Abstract
Muscle wasting during sepsis is at least in part regulated by glucocorticoids and is associated with increased transcription of genes encoding the ubiquitin ligases atrogin-1 and muscle-specific RING-finger protein-1 (MuRF1). Recent studies suggest that muscle atrophy caused by denervation is associated with reduced expression of the nuclear cofactor peroxisome proliferator-activated receptor-γ coactivator (PGC)-1β and that PGC-1β may be a repressor of the atrogin-1 and MuRF1 genes. The influence of other muscle-wasting conditions on the expression of PGC-1β is not known. We tested the influence of sepsis and glucocorticoids on PGC-1β and examined the potential link between downregulated PGC-1β expression and upregulated atrogin-1 and MuRF1 expression in skeletal muscle. Sepsis in rats and mice and treatment with dexamethasone resulted in downregulated expression of PGC-1β and increased expression of atrogin-1 and MuRF1 in the fast-twitch extensor digitorum longus muscle, with less pronounced changes in the slow-twitch soleus muscle. In additional experiments, adenoviral gene transfer of PGC-1β into cultured C2C12 myotubes resulted in a dose-dependent decrease in atrogin-1 and MuRF1 mRNA levels. Treatment of cultured C2C12 myotubes with dexamethasone or PGC-1β small interfering RNA (siRNA) resulted in downregulated PGC-1β expression and increased protein degradation. Taken together, our results suggest that sepsis- and glucocorticoid-induced muscle wasting may, at least in part, be regulated by decreased expression of the nuclear cofactor PGC-1β.
Keywords: peroxisome proliferator-activated receptor-γ coactivator-1β, muscle wasting, transcription factors, muscle mass
sepsis-induced muscle wasting is associated with increased transcription of genes in the ubiquitin-proteasome proteolytic pathway, in particular the genes encoding the ubiquitin ligases atrogin-1 and muscle-specific RING-finger protein-1 (MuRF1) (7, 10, 44). Because stimulated gene transcription is involved in muscle wasting, it is not surprising that increased expression and activity of certain transcription factors, including NF-κB (4, 28, 29, 40), CCAAT-enhancer-binding protein (C/EBP)-β and C/EBPδ (27, 46), and FOXO1 and FOXO3a (32, 35, 49), play a role in muscle wasting.
In addition to being regulated by transcription factors, gene transcription is also influenced by nuclear cofactors acting as activators or repressors (14). The potential role of nuclear cofactors in muscle wasting was illustrated in recent studies in our laboratory, in which we found evidence that glucocorticoid-induced muscle atrophy may, at least in part, be regulated by the nuclear cofactor p300 (47, 48).
An important group of nuclear cofactors that may also participate in the regulation of muscle mass are members of the peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1 (PGC-1) family (1, 20, 21, 31). Three members of this family have been described: PGC-1α, PGC-1β, and PGC-1-related coactivator (reviewed in Ref. 20). All three family members share a similar basic structure, with an NH2-terminal domain containing nuclear hormone receptor motifs, a COOH-terminal region containing RNA-binding motifs, and serine-arginine-rich domains. In a recent study, muscle atrophy induced by diabetes, uremia, experimental cancer, or denervation was associated with a substantial downregulation of PGC-1α in skeletal muscle (31). In the same study, overexpressing PGC-1α in skeletal muscle blunted the increase in atrogin-1 and MuRF1 caused by denervation or fasting, suggesting that PGC-1α is a repressor of atrogin-1 and MuRF1 gene transcription. Additional experiments provided evidence that PGC-1α represses atrogin-1 gene transcription secondary to reduced capacity of FOXO3a to activate the atrogin-1 promoter (31).
Less is known about the influence of catabolic conditions on the expression of PGC-1β in skeletal muscle. This is important, because although PGC-1α and -1β are close homologs and share extensive sequence identity, PGC-1α and -1β can influence the transcription of different genes and regulate individual metabolic pathways differentially in various tissues (20, 25, 36). Importantly, the influence of sepsis on the expression of PGC-1β in skeletal muscle is not known. We tested the hypothesis that sepsis downregulates the expression of PGC-1β in skeletal muscle. Because the catabolic effects of sepsis are, at least in part, mediated by glucocorticoids, we also examined the potential role of glucocorticoids in the regulation of PGC-1β expression in skeletal muscle.
MATERIALS AND METHODS
Animal experiments.
Sepsis was induced in male Sprague-Dawley rats (50–70 g) or C57BL/6 mice (18–24 g) by cecal ligation and puncture (CLP), as described previously (7, 27, 28, 44). Other animals underwent sham operation (laparotomy and manipulation, but no ligation or puncture of the cecum). Saline (10 ml/kg body wt sc) was injected on the back at the time of surgery to prevent hypovolemia and septic shock. Animals had free access to water, but food was withheld after the surgical procedures to avoid the influence of differences in food intake on metabolic changes in sham-operated and septic animals. Extensor digitorum longus (EDL) and soleus muscles were harvested 8 and 16 h after sham operation or CLP, immediately frozen in liquid nitrogen, and stored at −80°C. The septic model used here resulted in a reproducible increase in muscle protein breakdown and activation of the ubiquitin-proteasome proteolytic pathway in several previous reports from our laboratory (7, 38, 44).
In other experiments, male Sprague-Dawley rats (50–60 g) or C57BL/6 mice (18–24 g) were injected intraperitoneally with dexamethasone (10 mg/kg), and control animals were injected with an equal volume of vehicle (50:50 water-ethanol). Animals had free access to water, but food was withheld after injection. At 16 h after injection of dexamethasone or vehicle, EDL muscles were harvested, immediately frozen in liquid nitrogen, and stored at −80°C. We reported previously that treatment of rats with dexamethasone (10 mg/kg) resulted in sepsis-like metabolic changes in skeletal muscle (37, 46), reflecting the important role of glucocorticoids as mediators of sepsis-induced muscle wasting.
The experimental protocols were approved by the Institutional Animal Care and Utilization Committee at the Beth Israel Deaconess Medical Center. Animals were treated and cared for in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Cultured myotubes.
