Abstract
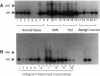
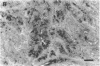
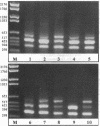
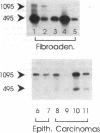
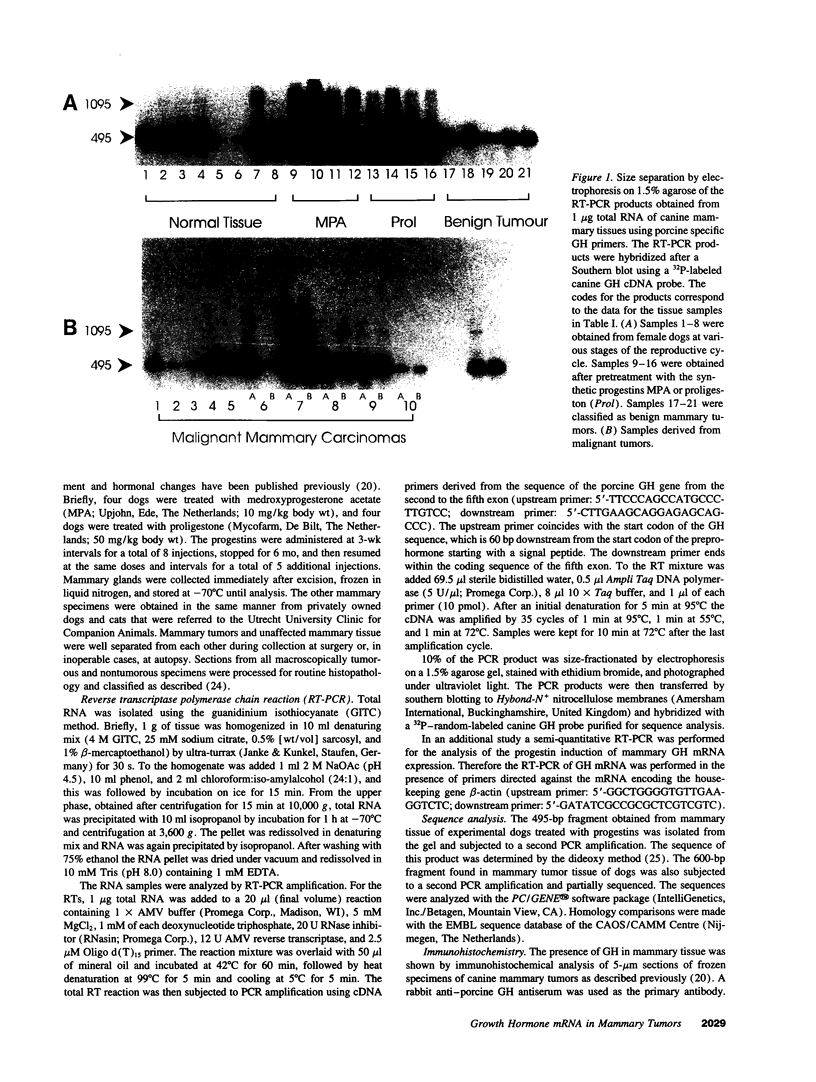
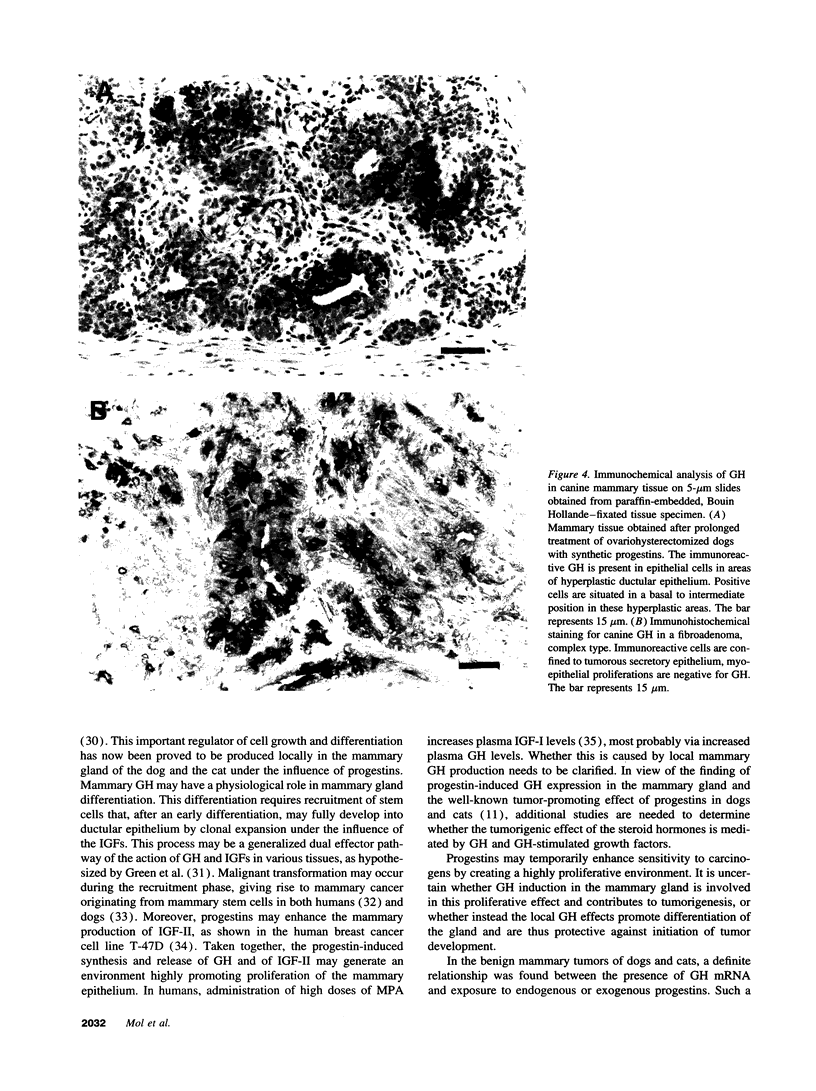
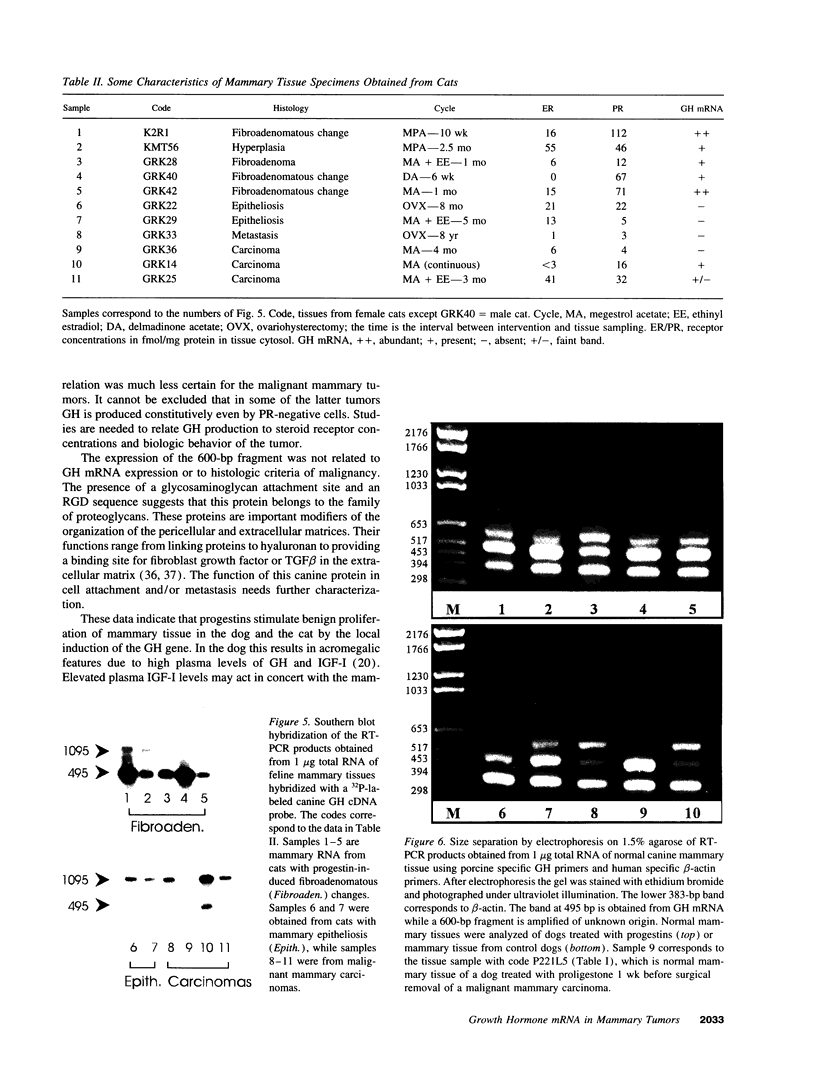
We have shown recently that in the dog progestin administration results in mammary production of immunoreactive growth hormone (GH). At present we demonstrate the expression of the gene encoding GH in the mammary gland of dogs and cats using reverse-transcriptase PCR. GH mRNA was found in the great majority of normal mammary tissues as well as benign and malignant mammary tumors of the dog and was associated with the presence of immunoreactive GH in cryostat sections. The mammary PCR product proved to be identical to that of the pituitary. The highest expression levels were found after prolonged treatment with progestins. In carcinomas GH mRNA was also found in progesterone receptor-negative tissue samples, indicating that after malignant transformation GH gene expression may become progestin independent. GH mRNA was also present in mammary tissues of cats with progestin-induced fibroadenomatous changes. It is concluded that GH gene expression occurs in normal, hyperplastic, and neoplastic mammary tissue of the dog. The expression in normal tissue is stimulated by progestins and might mediate the progestin-stimulated development of canine mammary tumors. The demonstration of progestin-stimulated GH expression in mammary tissue of cats indicates that the phenomenon is more generalized among mammals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenstein M. A., Maitimu-Smeele I., Donker G. H., Daroszewski J., Milewicz A., Thijssen J. H. On the significance of in situ production of oestrogens in human breast cancer tissue. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):891–896. doi: 10.1016/0960-0760(92)90443-m. [DOI] [PubMed] [Google Scholar]
- Boyd F. T., Cheifetz S., Andres J., Laiho M., Massagué J. Transforming growth factor-beta receptors and binding proteoglycans. J Cell Sci Suppl. 1990;13:131–138. doi: 10.1242/jcs.1990.supplement_13.12. [DOI] [PubMed] [Google Scholar]
