Abstract
Overexpression of the small-conductance calcium-activated K+ channel 3 (SK3) in transgenic mice compromises parturition, suggesting that the SK3 channel plays a role in pregnancy. In wild-type mouse myometrium, expression of SK3 transcript and protein is significantly reduced during pregnancy, but the mechanism(s) responsible for this attenuation of channel expression is unknown. The promoter region of the SK3-encoding mouse KCNN3 gene contains two binding sites for specificity protein (Sp) transcription factors, two of which are expressed in the uterus: Sp1, which enhances gene transcription in response to estrogen; and Sp3, which competes for the same binding motif as Sp1 and can repress gene expression. We investigated the hypothesis that Sp1 and Sp3 regulate SK3 channel expression during pregnancy. In mouse myometrium, Sp1 expression was reduced during late gestation, whereas Sp3 expression levels were constant throughout pregnancy. Using a reporter system, we found that Sp1 overexpression resulted in a significant increase in SK3 promoter activation and that Sp3 cotransfection reduced promoter activation to basal levels. These findings indicate that Sp3 outcompetes Sp1 to decrease SK3 transcription. To determine whether high levels of estrogen in vivo can affect the regulation of SK3 protein levels by Sp factors, ovariectomized mice were implanted with a 17β-estradiol or placebo pellet for 3 wk; estrogen-treated mice had reduced uterine SK3 protein expression compared with placebo-treated counterparts. In human myometrial cells overexpressing Sp1, estrogen treatment stimulated expression of the SK3 transcript. Overall, our findings indicate that Sp1 and Sp3 compete to regulate SK3 channel expression during pregnancy in response to stimulation by estrogen.
Keywords: small-conductance calcium-activated potassium channel 3, specificity protein 1 and 3, uterus, preterm labor
premature births account for ∼12.6% of all deliveries and are associated with ∼85% of neonatal deaths and morbidity (5). Presently used or approved tocolytics either are ineffective or prolong gestation for only 24–48 h, emphasizing the need for improved methods of preventing preterm labor. Potassium (K+) channels are logical targets for tocolytics since their activation produces repolarizing and hyperpolarizing currents that relax the myometrium. However, additional investigation is needed to confirm that manipulating K+ channel function can prevent premature labor contractions. One channel of particular interest is the small-conductance calcium-activated potassium channel 3 (SK3) (24). Seventy percent of mice that overexpress SK3 channels (SK3T/T mice) have a parturition defect that results in either delayed or failed delivery (2). When SK3T/T mice were induced to deliver prematurely with lipopolysaccharide or RU-486, they were unable to fully expel all fetuses, unlike their wild-type and functional knockout counterparts (26). Our laboratory has shown that uteri of SK3 mice produce less forceful contractions in vitro, and this may contribute to their inability to deliver (26).
In uterine tissue from wild-type mice, the expression of SK3 channel transcript and protein decreases as pregnancy progresses (26), suggesting that downregulation of the SK3 gene KCNN3 may be necessary for parturition. However, the mechanism responsible for the downregulation of SK3 expression is unknown. A change in promoter activity of the KCNN3 gene is one mechanism that could account for the downregulation of SK3 expression in the uterus prior to delivery. 17β-Estradiol (E2), a sex hormone required for the maintenance of pregnancy, enhances expression of the SK3 transcript despite the fact that KCNN3 does not contain an estrogen response element (3, 14). Rather, the promoter region of KCNN3 contains two GC-rich regions that represent binding sites for specificity protein (Sp) transcription factors (25, 27, 29). Of this class of transcription factors, Sp1 and Sp3 are expressed ubiquitously, have the same DNA-binding properties, and share a similar protein structure (4, 11). Sp1 is a transcriptional activator, whereas Sp3 activates or represses transcription depending on the environment of the promoter (11, 27). Sp3 has multiple isoforms, including short isoforms (Sp3si) that are transcriptionally inactive and long isoforms (Sp3li) whose transcriptional activities can change based on the promoter context. Estrogen receptor-α (ERα) enhances the ability of Sp1 and Sp3 to bind to DNA. KCNN3 transcription is increased in the presence of Sp1, and mutational analysis has shown that Sp binding sites on the SK3 promoter are necessary for ERα-mediated enhancement of SK3 expression (14). However, the mechanism underlying transcriptional regulation of this channel, which is a key contributor to myometrial quiescence during pregnancy, has not been identified. Here, we assess whether the Sp factors regulate the transcriptional activity and expression of SK3 channels during pregnancy.
MATERIALS AND METHODS
Animals and breeding protocol.