C2C12 cells, a mouse skeletal muscle cell line (45), were purchased from the American Type Culture Collection (Manassas, VA). Cells were grown and maintained in high-glucose DMEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a 10% CO2 humidified atmosphere at 37°C. Since the extent of myoblast fusion into myotubes declines with increasing passage, cells were used before passage 4 for all experiments to maintain the differentiation potential of the cultures. Cells grown to ∼60% confluence in T75 culture flasks were trypsinized and seeded into 6- or 12-multiwell culture plates for experiments. The cells were grown in the presence of 10% FBS until they reached ∼60% confluence, at which time the medium was replaced with DMEM containing 2% FBS for the induction of differentiation into myotubes. During this time (5–7 days), the myoblasts fused to form elongated, multinucleated myotubes. Myotubes were treated with 1 μM dexamethasone or a corresponding concentration of vehicle (0.01% ethanol) for 24 h, then protein degradation or mRNA levels were measured (see below).
Real-time PCR.
RNA was extracted from EDL and soleus muscles and from cultured C2C12 myotubes by the acid guanidinium thiocyanate-phenol-chloroform method (5) using TRI Reagent (Molecular Research Center, Cincinnati, OH). mRNA levels were determined by real-time RT-PCR using TaqMan analysis for rat PGC-1α, PGC-1β, atrogin-1, and MuRF1 and for mouse PGC-1α, PGC-1β, atrogin-1, and MuRF1. The sequences of the primer oligonucleotides and the double-labeled TaqMan oligonucleotide probes are given in Table 1. FOXO3a mRNA levels were determined using the TaqMan Gene Expression Assay (assay ID no. Rn01441087_m1, Applied Biosystems). Real-time RT-PCR using SYBR Green analysis was used to determine levels of gene expression of rat cox5b, cycs, nduf5, atp5o, and cox8h (Table 2). Primers were designed using Primer Express 1.5 (Applied Biosystems) and produced in an automated synthesizer according to the manufacturer's protocol. Forward and reverse primers were each designed in a different exon of the target gene sequence, thereby eliminating the possibility for amplifying genomic DNA. For each set of primers, a basic local alignment search tool indicated that sequence homology was acquired for the target gene only. To verify the specificity of the PCR, the PCR products were subjected to melting curve analyses.
Table 1.
Primers used for TaqMan real-time PCR analysis
| Gene | Primer Sequence, 5′–3′ |
|---|---|
| Rat | |
| PGC-1α | |
| Forward primer | GGCCCGGTACAGTGAGTGTT |
| Reverse primer | ATTGCTCCGGCCCTTTCTT |
| TaqMan probe | TGGTACCCAAGGCAGCCACTCCAC |
| PGC-1β | |
| Forward primer | TTACCTTCCGGTGTTCGGAG |
| Reverse primer | TTTCTCAGGGTAGCGCCGT |
| TaqMan probe | ATGCCGCCCTGTCCGTGAGG |
| Atrogin-1 | |
| Forward primer | CTTTCAACAGACTGGACTTCTCGA |
| Reverse primer | CAGCTCCAACAGCCTTACTACGT |
| TaqMan probe | TGCCATCCTGGATTCCAGAAGATTCAAC |
| MuRF1 | |
| Forward primer | GGACTCCTGCCGAGTGACC |
| Reverse primer | GCGTCAAACTTGTGGCTCAG |
| TaqMan probe | AGGAAAACAGCCACCAGGTGAAGGAGG |
| Mouse | |
| PGC-1α | |
| Forward primer | CATTTGATGCACTGACAGATGGA |
| Reverse primer | CCGTCAGGCATGGAGGAA |
| TaqMan probe | CCGTGACCACTGACAACGAGGCC |
| PGC-1β | |
| Forward primer | AGGAAGCGGCGGGAAA |
| Reverse primer | CTACAATCTCACCGAACACCTCAA |
| TaqMan probe | AGAGATTTCGAATGTATACCACACGGCCTTCA |
| Atrogin-1 | |
| Forward primer | CTTTCAACAGACTGGACTTCTCGA |
| Reverse primer | CAGCTCCAACAGCCTTACTACGT |
| TaqMan probe | TGCCATCCTGGATTCCAGAAGATTCAAC |
| MuRF1 | |
| Forward primer | TGTCTGGAGGTCGTTTCC G |
| Reverse primer | TGCCGGTCCATGATCACT T |
| TaqMan probe | TGCCCCTCGTGCCGCCAT |
PGC, peroxisome proliferator-activated receptor-γ coactivator; MuRF1, muscle-specific RING-finger protein-1.
Table 2.
Primers used for SYBR Green real-time PCR analysis
| Gene | Primer Sequence, 5′–3′ |
|---|---|
| Rat cox5b | |
| Forward primer | GGAGATCATGATAGCAGCACAG |
| Reverse primer | CTCTTCACAGATGCAGCCCAC |
| Rat cycs | |
| Forward primer | CTTGTCATAAAGTGGATATGATC |
| Reverse primer | CAATAGGTTTGAGGCGACACCCTC |
| Rat nduf5 | |
| Forward primer | CAGCTTCTACGTTCGATTGAGCGG |
| Reverse primer | CGCAAACGGCCAATCCCACCAGGCC |
| Rat atp5o | |
| Forward primer | GGTGTCCCTTGCTGTTCTGAACC |
| Reverse primer | GGTGTTGCCTAGGCGACC |
| Rat cox8h | |
| Forward primer | GGCCAAGGAAAGAGTGCGACCC |
| Reverse primer | CTGGCTTGGAAGAGATACGG |
cox5b and cox8h, members of the cytochrome oxidase complex; cycs, somatic cytochrome c; nduf5, member of the NADH dehydrogenase complex; atp5o, member of the ATPase complex.
For TaqMan analysis, multiplex real-time PCR was performed using the One Step PCR Master Mix Reagents Kit for quantitation of mRNA expression with simultaneous amplification of 18S RNA as endogenous controls to normalize the mRNA concentrations. TaqMan analyses and final calculations were performed with the PRISM 7700 Sequence Detection System (Applied Biosystems). For each sample, 100 ng of total RNA were subjected to real-time PCR (in duplicate) according to the protocol provided by the manufacturer.
For SYBR Green quantitative real-time PCR, total RNA was used as a template for reverse transcription using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), as described by the manufacturer. Briefly, each 20-μl reaction contained FastStart reaction mix with enzyme, 3 mM MgCl2, target forward and reverse cDNA-specific primers, and first-strand cDNA sample. The cDNA was then used for quantitative PCR, using the indicated oligonucleotide pairs, in a real-time PCR system (model 7300, Applied Biosystems). Each sample was run in duplicate, and all runs included a negative control. Amplification of hypoxanthine-guanine phosphoribosyl transferase RNA was used as endogenous control to normalize the mRNA concentrations. Analysis was performed using the ΔΔCT method.
