Abstract
Our objective was to investigate whether the direct bilateral infusion of the monounsaturated fatty acid (MUFA) oleic acid (OA) within the mediobasal hypothalamus (MBH) is sufficient to reproduce the effect of administration of OA (30 nmol) in the third cerebral ventricle, which inhibits glucose production (GP) in rats. We used the pancreatic basal insulin clamp technique (plasma insulin ∼20 mU/ml) in combination with tracer dilution methodology to compare the effect of MBH OA on GP to that of a saturated fatty acid (SFA), palmitic acid (PA), and a polyunsaturated fatty acid (PUFA), linoleic acid (LA). The MBH infusion of 200 but not 40 pmol of OA was sufficient to markedly inhibit GP (by 61% from 12.6 ± 0.6 to 5.1 ± 1.6 mg·kg−1·min−1) such that exogenous glucose had to be infused at the rate of 6.0 ± 1.2 mg·kg−1·min−1 to prevent hypoglycemia. MBH infusion of PA also caused a significant decrease in GP, but only at a total dose of 4 nmol (GP 5.8 ± 1.6 mg·kg−1·min−1). Finally, MBH LA at a total dose of 0.2 and 4 nmol failed to modify GP compared with rats receiving MBH vehicle. Increased availability of OA within the MBH is sufficient to markedly inhibit GP. LA does not share the effect of OA, whereas PA can reproduce the potent effect of OA on GP, but only at a higher dose. It remains to be determined whether SFAs need to be converted to MUFAs to exert this effect or whether they activate a separate signaling pathway to inhibit GP.
Keywords: nutrient sensing, diabetes, obesity, central nervous system
nutrient excess has been shown to contribute to the development of multiple aspects of the metabolic syndrome in animal models and in humans (14, 41, 57). Excessive dietary fat has historically been blamed as the major culprit in the development of obesity, inflammation, cardiovascular disease, hypertension, and diabetes, although current opinion favors total caloric excess rather than fat alone (5, 10, 17, 58, 62). The quality of fat may also have an impact on the cardiometabolic risk factors that result from nutrient excess. It has been shown that a switch from a diet high in saturated fat to one high in monounsaturated fat can actually improve insulin sensitivity and decrease diastolic blood pressure in patients suffering from these aspects of the metabolic syndrome (40, 60). Similarly, dietary supplementation with polyunsaturated ω-3 fatty acids has been shown to decrease inflammation in otherwise healthy humans (33).
Fatty acids provide more than nutritive value. They have been shown to act as signaling molecules themselves by directly activating various nuclear receptors and transcription factors to induce expression of genes involved in energy homeostasis and both glucose and fat metabolism (4, 46, 47, 59). Many of these proteins, such as AMP-activated protein kinase and stearoyl-CoA desaturase 1 (SCD1), have been implicated in the biochemical mechanisms that translate excess dietary fat of different types into overweight, obesity, and their sequelae (18, 45, 54). With prolonged nutrient excess, these sensing mechanisms become less effective.
Dietary and hormonal signals from the major organs involved in energy homeostasis are integrated through various networks that converge in part in the hypothalamus. The hypothalamus is sensitive to the adiposity hormones insulin and leptin, although this responsiveness is decreased in both older and obese humans and animal models given a high-fat diet (11, 16, 27, 48, 61). Furthermore, the hypothalamus is directly responsive to macronutrients such as glucose and long-chain fatty acids (LCFA) (22, 36). In lean young animals, third ventricle (icv) infusion of glucose, fatty acids, insulin, and leptin has been shown to decrease food intake and hepatic glucose production (12, 22, 36, 39). Thus far, only the monounsaturated long-chain fatty acid oleic acid has been shown to inhibit hepatic glucose production in lean, young animals via icv infusion, and diet-induced obesity impairs this hypothalamic sensitivity (30).
It is possible that all three nutrient-signaling pathways converge at some level within individual neurons, since insulin and leptin have been shown to activate the KATP channel in neurons, and blockade of this channel disrupts downstream effects of insulin, glucose, and fatty acids in the brain (22, 38, 51, 52).