- Clarke C. L., Sutherland R. L. Progestin regulation of cellular proliferation. Endocr Rev. 1990 May;11(2):266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- Concannon P., Altszuler N., Hampshire J., Butler W. R., Hansel W. Grwoth hormone, prolactin, and cortisol in dogs developing mammary nodules and an acromegaly-like appearance during treatment with medroxyprogesterone acetate. Endocrinology. 1980 Apr;106(4):1173–1177. doi: 10.1210/endo-106-4-1173. [DOI] [PubMed] [Google Scholar]
- Eigenmann J. E., Eigenmann R. Y., Rijnberk A., van der Gaag I., Zapf J., Froesch E. R. Progesterone-controlled growth hormone overproduction and naturally occurring canine diabetes and acromegaly. Acta Endocrinol (Copenh) 1983 Oct;104(2):167–176. doi: 10.1530/acta.0.1040167. [DOI] [PubMed] [Google Scholar]
- Eigenmann J. E., Rijnberk A. Influence of medroxyprogesterone acetate (Provera) on plasma growth hormone levels and on carbohydrate metabolism. I. Studies in the ovariohysterectomized bitch. Acta Endocrinol (Copenh) 1981 Dec;98(4):599–602. doi: 10.1530/acta.0.0980599. [DOI] [PubMed] [Google Scholar]
- Feldman M., Ruan W., Cunningham B. C., Wells J. A., Kleinberg D. L. Evidence that the growth hormone receptor mediates differentiation and development of the mammary gland. Endocrinology. 1993 Oct;133(4):1602–1608. doi: 10.1210/endo.133.4.8404600. [DOI] [PubMed] [Google Scholar]
- Fields K., Kulig E., Lloyd R. V. Detection of prolactin messenger RNA in mammary and other normal and neoplastic tissues by polymerase chain reaction. Lab Invest. 1993 Mar;68(3):354–360. [PubMed] [Google Scholar]
- Goldfine I. D., Papa V., Vigneri R., Siiteri P., Rosenthal S. Progestin regulation of insulin and insulin-like growth factor I receptors in cultured human breast cancer cells. Breast Cancer Res Treat. 1992;22(1):69–79. doi: 10.1007/BF01833335. [DOI] [PubMed] [Google Scholar]
- Green H., Morikawa M., Nixon T. A dual effector theory of growth-hormone action. Differentiation. 1985;29(3):195–198. doi: 10.1111/j.1432-0436.1985.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Hampe J. F., Misdorp W. Tumours and dysplasias of the mammary gland. Bull World Health Organ. 1974;50(1-2):111–133. [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Hayden D. W., Barnes D. M., Johnson K. H. Morphologic changes in the mammary gland of megestrol acetate-treated and untreated cats: a retrospective study. Vet Pathol. 1989 Mar;26(2):104–113. doi: 10.1177/030098588902600202. [DOI] [PubMed] [Google Scholar]