All animal procedures used in this study complied with the guidelines for the care and use of animals set by the National Institutes of Health and were approved by the Animal Care and Use Committee at the University of Iowa. Adult C57BL/6 female mice were mated between 8 wk and 8 mo of age. Day 0 of pregnancy was determined on the basis of the presence of a copulatory plug. Mice were euthanized by CO2 inhalation on specific days relative to pregnancy duration [nonpregnant (NP), days 7, 14, and 18 of gestation (P7, P14, P18), and 2 days postpartum].
Human tissue collection.
Human myometrial tissue from the lower uterine segment was obtained from patients who, in the absence [nonlaboring (NL)] or presence [laboring (L)] of labor contractions (spontaneous or induced), underwent Cesarean section during late pregnancy (38–40 wk gestation) while under spinal anesthesia. NP tissue was isolated from uteri removed for hysterectomies. All patients signed written consent forms approved by the University of Iowa's Internal Review Board (approval no. 199809066).
Immunoblotting.
Mouse and human uterine tissue was isolated, flash-frozen, and homogenized, and whole cell lysates were prepared as described previously (1). Following protein quantitation by BCA, 15 μg of protein was loaded and separated by SDS-PAGE. Mouse immunoblots were probed with rabbit polyclonal anti-Sp1 or anti-Sp3 (1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:3,000 dilution; Pierce, Rockford, IL). Human immunoblots were probed with rabbit polyclonal anti-SK3 NH2-terminal primary antibody (1:100 dilution; Alomone Laboratories, Jerusalem, Israel) and HRP-conjugated goat anti-rabbit Fc secondary antibody (1:3,000 dilution; Pierce). To assure equal loading, the blots were reprobed with anti-GAPDH primary antibody (1:1,000; Chemicon, Temecula, CA) and HRP-conjugated mouse anti-goat secondary antibody (1:3,000 dilution; Jackson ImmunoResearch, West Grove, PA). Signal was detected by chemiluminescence (ECL Western Blotting Detection Reagents, Buckinghamshire, UK; or SuperSignal West Femto Maximum Sensitivity Substrate Thermo Scientific, Rockford, IL). Sp1/Sp3 and SK3 protein expression was quantitated by densitometry (ImageJ; National Institutes of Health) and normalized to GAPDH.
Design of SK3 promoter construct.
An 875-bp fragment (containing both Sp binding sites) of the 5′ promoter region of the KCNN3 gene was prepared by PCR amplification of mouse uterine genomic DNA, using a sense primer containing a KpnI restriction site and an antisense primer containing a SacI restriction site (5′-CAGGAGTGGGTCCCTTCTGC-3′, 5′-TTGGGGCCTGGCTGAGTT-3′). The PCR product was purified by agarose gel electrophoresis, extracted from gel slices (QIAquick; Qiagen, Chatsworth, CA), and cloned into the pCRII vector (Invitrogen, San Diego, CA). Following restriction digestion with KpnI and SacI, the SK3 promoter fragment was directionally cloned into the pGL3-Basic luciferase reporter vector (Promega, Madison, WI) between unique KpnI and SacI sites to generate the SK3/luciferase reporter construct. The plasmid constructs pPac, pPac Sp1, pPac Sp3 complete (Sp3si), and pPac Sp3 new (Sp3li) were a kind gift from Dr. Guntram Suske.
Schneider's line 2 transfection and bioluminescence imaging.
Drosophila Schneider's line 2 (SL2) cells (Invitrogen) were cultured in Schneider's insect medium supplemented with 10% heat-inactivated fetal bovine serum and 50 U/ml penicillin-streptomycin at room temperature. SL2 cells (3 × 106 cells/well in 6-well plates) were seeded the day before transfection. Cells were transfected with the SK3-luciferase construct and with the pPac, pPac Sp1, pPac Sp3si, or pPac Sp3li using the calcium phosphate method. The medium was changed 24 h posttransfection, and 1 × 105 cells of each transfection were analyzed with the Luciferase Assay System (Promega) for bioluminescence, using an IVIS 100 imaging system (Caliper Life Sciences, Hopkinton, MA).
Transfection of adherent human myometrial cells.
Human myometrial tissue from NL patients was used to isolate human myometrial smooth muscle cells (hMSMCs) within 1–3 h of collection, as described previously (19). For these experiments, adherent cells were trypsinized, plated in fresh dishes, and grown for 2 additional days until they reached 70–80% confluency, at which point they were transfected with constructs pN3 and pN3-Sp1FL-Complete (gifts from Dr. Guntram Suske), using the Lipofectamine LTX and PLUS reagent (Invitrogen) according to the instructions provided.
RNA isolation and quantitative PCR analysis.