LCFA are a major component of the diet. The nutritional differences between LCFA of different lengths and saturation states make up a great body of (conflicting) literature (3, 7, 32, 44, 53, 56) that has only grown in recent years with the increased study of the metabolic syndrome in animal models (6, 20, 29, 43). Historically, saturated fatty acids (SFA) have been thought to be the most detrimental form of LCFA insofar as inducing insulin resistance and other facets of the metabolic syndrome (9, 42). However, polyunsaturated fatty acids (PUFA) have been shown to have the opposite effect, since several of these, such as linolenic acid and conjugated linoleic acid, are protective against the harmful effects of obesity (1, 26, 44, 64).
Given that there may be distinct contributions of saturated and unsaturated fatty acids to insulin resistance and the metabolic syndrome, we wanted to investigate the possibility that the ability of hypothalamic nutrient sensing to alter glucose production might vary as a function of LCFA chain length and/or saturation.
For LCFA to exert their effects on glucose homeostasis, they must be converted to long-chain fatty acyl-CoA (23). The present series of experiments was designed to evaluate the degree to which mediobasal hypothalamic LCFA infusion is sufficient to suppress hepatic glucose production. Furthermore, we examined the degree to which length and saturation state of mediobasal hypothalamus (MBH) LCFA infusions determined their impact on glucose homeostasis.
MATERIALS AND METHODS
Animal preparation.
Ten-week-old male Sprague-Dawley rats served as subjects in all experiments (Charles River Breeding Laboratories). Indwelling stainless steel bilateral cannulae were sterotaxically placed into the MBH (31) 3 wk before experiments to target the arcuate nucleus. Catheters were placed in the internal jugular vein and the carotid artery, and 1 wk later, basal insulin euglycemic pancreatic clamp procedures were performed (35–37). All study protocols were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
Clamp procedure in rats.
Infusion studies lasted a total of 360 min. At 0 min, a primed, continuous, intrahypothalamic (IH) infusion of the various study solutions was initiated and maintained at a rate of 0.33 ml/h. These consisted of various doses of the cyclodextrin-complexed long-chain fatty acids oleate, palmitate, and linoleate at doses of 4 nmol, 200 pmol, and, for oleate only, 40 pmol. Cyclodextrin was used, because this had been identified as an appropriate vehicle for the central delivery of fatty acids, and cyclodextrin alone has been shown to be without effect on hepatic glucose metabolism at 10- to 100-fold doses used in the present study (28). Cyclodextrin was complexed to individual fatty acids by Cyclodextrin Technologies Development (www.cyclodex.com; High Springs, FL) to form a water-soluble complex with 2.36% water content and 37–38 mg fatty acid/g of complex by spectrophotometry. Each fatty acid complex was then solubilized in water to a final concentration of 17 mM, as described previously (28). The resulting 4-hydroxy-1-(3-pyridyl)-1-butanone compound-fatty acid solution was diluted in artificial cerebrospinal fluid to the appropriate concentration used for each MBH injection. Based on the final nanomolar fatty acid concentrations used, the calculated osmolarities of the complexes are at physiological levels determined by the osmolarity of the artificial cerebrospinal fluid solvent (290–300 mOsm; Harvard Apparatus). The current fatty acid concentrations are consistent with or lower than those shown to nontoxcially excite or inhibit hypothalamic neurons in slice preparations without interfering with their glucose excitability (25). After 2 h of intrahypothalamic infusions, intravenous saline or lipids and a primed, continuous intravenous infusion of [3-3H]glucose (40 μCi bolus, 0.4 μCi/min; PerkinElmer) were begun and maintained throughout the study. Samples for determination of [3H]glucose-specific activity were obtained at 10-min intervals. For the final 2 h of the study, a pancreatic clamp was performed; this involved continuous infusions of insulin (1 mU·kg−1·min−1) and somatostatin (3 μg·kg−1·min−1) and a variable infusion of a 25% glucose solution, which was adjusted periodically to clamp the plasma glucose concentration at the basal level measured in the first part of the procedure. Plasma samples for determination of free fatty acid, insulin, and glucagon concentrations were obtained at 30-min intervals during the study. Ten minutes before the end of the studies, [U-14C]lactate (20 μCi bolus, 1.0 μCi/min; PerkinElmer) was administered intravenously to determine the contribution of gluconeogenesis to the pool of hepatic glucose 6-phosphate (2). At the end of the studies, rats were anesthetized (150 mg/kg ketamine) and tissue samples freeze-clamped with precooled aluminum tongs in situ. All tissue samples were stored at −80°C for further analysis.
Mediobasal hypothalamic wedge sampling.