PGC-1β gene silencing with siRNA and PGC-1β overexpression by adenoviral-mediated gene transfer.
The role of PGC-1β in the regulation of protein degradation and expression of atrogin-1 and MuRF1 was tested by knocking down the expression of PGC-1β using siRNA technology or by overexpressing PGC-1β using adenoviral-mediated gene transfer. Mouse PGC-1β siRNA (PPARGC1B, ON-TARGET plus SMART pool, Dharmacon, Lafayette, CO) and control siRNA (NON-TARGET plus SMART pool; Dharmacon) were used for gene silencing experiments. Myotubes grown in six-multiwell culture plates were transfected with 200 nM PGC-1β siRNA or control siRNA using DharmaFECT 3 reagent (4%) in DMEM without FBS or antibiotics for 24 h. The effectiveness of the siRNA treatment was assessed by measuring PGC-1β mRNA levels by real-time PCR.
In other experiments, adenoviral-mediated gene transfer was used to overexpress PGC-1β. Recombinant adenovirus particles containing PGC-1β were generated by cloning the gene into Lentilox vector pLL3.7 followed by transfection into 293FT cells, resulting in the production of intact adenoviral particles. Recombinant adenovirus particles containing green fluorescent protein were also generated for use as control adenovirus particles. Lysates of the 293FT cells containing the adenoviral particles were then reinfected into larger numbers of 293FT cells, resulting in amplification of the adenovirus. For infection, differentiated myotubes were incubated with adenovirus at 10–50 multiplicity of infection in differentiation medium.
Protein degradation in cultured myotubes.
The role of PGC-1β in the regulation of protein degradation was examined by measuring protein degradation rates in myotubes transfected with PGC-1β siRNA and in myotubes in which PGC-1β expression was increased by adenoviral gene transfer, as described above. After transfection with PGC-1β siRNA or adenoviral gene transfer, myotube proteins were labeled with l-[3,5-3H]tyrosine (1.0 μCi/ml) for 48 h. After the [3H]tyrosine-containing medium was removed, the myotubes were rinsed, and fresh differentiation medium containing a high concentration (2 mM) of unlabeled tyrosine (to limit reincorporation of [3H]tyrosine into proteins) was added. Protein degradation rates were determined by measuring the release of [3H]tyrosine into the medium and were calculated as described in detail previously (23, 42).
Statistical analysis.
Student's t-test was used for comparisons between two experimental groups. When comparisons were made between more than two groups, ANOVA followed by Tukey's or Student-Newman-Keuls test was used as appropriate. P < 0.05 was considered statistically significant. Most experiments were repeated at least three times to provide evidence of reproducibility. Values are means ± SE.
RESULTS
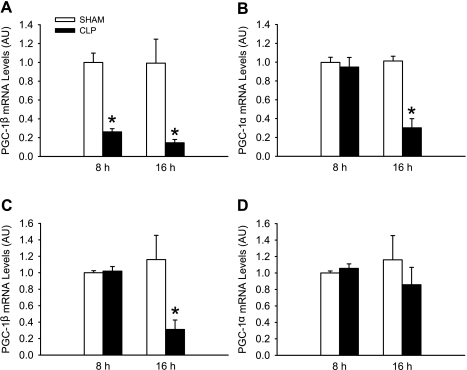
In initial experiments, PGC-1β mRNA levels were reduced by ∼70% and 80% in EDL muscles 8 and 16 h after induction of sepsis, respectively (Fig. 1A). These time points were based on previous experiments in which protein breakdown rates and the expression and activity of the ubiquitin-proteasome pathway were increased in EDL muscles 8 and 16 h after CLP in rats (7, 38, 44).
Fig. 1.
Sepsis in rats results in downregulated expression of peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and -1β in skeletal muscle. A and B: PGC-1β and 1α mRNA levels in extensor digitorum longus (EDL) muscles 8 and 16 h after induction of sepsis by cecal ligation and puncture (CLP) in rats. Control rats were sham-operated. C and D: PGC-1β and -1α mRNA levels in soleus muscles 8 and 16 h after sham operation or CLP. AU, arbitrary units. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. sham (by ANOVA).
To test whether sepsis downregulates the expression of PGC-1α, similar to PGC-1β, we next determined PGC-1α mRNA levels in EDL muscles 8 and 16 h after induction of sepsis. PGC-1α mRNA levels were reduced in EDL muscles 16 h, but not 8 h, after CLP (Fig. 1B), suggesting that PGC-1α expression may be less sensitive to the effects of sepsis than PGC-1β expression (cf. Fig. 1A).
Results in previous studies suggest that the catabolic effects of sepsis are more pronounced in white, fast-twitch skeletal (e.g., EDL) muscle than in red, slow-twitch (e.g., soleus) muscle (39). To test whether the response of PGC-1α and -1β to sepsis is different in slow- and fast-twitch muscle, we next measured PGC-1α and -1β mRNA levels in soleus muscles of sham-operated and septic rats (Fig. 1, C and D). Interestingly, PGC-1α mRNA levels were not affected by sepsis in soleus muscles at any of the time points studied, and PGC-1β mRNA was reduced only at 16 h after CLP in soleus muscles. Taken together, the results shown in Fig. 1 suggest that PGC-1β is more sensitive to the effects of sepsis than PGC-1α and that sepsis-induced changes in PGC-1β expression occur earlier in fast- than in slow-twitch muscle.
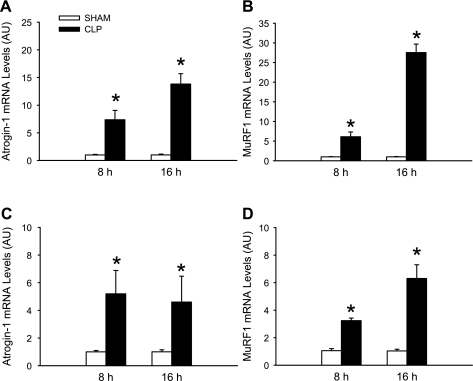
Although we reported previously that the gene expression of atrogin-1 and MuRF1 was increased in skeletal muscle after CLP in rats (7, 44), we wanted to confirm the same response in the present experiments. mRNA levels for atrogin-1 and MuRF1 were increased 8 and 16 h after CLP in the same EDL muscles that were analyzed in Fig. 1, A and B, suggesting a strong correlation between reduced PGC-1β expression and increased expression of atrogin-1 and MuRF1 (Fig. 2, A and B). As expected, the sepsis-induced changes in atrogin-1 and MuRF1 expression were less pronounced in soleus than EDL muscles (Fig. 2, C and D). Atrogin-1 and MuRF1 mRNA levels were increased 8 h after induction of sepsis, a time point at which PGC-1α mRNA was not altered in EDL or soleus muscles, and PGC-1β mRNA was not altered in soleus muscle (Fig. 1). Taken together, the results shown in Figs. 1 and 2 suggest (but do not prove) that downregulation of PGC-1β may be involved in the upregulation of atrogin-1 and MuRF1 in sepsis-induced wasting of white, fast-twitch skeletal muscle.
