts between differently aged lenses were confirmed by RT-PCR by comparing separately prepared 3-week-old Emory mouse lens RNA made from an additional 20 lenses with the original 7.5-month-old RNA preparation. The RT-PCR primers for αA-crystallin amplification are shown in Table 1. To reduce the possibility of artifacts, two separate sets of RT-PCR primers (ARK primer sets 1 and 2) were used for ARK amplification (Table 1).
Consistent with the differential display results (Fig. 2), ARK expression was almost entirely restricted to 7.5-month-old Emory mouse lenses (Fig. 3A). By contrast, αA-crystallin was detected at higher levels in 3-week- than in 7.5-month-old Emory mouse lenses (Fig. 3B). Production of ARK was linear up to 33 PCR cycles (compare Fig. 3A, 27–33 cycles) for amplification of both 3-week- and 7.5-month-old RNA preparations. Production of αA-crystallin was linear up to 27 PCR cycles with 7.5-month-old RNA and 25 PCR cycles with 3-week-old RNA (compare Fig. 3B, 22–27 cycles).
Figure 3.

Confirmation of differential ARK and αA-crystallin transcript levels in 3-week-old (3 Wk) and 7.5-month-old (7.5 M) Emory mouse lenses. Ethidium bromide–stained gels show the transcript levels detected by RT-PCR, using 43 ng of Emory mouse lens RNA at the indicated number of PCR cycles. (A) Ethidium bromide–stained gels show levels of ARK transcript obtained using primer set 1 (Table 1). (B) Levels of αA-crystallin transcript obtained with the same RNAs.
As a second confirmation, αA-crystallin and ARK expression were again compared between 3-week- and 7.5-month-old Emory mouse lenses by RT-PCR, using a second ARK primer set (primer set 2) to reduce the possibility of artifacts. Consistent with the differential display results (Fig. 2) and the previous RT-PCR results (Fig. 3), ARK expression was restricted to 7.5-month-old lenses (Fig. 4A), whereas αA-crystallin expression was significantly higher in 3-week-old lenses (Fig. 4B).
Figure 4.
Reconfirmation of differential ARK and αA-crystallin transcript levels in 3-week-old (3 Wk) and 7.5-month-old (7.5 M) Emory mouse lenses. Ethidium bromide–stained gels show the transcript levels detected by RT-PCR, using 100 ng of Emory mouse lens RNA at the indicated number of PCR cycles. (A) Levels of ARK transcript obtained using primer set 2 (Table 1). (B) Levels of αA-crystallin transcript obtained with the same RNAs.
Internal standards for RNA integrity were not evaluated in the confirmations, because increased expression of ARK and decreased expression of αA-crystallin in the same RNA sample makes RNA degradation an unlikely explanation for these observations.
Lens-Specific Upregulation of ARK
To determine the lens specificity of ARK expression between 3-week- and 7.5-month-old Emory mice, RNAs were isolated from the remainder of the eye and ARK levels between the remainder of the eye and the lens were compared by RT-PCR. In this case, the original lens RNAs used for the differential display were examined. In contrast to the lens, which exhibited increased expression of ARK in 7.5-month- relative to 3-week-old mice (Figs. 3, 4, and 5A), no differences were detected in the levels of ARK transcript in the remainder of the eye between 3-week- and 7.5-month-old eyes (Fig. 5B), indicating that increased expression of ARK in 7.5-month- relative to 3-week-old Emory mouse is specific to the lens.
Figure 5.

ARK levels in 3-week-old (3 Wk) and 7.5-month-old (7.5 M) Emory mouse lenses and the remainder of the eye minus the lens. Ethidium bromide–stained gels show the transcript levels detected by RT-PCR, using the indicated amounts of RNA obtained from Emory mouse lens (A) or the remainder of the eye minus the lens (B).
Expression of αA-Crystallin and ARK in Non–Emory Mouse Lenses
The expression levels of αA-crystallin and ARK were compared in lenses isolated from 3-week- and 7.5-month-old Emory mice and from three different non–Emory mouse strains (FVB/N, 129Sv, CD1), ranging in age from 4 weeks to 11.5 months (Fig. 6).
Figure 6.
Expression levels of ARK and αA-crystallin in Emory mouse versus non–Emory mouse lenses. Ethidium bromide-stained gels show transcript levels detected by RT-PCR, using 100 ng RNA isolated from the lenses of Emory and non-Emory mouse strains at indicated ages. W, week; M, month.