The MBH was sampled by dissecting a wedge of tissue to include the entire mediolateral and dorsoventral extent of the arcuate nuclei while minimizing ventromedial nucleus tissue, as described previously (23).
Measurement of long-chain acyl-CoA esters.
Long-chain acyl-CoA esters were extracted from the mediobasal hypothalamic wedge samples and measured as described previously (63).
Statistical analysis.
Statistical analysis was done by unpaired Student's t-test or analysis of variance.
RESULTS
Bilateral MBH infusion of oleic acid, targeting the arcuate nucleus, dose-dependently increased MBH oleyl-CoA levels (Fig. 1B). These infusions also significantly suppressed hepatic glucose production at the 0.2- and 4-nmol doses (Fig. 1, D and E) primarily by increasing the glucose infusion rate required to maintain euglycemia (Fig. 1C). In the presence of near basal insulin concentrations, there was a marginal need for exogenous infusion of glucose during IH infusion of vehicle and the 0.04-nmol dose of IH oleic acid (Fig. 1C). However, a significant infusion of exogenous glucose at a rate >6 mg·kg−1·min−1 was required to prevent hypoglycemia during IH infusion of both the 0.2- and 4-nmol doses of oleic acid (Fig. 1C). The average plasma glucose concentrations were similar in all groups before the start of the pancreatic insulin clamp studies (Table 1). In the presence of near basal insulin concentrations, increasing doses of oleic acid resulted in reciprocal decreases in glucose production, with the highest dose of oleic acid producing the only significant suppression (P < 0.05; Fig. 1E). For animals receiving both the medium and high doses of oleic acid, glucose production was significantly suppressed (by 60%, P < 0.05, and 57%, P < 0.001, respectively) during the clamp procedure, whereas the low dose of oleic acid did not suppress glucose production compared with vehicle. In contrast, MBH oleic acid failed to alter glucose uptake at any dose tested (Fig. 1F).
Fig. 1.
Mediobasal hypothalamus (MBH) oleic acid enhances insulin action and inhibits glucose production. A: schematic representation of experimental procedure. Surgical implantation of bilateral cannulae was performed on day 1 (∼3 wk before the clamp study). Full recovery of body weight and food intake was seen by day 7. On day 14, iv catheters were placed. Pancreatic insulin clamp studies were performed on day 21. Infusions lasted a total of 360 min. MBH infusions were started at t = 0 min and maintained throughout the study. At t = 120 min, an infusion of labeled glucose was started and maintained throughout the next 4 h of the study. At t = 240 min, the infusions of insulin and somatostatin to maintain a basal clamp, along with a varying dose of glucose as needed to maintain euglycemia, were begun and continued for the next 2 h until the end of the study. B: dose-response curve showing concentration of long-chain fatty acyl-CoA (LCFA-CoA) per gram of protein in a small half-wedge of mediobasal hypothalamic tissue in response to local infusions of the various doses of oleic acid. C: effect of different doses of oleic acid on glucose infusion rate. D: effect of intrahypothalamic (IH) oleic acid on %decrease of glucose production during the clamp. E: effect of IH oleic acid on the rate of glucose production during the clamp. F: effect of oleic acid on the rate of glucose uptake. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 1.
General characteristics of experimental groups before intrahypothalamic infusions and during the pancreatic basal insulin clamp studies
| Vehicle | High OA | Medium OA | Low OA | High PA | Medium PA | High LA | Med LA | |
|---|---|---|---|---|---|---|---|---|
| Basal | ||||||||
| Body weight, g | 313 ± 3 | 300 ± 4 | 307 ± 4 | 294 ± 9 | 304 ± 2 | 308 ± 8 | 310 ± 8 | 308 ± 11 |
| Glucose, mmol/l | 8.2 ± 0.1 | 8.0 ± 0.3 | 8.7 ± 0.4 | 8.7 ± 0.7 | 8.9 ± 0.2 | 8.7 ± 0.2 | 8.6 ± 0.2 | 8.5 ± 0.3 |
| Insulin, ng/ml | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.02 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| FFA, mmol/l | 1.2 ± 0.1 | 0.8 ± 0.02 | 0.7 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.02 | 0.8 ± 0.001 | 0.6 ± 0.02 | 0.7 ± 0.1 |
| Clamp | ||||||||
| Glucose, mmol/l | 7.8 ± 0.5 | 7.6 ± 0.3 | 7.7 ± 0.8 | 8.6 ± 0.2 | 7.7 ± 0.8 | 7.8 ± 0.4 | 8.5 ± 0.2 | 8.6 ± 0.7 |
| Insulin, ng/ml | 1.3 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.3 | 1.5 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 |
| FFA, mmol/l | 1.0 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.02 | 0.9 ± 0.05 | 0.7 ± 0.06 | 0.4 ± 0.1 | 0.6 ± 0.07 | 0.5 ± 0.07 |
Data are means ± SE. OA, oleic acid; PA, palmitic acid; LA, linoleic acid; FFA, free fatty acids. Values during the clamp studies are steady-state levels averaged from at least 6 plasma samples during the course of the study.