Fig. 2.
Sepsis in rats results in upregulated expression of the ubiquitin ligases atrogin-1 and muscle-specific RING-finger protein-1 (MuRF1) in skeletal muscle. A and B: atrogin-1 and MuRF1 mRNA levels in EDL muscles 8 and 16 h after sham operation or CLP in rats. C and D: atrogin-1 and MuRF1 mRNA levels in soleus muscles 8 and 16 h after sham operation or CLP in rats. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. sham.
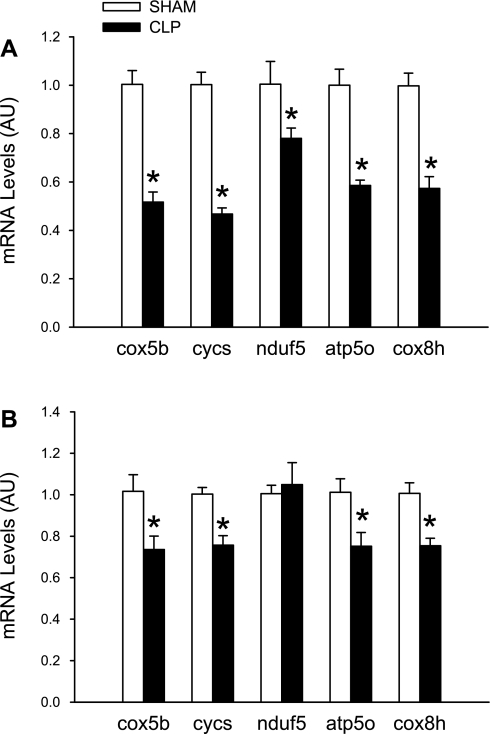
Whereas the atrogin-1 and MuRF1 genes may be negatively regulated by PGC-1β, there is evidence that PGC-1β (as well as PGC-1α) can increase the expression of other genes, in particular genes involved in energy metabolism and mitochondrial biogenesis and function (1, 20, 31). To examine whether sepsis-induced downregulation of PGC-1β is associated with reduced expression of genes regulating energy metabolism and mitochondrial abundance, mRNA levels for several of these genes were measured in EDL muscles from sham-operated and septic rats. The expression of cox5b, cycs, nduf5, atp5o, and cox8h was reduced by 20–50% in EDL muscles 16 h after CLP (Fig. 3A), suggesting that inhibited expression of PGC-1β (and perhaps PGC-1α as well) may result in reduced levels of gene products involved in mitochondrial biogenesis and oxidative metabolism in fast-twitch muscle. Similar, but less pronounced, changes in the expression of cox5b, cycs, atp5o, and cox8h were seen in soleus muscles from septic rats (Fig. 3B). The expression of nduf5 was not altered in septic soleus muscles. Thus, reduced expression of PGC-1β may be accompanied by multiple changes in gene expression in different types of skeletal muscle, in addition to increased expression of atrogin-1 and MuRF1, many of which may participate in the development of muscle wasting. Because PGC-1α mRNA levels were not altered in soleus muscles during sepsis (Fig. 1D), it is possible that reduced expression of PGC-1β is more important for the downregulation of genes involved in mitochondrial biogenesis and oxidative metabolism, at least in red, slow-twitch muscle.
Fig. 3.
Sepsis in rats downregulates expression of several genes involved in energy metabolism and mitochondrial biogenesis in EDL (A) and soleus (B) muscles 16 h after sham operation or CLP. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. sham. Cox5b and cox8h, members of the cytochrome oxidase complex; cycs, somatic cytochrome c; nduf5, a member of the NADH dehydrogenase complex; atp5o, a member of the ATPase complex.
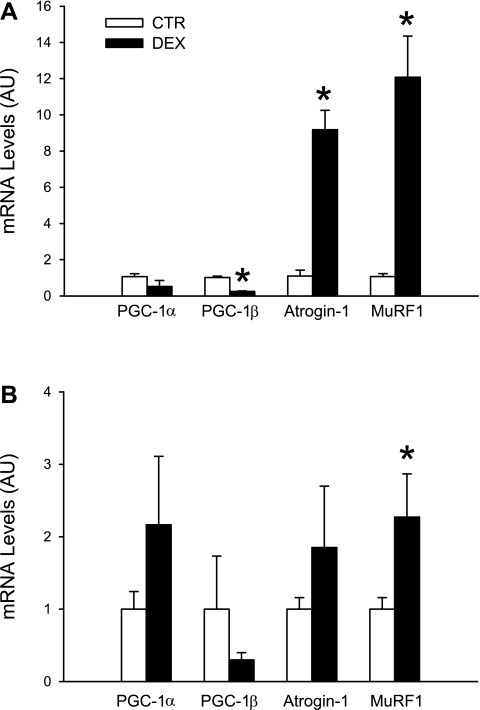
Because several sepsis-induced catabolic responses are regulated by glucocorticoids (13, 33), we next wanted to determine whether glucocorticoids can regulate the expression of PGC-1β in skeletal muscle. First, we examined the effects of glucocorticoids on muscle PGC-1β expression by treating rats with dexamethasone (10 mg/kg). We found in previous reports that this treatment resulted in increased expression of the ubiquitin-proteasome pathway and stimulated muscle proteolysis (37). Here we found that treatment of rats with dexamethasone reduced the expression of PGC-1β in EDL muscles by ∼70% and upregulated atrogin-1 and MuRF1 mRNA levels approximately fivefold (Fig. 4A). Interestingly, the expression of PGC-1α in EDL muscles was not influenced by dexamethasone treatment (Fig. 4A). In addition, treatment of rats with dexamethasone increased the expression of atrogin-1 and MuRF1 five- to sixfold in soleus muscles but did not result in changes in PGC-1α or -1β mRNA levels in the same muscles (Fig. 4B). Taken together, the results in Fig. 4 suggest that PGC-1β is differentially regulated by glucocorticoids in fast- and slow-twitch skeletal muscle and that PGC-1α is not regulated in skeletal muscle by glucocorticoids alone.