The Total RNA mini kit (Bio-Rad, Hercules, CA) was used to obtain total RNA from human uterine tissue and cultured hMSMCs. Total RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad) to generate cDNA. The cDNA was amplified in triplicate with SYBR Green Supermix (Bio-Rad) using primers specific for human SK3 channels (5′-GAGCGTCAAGATGGAACAGA-3′, 5′-ATCTTGGAAAGGTCCACCAG-3′), Sp1 (5′-GCTACCTTGACTCCCAGCTC-3′, 5′-TGGAACTGGCTTGTGATGAT-3′), or GAPDH (5′-TCAAGAAGGTGGTGAAGCAG-3′, 5′-CGCTGTTGAAGTCAGAGGAG). GAPDH served as a standard to normalize gene expression.
Ovariectomy and E2 pellet implantation of C57BL/6 mice.
Mice were anesthetized with ketamine (91 mg/kg) and xylazine (9.1 mg/kg ip) and ovariectomized, as described previously (13). Twenty-one-day release E2 0.5-mg pellets or placebo pellets (Innovative Research, Sarasota, FL) were implanted subcutaneously at the time of the surgery. Three weeks postsurgery, the mice were euthanized and blood was extracted for the measurement of serum estradiol using the EIA kit (Cayman Chemical, Ann Arbor, MI). The uterus was removed, flash-frozen in liquid nitrogen, and stored at −80°C for protein isolation (for placebo-treated mice, 3 uteri/sample were pooled together) and immnuoblotting. Alternatively, uterine tissue was collected and fixed in 4% paraformeldehyde overnight and then embedded in OCT compound. Sections were cut (10 μm) and then permeabolized in 0.2% Triton X with 0.3 M sucrose for 10 min, and then they were blocked in egg substitute followed by 5% milk for 10 min each. Mouse blocking solution (Vector M. O. M. kit; Vector Laboratories) was applied for 1 h, followed by diluent for 5 min. Tissue was then incubated in primary antibody (SK3, 1:50; Millipore, Billerica, MA; and α-actin, 1:400; Sigma, St. Louis, MO) overnight and then treated with biotinylated anti-mouse IgG reagent (Vector M. O. M. kit) and Strep-Alexa 568/goat anti-rabbit 488 (Invitrogen) for 10 min prior to being mounted in Vectashield (Vector Laboratories). Images were taken with a Zeiss 710 Scanning Confocal microscope (Carl Zeiss Microimaging).
Statistical analysis.
All data are presented as means ± SE. Statistical significance was determined by one-way and two-way ANOVA where appropriate, followed by post hoc comparison using Student t-tests. Nonparametric data analysis was used to evaluate hMSMC transfection experiments, with “0” assigned for a <10% change in transcript expression, “+” for a 10% increase in transcript expression, and “−” for a 10% decrease in transcript expression compared with that obtained with the control vector. A 10% change in expression was used for these assignments of “change” because the variance between samples tested in triplicate never exceeded 10%. Significance was determined at P < 0.05; n refers to the number of animals in all cases.
RESULTS
The Sp1/Sp3 protein ratio shifts during mouse gestation.
We began our study by assessing whether the levels of Sp1 and Sp3 protein change during pregnancy. Western blotting revealed that expression of the Sp1 protein was prominent in NP and P7 myometrium but decreased significantly by P14 and remained low until after delivery (Fig. 1A). In contrast, Sp3si levels remained at the level seen in NP tissue throughout gestation. We did not detect Sp3li in the mouse uterus by Western blotting (data not shown). Densitometry-based quantification of immunoblots confirmed that Sp1 was significantly downregulated by P14 compared with those in NP tissue, whereas Sp3si levels were consistent in all tissues tested (n = 3–7; Fig. 1B). Thus, in the mouse uterus, Sp1 protein decreased from the levels detected in NP tissue through midgestation and remained low throughout pregnancy, whereas Sp3si expression was sustained.
Fig. 1.
Western blot analysis of whole cell lysates from mouse uteri. Protein was extracted at different stages of pregnancy [nonpregnant (NP), day 7 (P7), day 14 (P14), day 18 (P18), and 2 days postpartum (PP2)] and separated by SDS-PAGE. Blots were probed with antibodies against Sp1, Sp3, and GAPDH, which served as a loading control. A: specificity protein (Sp)1 protein was prominent only in NP and P7 tissue, decreasing by P14, whereas Sp3 protein levels were similar in all tissues. B: quantification of Sp1 and Sp3 protein levels, with data normalized to GAPDH protein levels (n = 3–7). Means ± SE. *P < 0.05 vs. NP; †P < 0.05 vs. Sp1. Sp3si, Sp3 short isoform.
KCNN3 promoter activation in mouse is regulated by the Sp1/Sp3 protein ratio.