We next examined whether MBH administration of comparable 0.2- and 4-nmol doses of palmitic acid (C16:0; as a model SFA) and linoleic acid (C18:2; as a model PUFA) would affect hepatic glucose production. During the basal period, MBH oleic acid administration significantly reduced plasma glucose (P < 0.001 for the 4-nmol dose and P < 0.05 for the 0.2-nmol dose), whereas palmitic and linoleic acid infusions were without effect (Fig. 2).
Fig. 2.
Response of plasma glucose to IH fatty acids. Each bar is an average of at least 7 measurements between 2 and 4 h after the start of the IH infusion; n = 5 animals. A: oleic acid. Both the high and the medium doses of oleic acid caused a decrease in circulating glucose levels compared with IH vehicle and low-dose oleic acid. B: palmitic acid. Both the high and low dose of palmitic acid caused a trend toward decreased circulating glucose levels. C: linoleic acid. Neither low nor high doses of linoleic acid significantly decreased the circulating plasma glucose following 2 h of infusion. *P < 0.05, ***P < 0.001.
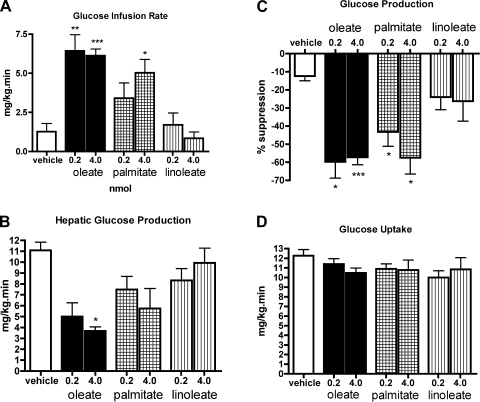
During the clamp, both high and medium doses of oleic acid significantly increased the glucose infusion rate required to maintain euglycemia. This was replicated only by the 4-nmol dose of palmitic acid, with a significant but not quite as marked glucose infusion rate required (5 mg·kg−1·min−1, P < 0.05; Fig. 3A). Although the net decrease in plasma glucose levels resulting from MBH palmitic acid infusion was not significant, the percent suppression of glucose production compared with basal rates was significant for both the 4- (58%, P < 0.05) and 0.2-nmol (43%, P < 0.05) doses. In fact, the high dose of palmitic acid fully replicated the percent suppression seen in animals treated with the high dose of oleic acid (57%; Fig. 3C). The 0.2-nmol dose of palmitic acid, which caused a significant suppression of glucose production compared with basal rates, did not reach the degree of suppression seen in animals treated with the 0.2-nmol dose of oleic acid (60%; Fig. 3, B and C). In contrast, neither dose of linoleic acid tested required more than a marginal amount of exogenous glucose to be infused to maintain euglycemia (Fig. 3A), and neither dose suppressed glucose production at all (Fig. 3, B and C). Glucose uptake was not significantly affected by any dose of palmitic, linoleic, or oleic acid compared with the control group (Fig. 3D).
Fig. 3.
MBH palmitic acid, but not linoleic acid, enhances insulin action and inhibits glucose production, albeit not as potently as oleic acid. A: effect of high- and low-dose palmitic and linoleic acids on glucose infusion rate compared with the same doses of oleic acid. B: effect of MBH palmitic and linoleic acids on the rate of glucose production during the clamp. The rates of glucose production were similar in all groups before the start of the pancreatic insulin clamp studies. C: effect of IH palmitic and linoleic acids on %decrease of glucose production during the clamp. D: effect of palmitic and linoleic acids on the rate of glucose uptake. *P < 0.05, **P < 0.01, ***P < 0.001.