Fig. 4.
Treatment of rats with dexamethasone (Dex) downregulates expression of PGC-1β in EDL muscles and upregulates expression of atrogin-1 and MuRF1 in EDL and soleus muscles. Rats were injected intraperitoneally with dexamethasone (10 mg/kg) or corresponding volume of solvent (CTR), and mRNA levels were measured in EDL (A) and soleus (B) muscles after 16 h. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. CTR.
The experiments described above were performed in rats. To test whether sepsis-induced downregulation of PGC-1α and -1β expression in skeletal muscle is species-specific, we next determined the influence of sepsis on muscle PGC-1α and -1β mRNA levels in mice. Similar to our observation in septic rats, sepsis induced by CLP in mice resulted in a ∼60% reduction of PGC-1α and a ∼80% reduction of PGC-1β mRNA in EDL muscles, and these effects on PGC-1α and -1β were accompanied by a 2.5- and 5-fold increase in atrogin-1 and MuRF1 expression, respectively (Fig. 5A). The response to sepsis was similar in soleus muscles with regard to PGC-1α and -1β expression, whereas the increase in atrogin-1 mRNA levels was less pronounced and MuRF1 expression did not change in soleus muscles during sepsis (Fig. 5B).
Fig. 5.
Sepsis in mice results in downregulated expression of PGC-1α and -1β and upregulated ubiquitin ligase expression in skeletal muscle. Sepsis was induced by CLP in mice, and control mice were sham-operated. After 16 h, mRNA levels were measured by real-time PCR in EDL (A) and soleus (B) muscles. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. sham.
Also, in EDL muscles, the response of PGC-1β, atrogin-1, and MuRF1 mRNA expression to dexamethasone (10 mg/kg) was similar in mice and rats (cf. Fig. 6A with Fig. 4A). Similar to the observation in dexamethasone-treated rats, PGC-1α and -1β expression was not influenced by dexamethasone in mouse soleus muscles (cf. Fig. 6B with Fig. 4B). Atrogin-1 expression was not altered in soleus muscles of dexamethasone-treated mice, whereas MuRF1 mRNA levels were increased ∼2.2-fold (Fig. 6B).
Fig. 6.
Treatment of mice with dexamethasone downregulates expression of PGC-1β and upregulates expression of atrogin-1 and MuRF1 in EDL muscles. Mice were injected intraperitoneally with dexamethasone (10 mg/kg) or corresponding volume of solvent (CTR), and mRNA levels were determined in EDL (A) and soleus (B) muscles after 16 h. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. CTR.
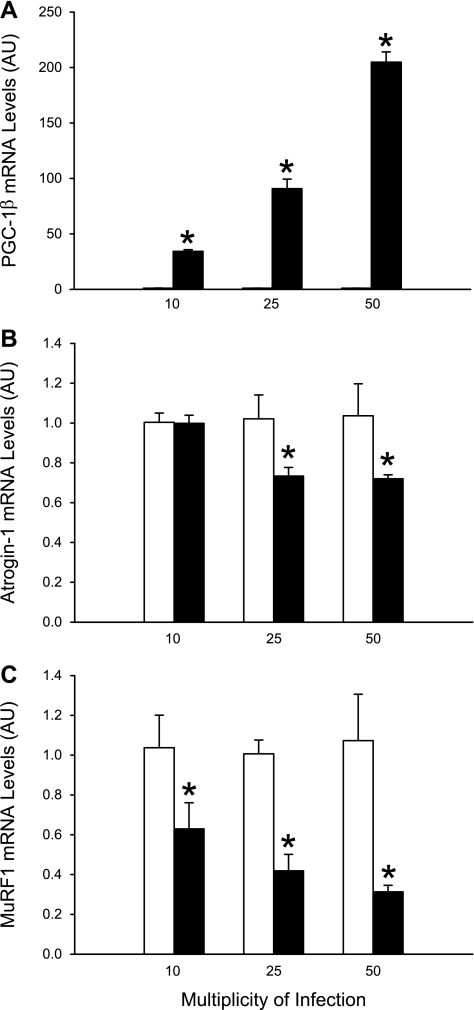
To further examine the role of PGC-1β in the regulation of muscle atrogin-1 and MuRF1 expression, we next overexpressed PGC-1β in cultured C2C12 myotubes by adenoviral transfer of PGC-1β cDNA. The adenoviral gene transfer resulted in a dose-dependent increase in PGC-1β mRNA levels (Fig. 7A) and an opposite, dose-dependent decrease in atrogin-1 and MuRF1 mRNA levels (Fig. 7, B and C). These results strongly suggest that PGC-1β is a repressor of atrogin-1 and MuRF1 gene transcription in skeletal muscle. Interestingly, the reduction of atrogin-1 expression (∼30%) was less pronounced than the reduction of MuRF1 expression (∼70%) and required a higher level of PGC-1β expression, suggesting that atrogin-1 gene transcription is less sensitive to PGC-1β-mediated repression than MuRF1 gene transcription. In an additional experiment, we measured protein degradation in myotubes transfected with PGC-1β, hypothesizing that overexpression of PGC-1β would reduce protein degradation (perhaps secondary to reduced expression of atrogin-1 and MuRF1). Surprisingly, protein degradation was not influenced by PGC-1β overexpression but was 23 ± 1.3%/24 h in PGC-1β-transfected myotubes (50 multiplicity of infection) and 20 ± 0.96%/24 h in myotubes transfected with control vector (not significant). This result suggests that increased expression of PGC-1β (and reduced atrogin-1 and MuRF1 expression) is not sufficient to inhibit basal protein degradation in cultured muscle cells.
Fig. 7.
Overexpression of PGC-1β in cultured myotubes results in downregulated expression of atrogin-1 and MuRF1. PGC-1β was overexpressed in cultured C2C12 myotubes by adenoviral transfer of PGC-1β cDNA (filled bars). Other myotubes were infected with the same concentrations of adenoviral particles that did not contain cDNA (open bars). Adenoviral gene transfer resulted in a dose-dependent increase in PGC-1β mRNA levels (A) and dose-dependent decrease in atrogin-1 and MuRF1 mRNA levels (B and C). Values are means ± SE; n = 6–8 in each group. *P < 0.05 vs. control.