Consistent with the previous comparisons (Figs. 2, 3, and 4), αA-crystallin transcript was increased, and ARK transcript was decreased in 3-week- compared with 7.5-month-old Emory mouse lenses, when the same RNAs examined in the first RT-PCR confirmations (Fig. 6) were used. By contrast, almost identical levels of αA-crystallin were detected between 3-week-old Emory mouse lenses and 4-week- to 11.5-month-old non–Emory mouse lenses. We were surprised to note that increased expression of ARK was confined to the 7.5-month-old Emory lenses compared with the originally examined 3-week-old Emory lenses or non–Emory mouse lenses aged 4 weeks to 11.5 months (Fig. 6).
Discussion
In the current study, decreased expression of αA- and βA3/A1-crystallins and increased expression of ARK were demonstrated between 3-week- and 7.5-month-old Emory mouse lenses. The ages of lenses examined were chosen based on previous work that demonstrated that initial opacification in the Emory mouse begins after 3 weeks and is almost complete by 8.5 months.1,2 Our results are consistent with previous studies demonstrating decreased crystallin mRNA synthesis in the Emory mouse at the same ages as those examined in the present study.8 We do not know whether our results are specific for the Emory mouse cataract, because other age-dependent cataract onset mouse models were not examined in the present work, and genetic mouse models are not likely to be equivalent. We also do not know how many other transcripts are differentially expressed between 3-week- and 7.5-month-old Emory mice, because only one differential display primer set was used, and each primer set is expected to amplify only a small fraction of the total number of transcripts likely to be expressed in mouse lens.
At this time, the functional significance of these changes in gene expression are not known. It is not possible from the present data to determine whether increased expression of ARK and/or decreased expression of crystallins is a directed response of the lens to the presence of cataract or is even a secondary effect of the cataracts.
Although the changes in α- and β-crystallin and ARK mRNA levels could contribute to, or even cause, the cataract, future developmental, transgenic, and functional analyses are needed to determine the role of these genes in Emory mouse cataractogenesis and to establish a direct cause-and-effect relationship between the levels of these genes and cataract in the Emory mouse.
Both crystallins and ARK have properties consistent with their playing important roles in lens maintenance and Emory mouse cataract. Crystallins play essential structural roles in the lens,10 –13 and αA-crystallin is a molecular chaperone.14 ARK is a member of receptor tyrosine kinases that are responsible for transmembrane signal transduction. Activated receptor tyrosine kinases rapidly associate with the SH2 domains of cellular proteins, triggering phosphorylation-mediated second-messenger cascades that lead to cell division and differentiation.21 ARK receptor tyrosine kinase has been implicated in cell– cell adhesion and is a transmembrane protein whose extracellular domain contains two immunoglobulin G (IgG)–like domains and two fibronectin (FN) type III motifs16 that are also found in neuronal adhesion molecules (NCAMs).22 The cytoplasmic domain of ARK is similar in structure to that of the insulin-like and fibroblast growth factor receptor tyrosine kinases. Increased expression of ARK may be related to cell-adhesion and/or phosphorylation essential in lens growth and maintenance.
The activity of ARK is likely to be dependent on its activating ligand, which has been identified as the growth arrest–specific gene Gas6, which is upregulated in serum-starved fibroblast cell lines.17,18 Because homophilic interactions of ectopically expressed AXL/UFO receptors lead to cell aggregation16 and Gas6 has been shown to inhibit the adhesion of granulocytes to endothelial cells,23 ARK (AXL/UFO) and Gas6 are believed to regulate cell adhesion. Differential expression of AXL/UFO has been demonstrated in transformed leukemia–lymphoma24 and metastatic prostate carcinoma25 cell lines, suggesting a role in differentiation and transformation. It is interesting to speculate that any one of these properties may be necessary for cataract development in the Emory mouse.
Regardless of the specific roles of αA-crystallin or ARK in Emory mouse cataract, the present results provide evidence that age-onset cataract in the Emory mouse is associated with increased expression of the ARK membrane receptor tyrosine kinase and a concomitant decrease in the levels of αA- and βA3/A1-crystallins. Future studies will involve determining the function of the identified genes by identifying the targets of ARK phosphorylation in the Emory mouse lens and by evaluating the role of ARK in lens growth and maintenance.
Acknowledgments
The authors thank John Hawse, Ekta Choudhary, Weiyan Zhang, Kveta Cveklova, and Sumanto Goswami for assistance and advice during this work; and the Analytical Imaging Facility of Albert Einstein College of Medicine for lending photographic expertise.
Supported by National Eye Institute Grants EY13022 (MK) and EY12200 (AC). AC is the recipient of a Research to Prevent Blindness Career Development Award.
Footnotes
Commercial relationships policy: N.