Thus, MBH SFA infusion during basal pancreatic insulin clamp studies suppresses glucose production, but not as potently as the monounsaturated fatty acid. As was the case for oleic acid, SFA altered glucose homeostasis exclusively by reducing glucose production without increasing peripheral glucose uptake.
Surprisingly, MBH linoleate administration had no effect on glucose infusion rate or hepatic glucose production and did not alter glucose uptake relative to vehicle infusion. These results show that the w-6 PUFA linoleic acid cannot reproduce the effects of centrally infused monounsaturated fatty acid on peripheral glucose metabolism.
To further understand the mechanism of action of intrahypothalamic fatty acids on glucose metabolism in the liver, we studied the in vivo hepatic glucose flux through glucose-6-phosphatase (G-6-Pase; also called futile glucose cycling) and the relative contributions of this along with gluconeogenesis and glycogenolysis to total glucose output for animals treated with the 0.2-nmol dose of each of the fatty acids.
The fluxes through G-6-Pase, gluconeogenesis, and glycogenolysis were all significantly decreased in response to MBH treatment with oleic acid, with gluconeogenesis being most depressed (Fig. 4, A–D). MBH infusion of oleate significantly reduced gluconeogenesis (0.7 ± 0.2 mg·kg−1·min−1, P < 0.05; Fig. 4A) and markedly reduced glycogenolysis compared with vehicle (5.2 ± 1.3; Fig. 4B). MBH palmitate had no effect on gluconeogenesis (1.8 ± 0.7 mg·kg−1·min−1; Fig. 4A) but did reduce glycogenolysis to a similar extent as MBH oleate (5.0 ± 1.2; Fig. 4B). Interestingly, IH linoleate did markedly suppress gluconeogenesis compared with vehicle (0.9 ± 0.3 mg·kg−1·min−1; Fig. 4A) but had no effect on glycogenolysis compared with vehicle (Fig. 4B). Compared with vehicle, only MBH oleic acid significantly reduced the futile cycling of glucose, by ∼50% (P < 0.05), although IH palmitic acid showed a trend toward decreased glucose cycling (Fig. 4C). The flux through G-6-Pase was decreased mostly by IH oleic acid, by ∼30% compared with vehicle. It was also decreased, albeit less so, by palmitic acid. As with glucose cycling, IH linoleic acid had no effect on the flux through G-6-Pase (Fig. 4D). Overall, at the 0.2-nmol dose, only the monounsaturated fatty acid oleic acid had a significant effect on glucose fluxes in the liver, thereby explaining its potent effects on glucose production seen in the clamp studies.
Fig. 4.
MBH oleic and palmitic acids decrease hepatic glucose fluxes, but linoleic acid does not. Data shown are from animals treated with the medium dose, 200 pmol, of each fatty acid. A: gluconeogenesis. B: glycogenolysis. C: glucose cycling. D: total glucose output. *P < 0.05.
DISCUSSION
These results identify a role for mediobasal hypothalamic LCFA in the control of hepatic glucose metabolism. The mode of LCFA entry into neurons remains a subject of active investigation. LCFA may enter neurons by flip-flop mechanisms (19, 21) or, alternatively, rely on the neuronal expression of fatty acid-binding proteins (25), which are regulated by transcription factors activated by LCFA (50). Fatty acid-binding proteins fuction as transcription factors themselves as well to help activate peroxisome proliferator-activated receptors, which are known to upregulate lipid metabolic genes (15, 55). Because circulating free fatty acids have easy access to the central nervous system, there is likely a physiological role for LCFA in the hypothalamic control of peripheral glucose homeostasis.
The present results demonstrate that the monounsaturated fatty acid oleic acid is the most potent among the various types of fatty acids tested. This is consistent with demonstrations that monounsaturated fatty acids 1) have beneficial metabolic effects, 2) activate the KATP channels implicated in hepatic glucose meabolism, and 3) abrogate the effects of SFA and high glucose on pancreatic β-cells (24, 28, 36). Results from the present studies do not support a role for the w-6 polyunsaturated fatty acid linoleic acid in the hypothalamic control of glucose homeostasis. This was somewhat unexpected, although it may be a function of the number or placement of double bonds in the molecule. Recent literature has shown that different types of polyunsaturated fatty acids have different effects, specifically that the two classes of dietary essential fatty acids, the w-3 and w-6, can have radically different nutritional and metabolic outcomes. A diet enriched in w-6 fatty acids promotes increased risk of cancer and autoimmune and cardiovascular disease, whereas a diet enriched in w-3 PUFA exerts a suppressive effect on those conditions (8, 49). Studies of w-3 polyunsaturated LCFA will be important to identify the degree to which they share the effects of w-6 sitmuli. The role of polyunsaturated fatty acids may be more relevant for cellular integrity and maintenance rather than performing roles in nutrient signaling important in the central control of peripheral glucose homeostasis.