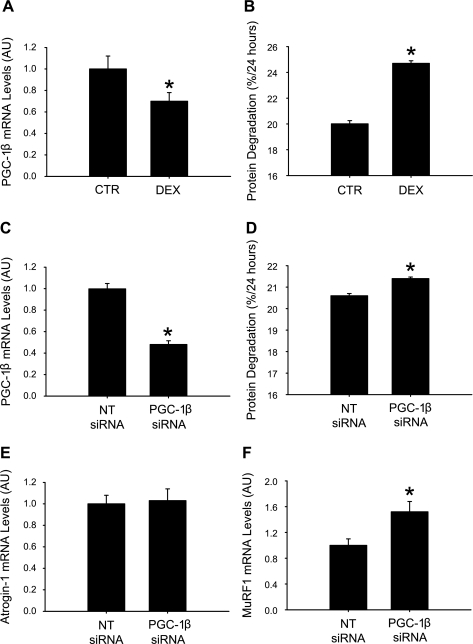
Although increased PGC-1β expression (resulting in reduced atrogin-1 and MuRF1 expression) was not sufficient to reduce protein breakdown rates in the present experiments, the results, of course, do not rule out that PGC-1β may regulate muscle protein breakdown. To further test that notion, we next tested whether downregulation of PGC-1β expression may increase muscle protein degradation. We first examined whether there is a correlation between PGC-1β expression and protein degradation in dexamethasone-treated cultured C2C12 myotubes. We and others reported previously that dexamethasone-treated cultured myotubes can be used as an in vitro model of muscle wasting characterized by increased protein degradation and upregulated expression and activity of atrogin-1 and MuRF1 (2, 12, 23, 42). Here, we found that treatment of C2C12 myotubes with 1 μM dexamethasone for 24 h resulted in a ∼25% reduction of PGC-1β mRNA and a ∼25% increase in protein degradation (Fig. 8, A and B). To more definitively test the link between reduced PGC-1β expression and protein degradation, we next measured protein degradation in myotubes in which the PGC-1β expression was downregulated by PGC-1β siRNA. This treatment resulted in a 50% reduction of PGC-1β mRNA and stimulated protein degradation (Fig. 8, C and D). The increase in protein degradation was less pronounced in myotubes treated with PGC-1β siRNA than dexamethasone (∼5% vs. 25%), despite a more pronounced downregulation of PGC-1β expression (50% vs. 25%). One explanation for the less pronounced increase in protein degradation in myotubes transfected with PGC-1β siRNA than in dexamethasone-treated myotubes may be that mechanisms involved in the regulation of muscle mass may be differentially regulated in dexamethasone-treated and PGC-1β siRNA-transfected myotubes. In particular, previous reports suggest that treatment of cultured myotubes with dexamethasone results in a robust increase in the expression of atrogin-1 and MuRF1 (23). To test whether downregulation of PGC-1β with siRNA results in an upregulation of atrogin-1 and MuRF1 expression similar to that in dexamethasone-treated myotubes, we next measured atrogin-1 and MuRF1 mRNA levels in myotubes transfected with nontargeting or PGC-1β-specific siRNA. Surprisingly, downregulation of PGC-1β with siRNA increased the expression of MuRF1 but did not influence atrogin-1 mRNA levels (Fig. 8, E and F). This observation is consistent with the results in Fig. 7 suggesting that expression of MuRF1 is more sensitive to regulation by PGC-1β than atrogin-1.
Fig. 8.
Downregulation of PGC-1β with dexamethasone or PGC-1β small interfering RNA (siRNA) is associated with increased protein degradation and MuRF1 expression in cultured myotubes. Treatment of cultured C2C12 myotubes with 1 μM dexamethasone or corresponding concentration of solvent (CTR) for 24 h resulted in reduced mRNA levels for PGC-1β (A) and increased protein degradation (B). Treatment of cultured C2C12 myotubes with 200 nM PGC-1β siRNA or nontargeting (NT) siRNA for 24 h resulted in downregulated expression of PGC-1β (C), increased protein degradation (D), unchanged atrogin-1 expression (E), and increased MuRF1 expression (F). Values are means ± SE; n = 6–8 in each group. *P < 0.05 vs. CTR or NT siRNA.
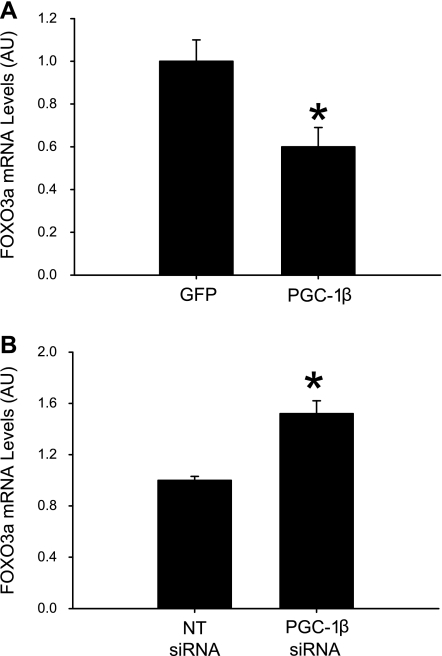
A recent report suggests that PGC-1α may regulate muscle mass secondary to changes in FOXO3a expression and activity (31). The influence of PGC-1β on FOXO3a expression is not known. To address that question, we next determined FOXO3a expression in myotubes in which PGC-1β was overexpressed by adenoviral gene transfer or silenced by siRNA. FOXO3a expression was decreased in myotubes with a high expression of PGC-1β and increased in myotubes transfected with PGC-1β siRNA (Fig. 9, A and B). These observations suggest that FOXO3a expression is, at least in part, regulated by PGC-1β in skeletal muscle and that some of the PGC-1β-mediated changes in atrogin-1 and MuRF1 mRNA levels may be secondary to changes in FOXO3a expression.
Fig. 9.
PGC-1β regulates FOXO3a expression in cultured myotubes. PGC-1β was overexpressed by adenoviral gene transfer (A) or silenced by PGC-1β siRNA (B), and FOXO3a mRNA levels were determined. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. control [green fluorescent protein (GFP) or NT siRNA].
DISCUSSION
In the present study, induction of sepsis in rats or mice by CLP or treatment with dexamethasone resulted in reduced expression of the nuclear cofactor PGC-1β in white, fast-twitch skeletal muscle. In the same experiments, expression of the muscle-specific ubiquitin ligases atrogin-1 and MuRF1 was increased, consistent with the concept that PGC-1β is a repressor of atrogin-1 and MuRF1 gene transcription. More direct evidence for a role of PGC-1β in the regulation of the atrogin-1 and MuRF1 genes was found in cultured myotubes in which overexpression of PGC-1β resulted in downregulation of atrogin-1 and MuRF1 mRNA levels, and silencing of the PGC-1β gene resulted in increased protein degradation. The present observations are important, because they provide the first evidence that sepsis results in reduced expression of PGC-1β in skeletal muscle and that downregulated PGC-1β expression may be involved in increased expression of atrogin-1 and MuRF1 in skeletal muscle.