References
- 1.Kuck JFR. Late onset of hereditary cataract of the Emory mouse: a model for human senile cataract. Exp Eye Res. 1990;50:659–664. doi: 10.1016/0014-4835(90)90110-g. [DOI] [PubMed] [Google Scholar]
- 2.Takizawa A, Kazuyuki S. In vivo observations of cataract development in Emory mouse. Ophthalmic Res. 1986;18:243–247. doi: 10.1159/000265441. [DOI] [PubMed] [Google Scholar]
- 3.Kuck JFR, Kuck KD. The Emory mouse cataract: loss of soluble protein, glutathione, protein and other changes. Exp Eye Res. 1983;36:351–362. doi: 10.1016/0014-4835(83)90117-3. [DOI] [PubMed] [Google Scholar]
- 4.Bhuyan K, Bhuyan D, Kuck J, Kuck K, Kern H. Increased lipid peroxidation and altered membrane functions in Emory mouse cataract. Curr Eye Res. 1982;2:597–606. [PubMed] [Google Scholar]
- 5.Rathburn WB, Kuck JFR, Kuck KD. Glutathione metabolism in lenses of Emory and cataract-resistant mice: activity of five enzymes. Curr Eye Res. 1986;5:189–194. doi: 10.3109/02713688609020042. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto L, Kuck J, Kuck K. Changes in the major polypeptide (MIP26K) during opacification of the Emory mouse lens. Exp Eye Res. 1988;47:329–336. doi: 10.1016/0014-4835(88)90015-2. [DOI] [PubMed] [Google Scholar]
- 7.Takemto L, Horwitz J, Kuck J, Kuck K. A covalent change in alpha crystallin during opacification of the Emory mouse lens. Lens Eye Toxicity Res. 1989;6:431–441. [PubMed] [Google Scholar]
- 8.Shi S, Bekhor I. Profile of messenger RNA decay in the Emory mouse lens in cataractogenesis and in aging. Exp Eye Res. 1992;55:629–636. doi: 10.1016/s0014-4835(05)80175-7. [DOI] [PubMed] [Google Scholar]
- 9.Kantorow M, Kays T, Horwitz J, et al. Differential display detects altered gene expression between cataractous and normal human lenses. Invest Ophthalmol Vis Sci. 1998;39:2344–2354. [PubMed] [Google Scholar]
- 10.Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- 11.Bloemendal H, de Jong WW. Lens proteins and their genes. Prog Nucleic Acid Res Mol Biol. 1991;41:259–281. doi: 10.1016/s0079-6603(08)60012-4. [DOI] [PubMed] [Google Scholar]
- 12.Lubsen NH, Aarts HJM, Schoenmkers JGG. The evolution of lenticular proteins: the beta- and gamma-crystallin super gene family. Prog Biophys Mol Biol. 1988;51:47–76. doi: 10.1016/0079-6107(88)90010-7. [DOI] [PubMed] [Google Scholar]
- 13.Caspers GJ, Leunissen JA, de Jong WW. The expanding small heat-shock protein family, and structure predictions of the conserved “alpha-crystallin domain”. J Mol Evol. 1995;40:238–248. doi: 10.1007/BF00163229. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz J. α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz AS, Schleithoff L, Faust M, Bartram CR, Janssen JWG. Short report: the genometric structure of the human UFO receptor. Oncogene. 1993;8:509–513. [PubMed] [Google Scholar]
- 16.Bellosta P, Costa M, Lin D, Basilico C. The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol Cell Biol. 1995;15:614–625. doi: 10.1128/mcb.15.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 18.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen JW, Schulz AS, Steenvoorden AC, et al. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene. 1991;6:2113–2120. [PubMed] [Google Scholar]
- 20.O’Bryan JP, Frye RA, Cogswell PC, et al. AXL, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlessinger J, Ullrich A. Growth factor signaling by receptor tyrosine kinase. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 22.Williams AF, Barclay AN. The immunoglobulin superfamily-domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 23.Avanzi GC, Gallicchio M, Bottarel F, et al. GAS inhibits granulocyte adhesion to endothelial cells. Blood. 1998;91:2334–2340. [PubMed] [Google Scholar]
- 24.Challier C, Upoff CC, Janssen JWG, Drexler HG. Differential expression of the UFO/AXL oncogene in human leukemia-lymphoma cell lines. Leukemia. 1996;10:781–787. [PubMed] [Google Scholar]
- 25.Jacob A, Kalapurakal J, Davidson W, et al. A receptor tyrosine kinase, UFO/AXL, and other genes isolated by a modified differential display PCR are overexpressed in metastatic prostatic carcinoma cell line DU145. Cancer Detect Prevent. 1999;23:325–332. doi: 10.1046/j.1525-1500.1999.99034.x. [DOI] [PubMed] [Google Scholar]