- Hayden D. W., Johnson K. H., Ghobrial H. K. Ultrastructure of feline mammary hypertrophy. Vet Pathol. 1983 May;20(3):254–264. doi: 10.1177/030098588302000302. [DOI] [PubMed] [Google Scholar]
- Hellmén E., Lindgren A. The expression of intermediate filaments in canine mammary glands and their tumors. Vet Pathol. 1989 Sep;26(5):420–428. doi: 10.1177/030098588902600507. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. The molecular biology of RU486. Is there a role for antiprogestins in the treatment of breast cancer? Endocr Rev. 1992 May;13(2):146–163. doi: 10.1210/edrv-13-2-146. [DOI] [PubMed] [Google Scholar]
- Kay C. R., Hannaford P. C. Breast cancer and the pill--a further report from the Royal College of General Practitioners' oral contraception study. Br J Cancer. 1988 Nov;58(5):675–680. doi: 10.1038/bjc.1988.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. J. Biology of female sex hormone action in relation to contraceptive agents and neoplasia. Contraception. 1991 Jun;43(6):527–542. doi: 10.1016/0010-7824(91)90002-w. [DOI] [PubMed] [Google Scholar]
- Lee N. C., Rosero-Bixby L., Oberle M. W., Grimaldo C., Whatley A. S., Rovira E. Z. A case-control study of breast cancer and hormonal contraception in Costa Rica. J Natl Cancer Inst. 1987 Dec;79(6):1247–1254. [PubMed] [Google Scholar]
- Misdorp W. Progestagens and mammary tumours in dogs and cats. Acta Endocrinol (Copenh) 1991;125 (Suppl 1):27–31. [PubMed] [Google Scholar]
- Mol J. A., Kwant M. M., Arnold I. C., Hazewinkel H. A. Elucidation of the sequence of canine (pro)-calcitonin. A molecular biological and protein chemical approach. Regul Pept. 1991 Sep 3;35(3):189–195. doi: 10.1016/0167-0115(91)90082-r. [DOI] [PubMed] [Google Scholar]
- Owen L. N., Briggs M. H. Contraceptive steroid toxicology in the Beagle dog and its relevance to human carcinogenicity. Curr Med Res Opin. 1976;4(5):309–329. doi: 10.1185/03007997609109324. [DOI] [PubMed] [Google Scholar]
- Paul C., Skegg D. C., Spears G. F. Depot medroxyprogesterone (Depo-Provera) and risk of breast cancer. BMJ. 1989 Sep 23;299(6702):759–762. doi: 10.1136/bmj.299.6702.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. E. Effects of megestrol acetate on glucose tolerance and growth hormone secretion in the cat. Res Vet Sci. 1987 May;42(3):354–357. [PubMed] [Google Scholar]