The present results reveal that palmitic acid acts in the arcuate nucleus of the mediobasal hypothalamus to decrease hepatic glucose production in an acute injection study but does not fully recapitulate the significant suppression of hepatic glucose production seen in response to central administration of the same doses of oleic acid. Two possible reasons for this are 1) the fact that these fatty acids have different saturation states and 2) the fact that they have different chain lengths. Either explanation would implicate the involvement of a number of enzymes known to play roles in the pathway of fatty acid biosynthesis, namely SCD and LCFA elongase (Elovl). These two proteins work together in a stepwise fashion to convert palmitic acid to oleic acid in mammals. Certain isoforms of each protein, SCD1 and Elovl6, have already been shown to be involved in mediating insulin resistance in the liver (13, 18, 29, 34). They may also be involved in the regulation of liver glucose metabolism by the brain. This makes them intriguing proteins to target for study in the continued work to elucidate the nutrient-based sensing pathway of the hypothalamus.
GRANTS
This work is supported by research grants to G. J. Schwartz by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK; DK-47208 and DK-45024) and the Skirball Institute and the AECOM Diabetes Research and Tranaing Center (NIH/NIDDK 5P60 DK-20541). R. A. Ross is supported by the Ruth L. Kirschstein National Research Service Award Individual Fellowship (1F30AG029713-01).
DISCLOSURES
The authors have no competing financial interests to disclose.
ACKNOWLEDGMENTS
We acknowledge Drs. Silvana Obici and Roger Gutierrez-Juarez for their helpful discussions throughout the course of the experiments.
REFERENCES
- 1. Barre DE. The role of consumption of alpha-linolenic, eicosapentaenoic and docosahexaenoic acids in human metabolic syndrome and type 2 diabetes—a mini-review. J Oleo Sci 56: 319– 325, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest 100: 3105– 3110, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 54: 3458– 3465, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bragt MC, Popeijus HE. Peroxisome proliferator-activated receptors and the metabolic syndrome. Physiol Behav 94: 187– 197, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Browning LM, Jebb SA. Nutritional influences on inflammation and type 2 diabetes risk. Diabetes Technol Ther 8: 45– 54, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 36: 485– 501, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol 153: 240– 251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr 83: 1499S– 1504S, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283: 11107– 11116, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr 85: 662– 677, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev 5: 13– 19, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Davis JD, Wirtshafter D, Asin KE, Brief D. Sustained intracerebroventricular infusion of brain fuels reduces body weight and food intake in rats. Science 212: 81– 83, 1981 [DOI] [PubMed] [Google Scholar]
- 13. Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 101: 6409– 6414, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56: 35– 45, 2006 [PubMed] [Google Scholar]
- 15. Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7: 489– 503, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes 51: 1016– 1021, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Gaesser GA. Carbohydrate quantity and quality in relation to body mass index. J Am Diet Assoc 107: 1768– 1780, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gutiérrez-Juárez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest 116: 1686– 1695, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamilton JA, Guo W, Kamp F. Mechanism of cellular uptake of long-chain fatty acids: Do we need cellular proteins? Mol Cell Biochem 239: 17– 23, 2002 [PubMed] [Google Scholar]
- 20. Han P, Zhang YY, Lu Y, He B, Zhang W, Xia F. Effects of different free fatty acids on insulin resistance in rats. Hepatobiliary Pancreat Dis Int 7: 91– 96, 2008 [PubMed] [Google Scholar]
- 21. Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids 75: 149– 159, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 309: 943– 947, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320– 327, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Larsson O, Deeney JT, Branstrom R, Berggren PO, Corkey BE. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J Biol Chem 271: 10623– 10626, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE. Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am J Physiol Regul Integr Comp Physiol 297: R655– R664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li JJ, Huang CJ, Xie D. Anti-obesity effects of conjugated linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid. Mol Nutr Food Res 52: 631– 645, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Ma XH, Muzumdar R, Yang XM, Gabriely I, Berger R, Barzilai N. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. J Gerontol A Biol Sci Med Sci 57: B225– B231, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 52: 726– 733, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, Iwasaki H, Iwasaki Y, Karasawa T, Kumadaki S, Matsui T, Sekiya M, Ohashi K, Hasty AH, Nakagawa Y, Takahashi A, Suzuki H, Yatoh S, Sone H, Toyoshima H, Osuga J, Yamada N. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med 13: 1193– 1202, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem 279: 31139– 31148, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Morton GJ, Niswender KD, Rhodes CJ, Myers MG, Jr, Blevins JE, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky [fa(k)/fa(k)] rats. Endocrinology 144: 2016– 2024, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Moussavi N, Gavino V, Receveur O. Could the quality of dietary fat, and not just its quantity, be related to risk of obesity? Obesity (Silver Spring) 16: 7– 15, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Nälsén C, Vessby B, Berglund L, Uusitupa M, Hermansen K, Riccardi G, Rivellese A, Storlien L, Erkkilä A, Ylä-Herttuala S, Tapsell L, Basu S. Dietary (n-3) fatty acids reduce plasma F2-isoprostanes but not prostaglandin F2alpha in healthy humans. J Nutr 136: 1222– 1228, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99: 11482– 11486, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9: 756– 761, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271– 275, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest 108: 1079– 1085, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434: 1026– 1031, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54: 3182– 3189, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Rasmussen BM, Vessby B, Uusitupa M, Berglund L, Pedersen E, Riccardi G, Rivellese AA, Tapsell L, Hermansen K. Effects of dietary saturated, monounsaturated, and n-3 fatty acids on blood pressure in healthy subjects. Am J Clin Nutr 83: 221– 226, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 23: 447– 456, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Risérus U. Fatty acids and insulin sensitivity. Curr Opin Clin Nutr Metab Care 11: 100– 105, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Rivellese AA, De Natale C, Lilli S. Type of dietary fat and insulin resistance. Ann NY Acad Sci 967: 329– 335, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Sabin MA, De Hora M, Holly JM, Hunt LP, Ford AL, Williams SR, Baker JS, Retallick CJ, Crowne EC, Shield JP. Fasting nonesterified fatty acid profiles in childhood and their relationship with adiposity, insulin sensitivity, and lipid levels. Pediatrics 120: e1426– e1433, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem 282: 2483– 2493, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr Rev 62: 333– 339, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr 25: 317– 340, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Scarpace PJ, Matheny M, Moore RL, Tumer N. Impaired leptin responsiveness in aged rats. Diabetes 49: 431– 435, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 60: 502– 507, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Spann NJ, Kang S, Li AC, Chen AZ, Newberry EP, Davidson NO, Hui ST, Davis RA. Coordinate transcriptional repression of liver fatty acid-binding protein and microsomal triglyceride transfer protein blocks hepatic very low density lipoprotein secretion without hepatosteatosis. J Biol Chem 281: 33066– 33077, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521– 525, 1997 [DOI] [PubMed] [Google Scholar]
- 52. Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757– 758, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev 45: 559– 579, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun 326: 851– 858, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, Wahli W, Noy N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol 22: 5114– 5127, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tierney AC, Roche HM. The potential role of olive oil-derived MUFA in insulin sensitivity. Mol Nutr Food Res 51: 1235– 1248, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, Kawamura M, Ebihara K, Onji M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 23: 46– 52, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 3: 393– 402, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92: 476– 489, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nälsén C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH, KANWU Study Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 44: 312– 319, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50: 2786– 2791, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406: 457– 467, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Woldegiorgis G, Spennetta T, Corkey BE, Williamson JR, Shrago E. Extraction of tissue long-chain acyl-CoA esters and measurement by reverse-phase high-performance liquid chromatography. Anal Biochem 150: 8– 12, 1985 [DOI] [PubMed] [Google Scholar]
- 64. Zhou XR, Sun CH, Liu JR, Zhao D. Dietary conjugated linoleic acid increases PPAR gamma gene expression in adipose tissue of obese rat, and improves insulin resistance. Growth Horm IGF Res 18: 361– 368, 2008 [DOI] [PubMed] [Google Scholar]