Further evidence for a role of PGC-1β in the regulation of muscle mass was reported in a recent report published after the submission of the current work (3). In that report (3), PGC-1β overexpression reduced FOXO3-induced increases in atrogin-1 and MuRF1 gene transcription. Interestingly, in the same report (3), overexpression of PGC-1β resulted in a 15–25% reduction of protein degradation, which differs from the observation in the current report of unchanged protein degradation rates in myotubes overexpressing PGC-1β. We do not have an explanation for these apparently contradictory results.
Although not reported previously in sepsis- and glucocorticoid-induced muscle wasting, reduced PGC-1β expression was reported recently in muscle atrophy caused by denervation (30). Thus it is possible that downregulation of PGC-1β expression is a generalized response to muscle-wasting conditions, suggesting that reduced PGC-1β may play an important role in the loss of muscle mass during various catabolic conditions.
In addition to determining the influence of sepsis on PGC-1β expression, we also examined PGC-1α mRNA levels in the same experimental model. Reduced muscle PGC-1α expression was reported previously in several conditions characterized by muscle atrophy, including diabetes, uremia, cancer, starvation, and denervation (21, 30, 31), but the influence of sepsis on muscle PGC-1α expression is not well understood. Although sepsis resulted in reduced PGC-1α mRNA levels in EDL muscles, several observations reported here suggest that reduced PGC-1β expression may be more important than reduced PGC-1α expression in sepsis-induced muscle wasting. For example, the expression of PGC-1β, but not PGC-1α, was reduced in EDL muscles 8 h after induction of sepsis, a time point at which atrogin-1 and MuRF1 mRNA levels were increased six- to eightfold in the present experiments and muscle protein breakdown rates and ubiquitin mRNA levels were increased in previous reports from our laboratory (16, 39). In addition, PGC-1α mRNA levels were not affected in soleus muscles, whereas PGC-1β expression was reduced by ∼70%. Although the response to various catabolic conditions is typically less pronounced in slow-twitch (e.g., soleus) than in fast-twitch (e.g., EDL) muscle (15, 24, 39), in previous experiments, we found that total protein breakdown rates and ubiquitin mRNA levels were increased in soleus muscle of rats made septic by CLP, although those changes were substantially less pronounced than in EDL muscles (39). Thus the correlation between changes in PGC-1β expression and muscle wasting-related changes seems to be more robust than the corresponding correlation for PGC-1α.
Upregulation of the ubiquitin-proteasome pathway and stimulation of protein degradation in muscle wasting caused by sepsis and several other catabolic conditions are, at least in part, regulated by glucocorticoids (13, 33, 37). Results in the present study suggest that glucocorticoids can regulate muscle PGC-1α and -1β expression as well. Evidence for hormonal control of PGC-1 cofactors has been reported by others (11, 20, 22), but the present study provides the first evidence that glucocorticoids downregulate the expression of PGC-1β in skeletal muscle.
Although our experiments in septic and dexamethasone-treated rats and mice suggest that downregulated PGC-1β expression may be involved in the upregulation of atrogin-1 and MuRF1, this conclusion was based on circumstantial evidence. The observations in cultured myotubes that overexpression of PGC-1β downregulated atrogin-1 and MuRF1 mRNA levels and silencing of PGC-1β resulted in increased protein degradation provided more direct evidence for a role of PGC-1β in the expression of atrogin-1 and MuRF1 and in the regulation of muscle protein degradation.
The less pronounced increase in protein degradation noticed in PGC-1β siRNA- than in dexamethasone-treated myotubes may seem surprising considering the more pronounced reduction in PGC-1β mRNA levels in PGC-1β siRNA-treated myotubes. The observation, however, may have several potential explanations. First, it is likely that dexamethasone-induced protein degradation is mediated by multiple mechanisms in addition to reduced PGC-1β expression, including increased expression of the transcription factors FOXO1 and FOXO3a (32, 35) and C/EBPβ and C/EBPδ (27, 46), as well as increased expression and activity of the nuclear cofactor p300 (47, 48). When these (and other) factors work in concert, the increase in protein degradation may be more pronounced than if only PGC-1β is downregulated. In addition, it is possible that silencing of the PGC-1β gene by PGC-1β siRNA was counteracted by a compensatory increase in the expression of PGC-1α, as suggested by results in PGC-1β-knockout mice (18). The important conclusion from the experiments in PGC-1β siRNA-treated myotubes and in myotubes overexpressing PGC-1β is that downregulation of PGC-1β may result in increased protein degradation, possibly secondary to increased atrogin-1 and MuRF1 expression.
Whereas the present experiments were focused on the potential role of PGC-1β in the regulation of atrogin-1, MuRF1, and protein degradation in skeletal muscle, it should be noted that PGC-1 nuclear cofactors may also regulate other cellular functions of potential significance for muscle wasting. Importantly, reduced PGC-1β (and PGC-1α) levels may result in impaired mitochondrial biogenesis, fusion, and function (19, 20), which may be of significance, in light of recent reports of mitochondrial dysfunction in skeletal muscle during sepsis (8, 9).
An important limitation of the present study is the fact that it is not known if the reduced expression of PGC-1α and -1β noticed in skeletal muscle of septic rodents reflects similar changes in septic patients. Fredriksson et al. (9) reported recently that the expression of PGC-1α and -1β and several mitochondrial genes was not altered in intensive care unit patients with sepsis induced by multiple organ and failure. Although that report may suggest that the findings in the present study are not relevant for the situation in patients with sepsis, there may be other explanations for the apparent discrepancies between the present study and the report by Fredrisksson et al. First, among the 17 patients included in the study by Fredriksson et al., only 3 patients were listed as being septic (see Table 1 in Ref. 9). Second, only 2 of the 3 patients with sepsis had abdominal sepsis (which corresponds to the CLP model used here). Therefore, at this point, it is not known if downregulated expression of PGC-1α and -1β, as noted in rats and mice with septic peritonitis in the present study, reflects the situation in patients with septic peritonitis. More studies are needed to address this important question.