- Pike M. C., Henderson B. E., Casagrande J. T., Rosario I., Gray G. E. Oral contraceptive use and early abortion as risk factors for breast cancer in young women. Br J Cancer. 1981 Jan;43(1):72–76. doi: 10.1038/bjc.1981.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Henderson B. E., Krailo M. D., Duke A., Roy S. Breast cancer in young women and use of oral contraceptives: possible modifying effect of formulation and age at use. Lancet. 1983 Oct 22;2(8356):926–930. doi: 10.1016/s0140-6736(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Reed M. J., Christodoulides A., Koistinen R., Seppälä M., Teale J. D., Ghilchik M. W. The effect of endocrine therapy with medroxyprogesterone acetate, 4-hydroxyandrostenedione or tamoxifen on plasma concentrations of insulin-like growth factor (IGF)-I, IGF-II and IGFBP-1 in women with advanced breast cancer. Int J Cancer. 1992 Sep 9;52(2):208–212. doi: 10.1002/ijc.2910520209. [DOI] [PubMed] [Google Scholar]
- Rijnberk A., Eigenmann J. E., Belshaw B. E., Hampshire J., Altszuler N. Acromegaly associated with transient overproduction of growth hormone in a dog. J Am Vet Med Assoc. 1980 Sep 15;177(6):534–537. [PubMed] [Google Scholar]
- Rutteman G. R. Contraceptive steroids and the mammary gland: is there a hazard?--Insights from animal studies. Breast Cancer Res Treat. 1992;23(1-2):29–41. doi: 10.1007/BF01831473. [DOI] [PubMed] [Google Scholar]
- Selman P. J., Mol J. A., Rutteman G. R., van Garderen E., Rijnberk A. Progestin-induced growth hormone excess in the dog originates in the mammary gland. Endocrinology. 1994 Jan;134(1):287–292. doi: 10.1210/endo.134.1.7506206. [DOI] [PubMed] [Google Scholar]
- el-Etreby M. F., Gräf K. J. Effect of contraceptive steroids on mammary gland of beagle dog and its relevance to human carcinogenicity. Pharmacol Ther B. 1979;5(1-3):369–402. doi: 10.1016/0163-7258(79)90107-4. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F. E. Epidemiologic aspects of exogenous progestagens in relation to their role in pathogenesis of human breast cancer. Acta Endocrinol (Copenh) 1991;125 (Suppl 1):13–26. [PubMed] [Google Scholar]