Regardless of limitations, the present report is important, because it further supports the concept that muscle wasting in various catabolic conditions is regulated at the molecular level by transcription factors and nuclear cofactors (14, 34). Previous studies suggest that PGC-1 cofactors can interact with transcription factors involved in muscle wasting, such as FOXO transcription factors (31), the glucocorticoid receptor (17), and C/EBPβ (43). In addition, there is evidence that PGC-1α may interact with nuclear cofactors regulating the acetylation and deacetylation of nuclear proteins, including p300 (41) and the deacetylating enzymes SIRT1 (26) and histone deacetylase (HDAC5) (6). It will be important in future experiments to determine whether interactions between PGC-1β and various nuclear regulatory proteins regulate muscle mass.
GRANTS
This work was supported in part by National Institutes of Health Grants R01 DK-37908 and R01 NR-08545. Z. P. Arany was supported by the Department of Clinical Medicine, Sapienza, University of Rome (Rome, Italy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIx fibers in skeletal muscle. Cell Metab 5: 35–46, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bodine SC, Latres E, Baumheuter S, Lai VK, Nunez L, Clarke BA, Poueymirous WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Brault JJ, Jespersen JG, Goldberg AL. Peroxisome proliferator-activated receptor γ coactivator 1α or 1β overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J Biol Chem 285: 19460–19471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai D, Frantz JD, Tawa NE, Melendez PA, Oh BC, Lidov HGW, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 6. Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci USA 100: 1711–1716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290: R1589–R1597, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Fredriksson K, Hammarqvist F, Strigard K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab 291: E1044–E1050, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Fredriksson K, Tjader I, Keller P, Petrovic N, Ahlman B, Scheele C, Wernerman J, Timmons JA, Rooyackers O. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. Plosone 3: e3686, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frost RA, Nystrom GJ, Jefferson LS, Lang CH. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab 292: E501–E512, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29: 677–696, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care 2: 201–205, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Hasselgren PO. Transcription factors and nuclear cofactors in muscle wasting. In: Yearbook of Intensive Care and Emergency Medicine, edited by Vincent JL. Heidelberg, Germany: Springer-Verlag, 2007, p. 229–237 [Google Scholar]
- 15. Hasselgren PO, James JH, Benson DW, Hall-Angeras M, Angeras U, Hiyama DT, Li S, Fischer JE. Total and myofibrillar protein breakdown in different types of rat skeletal muscle: effects of sepsis and regulation by insulin. Metabolism 38: 634–640, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Hasselgren PO, Talamini MA, James JH, Fischer JE. Protein metabolism in different types of skeletal muscle during early and late sepsis in rats. Arch Surg 121: 918–923, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol 20: 2411–2422, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meihaeghe A, Bohlooly YM, Storlien L, Stromstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A. Ablation of PGC-1β results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4: 2042–2056, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liesa M, Borda-d'Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, Palacin M, Vidal-Puig A, Zorzano A. Mitochondrial fusion is increased by the nuclear coactivator PGC-1β. Plosone 3: e3613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1α and PGC-1β gene expression in twins. J Clin Invest 114: 1518–1526, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menconi M, Gonnella P, Petkova V, Lecker S, Hasselgren PO. Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J Cell Biochem 105: 353–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, Hesselink MK. Skeletal muscle wasting and contractile performance in septic rats. Muscle Nerve 31: 339–348, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Mortensen OH, Frandsen L, Schjerling P, Nishimura E, Grunnet N. PGC-1α and PGC-1β have both similar and distinct effects on myofiber switching toward an oxidative phenotype. Am J Physiol Endocrinol Metab 291: E807–E816, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Nemoto S, Ferguson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Penner G, Gang G, Sun X, Wray C, Hasselgren PO. C/EBP DNA binding activity is upregulated by a glucocorticoid-dependent mechanism in septic muscle. Am J Physiol Regul Integr Comp Physiol 282: R439–R449, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Penner CG, Gang G, Wray C, Fischer JE, Hasselgren PO. The transcription factors NF-κB and AP-1 are differentially regulated in skeletal muscle during sepsis. Biochem Biophys Res Commun 281: 1331–1336, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Poylin V, Fareed MU, O'Neal P, Alamdari N, Reilly N, Menconi M, Hasselgren PO. The NF-κB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediat Inflam 2008: 317851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic disease. FASEB J 21: 140–155, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Sandri M, Lin J, Handschin C, Yang W, Arany Z, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO action and atrophy specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 197: 1–10, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Spiegelman BM, Heinrich R. Biological control through regulated transcriptional coactivators. Cell 119: 157–167, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 36. St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome prolifeator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J Biol Chem 278: 26597–26603, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest 97: 339–348, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tiao G, Fagan JM, Samuels N, James JH, Hudson K, Lieberman M, Fischer JE, Hasselgren PO. Sepsis stimulates non-lysosomal energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest 94: 2255–2264, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tiao G, Lieberman MA, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol Regul Integr Comp Physiol 272: R849–R856, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Van Gummeren D, Damrauer JS, Jackman RW, Kandarian S. The IκB kinases IKKα and IKKβ are necessary and sufficient for skeletal muscle atrophy. FASEB J 23: 362–370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wallberg A, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1α. Mol Cell 12: 1137–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Luo GJ, Wang JJ, Hasselgren PO. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock 10: 298–306, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Wang H, Peiris TH, Mowery A, Le Lay J, Gao Y, Greenbaum LE. CCAAT/enhancer binding protein-β is a transcriptional regulator of peroxisome proliferator-activated receptor-γ coactivator-1α in the regenerating liver. Mol Endocrinol 22: 1596–1605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35: 698–705, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727, 1977 [DOI] [PubMed] [Google Scholar]
- 46. Yang H, Mammen J, Wei W, Menconi M, Evenson A, Fareed M, Petkova V, Hasselgren PO. Expression and activity of C/EBPβ and δ are upregulated by dexamethasone in skeletal muscle. J Cell Physiol 204: 219–226, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Yang H, Menconi M, Wei W, Petkova V, Hasselgren PO. Dexamethasone upregulates the expression and activity of the nuclear cofactor p300 and its interaction with C/EBPβ in cultured myotubes. J Cell Biochem 94: 1058–1067, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Yang H, Wei W, Menconi M, Hasselgren PO. Dexamethasone-induced protein degradation in cultured myotubes is p300/HAT dependent. Am J Physiol Regul Integr Comp Physiol 292: R337–R344, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal pathways in atrophying muscle. Cell Metab 6: 472–483, 2007 [DOI] [PubMed] [Google Scholar]