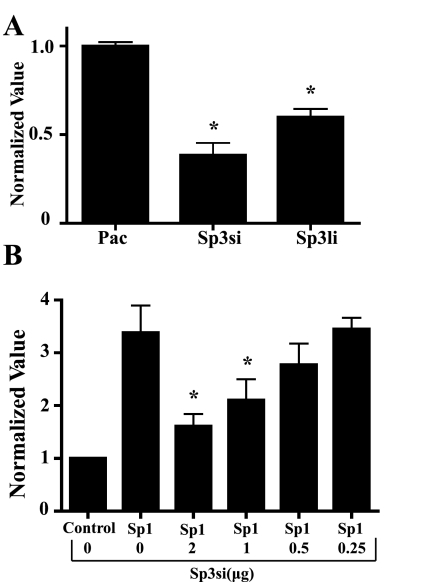
To investigate how Sp1 and Sp3 regulate the expression of endogenous SK3 channels, we generated a luciferase reporter plasmid in which expression is driven by the mouse KCNN3 promoter. In this system, fold changes in luciferase activity, as measured by bioluminescence imaging, correspond to changes in SK3 channel expression. The reporter plasmid was cotransfected with plasmids expressing Sp1, Sp3si, or Sp3li into Drosophila SL2 cells, which lack endogenous Sp transcription factors. Although both the short and long isoforms of Sp3 bind the promoter and reduce promoter activation (Fig. 2A), only the short isoform was detected in mouse myometrium by immunoblotting and was pursued in these experiments (data not shown). As illustrated in Fig. 2B, Sp1 enhanced luciferase expression ∼3.5-fold by 24 h after transfection, and Sp3si alone reduced it 2.6-fold (Fig. 2A) with respect to luminescence detected in the vector control. Furthermore, in the presence of Sp1, Sp3si suppressed SK3 transcriptional activation in a concentration-dependent manner (n = 6). Thus, Sp1 expression enhanced SK3 promoter activation, whereas Sp3si reduced Sp1-induced SK3 promoter activation.
Fig. 2.
Effects of Sp3 on small-conductance calcium-activated potassium channel 3 (SK3) promoter activation in the presence and absence of Sp1. Drosophila Schneider's line 2 cells were cotransfected with an SK3 promoter-driven luciferase gene and 1 μg of the Pac vector (control), Sp3si, and Sp3 long isoform (Sp3li) (A) or Sp1 plus Sp3si at various concentrations (B) as indicated. Bioluminescence was quantified, revealing that Sp3si and Sp3li alone can reduce SK3 promoter activation (n = 4–6). Means ± SE. A: *P < 0.05 vs. Pac. B: Sp3si dose-dependently reduced Sp1 activation of the SK3 promoter (n = 6). Means ± SE. *P < 0.05 vs. Sp1.
SK3 expression decreases in estrogen-treated mice.
Estrogen levels increase during pregnancy and may contribute to the downregulation of SK3 channels by modulating the binding of Sp1/Sp3 to the SK3 promoter. We used an in vivo approach to test whether estrogen is an endogenous regulator of SK3. Mice were ovariectomized and implanted with placebo or an E2 pellet to assess whether estrogen treatment regulates expression of the SK3 channel protein in the uterus. Three weeks after ovariectomy and pellet implantation, serum samples were taken, uteri were isolated, and total lysates were prepared from the uteri. In E2-treated mice (n = 6) estradiol levels were 961 ± 76 pg/ml, whereas in placebo-treated mice (n = 11) estradiol levels were below the limit of detection (<20 pg/ml). Immunoblotting showed that whereas SK3 channel protein was present in the placebo-treated mice, the levels were reduced in the estradiol-treated mice (Fig. 3A); quantitation revealed that SK3 protein was decreased by 42% in the uteri of the estradiol-treated mice (Fig. 3B). Immunohistochemistry was performed to confirm the location of SK3 channels in the uterus with and without estrogen (n = 6). This analysis revealed that the channel was expressed in all layers and that estrogen treatment did not alter channel localization (green was adjusted equally for each image to enhance visualization; Fig. 3C). Hence, estrogen reduced SK3 protein expression in mouse uteri in vivo.
Fig. 3.
Effects of estrogen treatment on SK3 channel expression in uteri of ovariectomized mice. Uterine whole cell lysates were prepared 3 wk after ovariectomy and pellet implantation (17β-estradiol or placebo), and proteins were separated by SDS-PAGE. Blots were probed with antibodies against SK3 and GAPDH (loading control). A: SK3 protein decreased in the presence of estrogen but remained constant with placebo treatment. B: quantification of SK3 protein levels normalized to GAPDH loading control (n = 6). Means ± SE. *P < 0.05 vs. placebo. C: immunohistochemistry of the uterus 3 wk post-placebo or -estradiol treatment, with localization of SK3 channel expression shown in green and α-smooth muscle actin (SMA) in red.
Uterine SK3 channel expression decreases during pregnancy in humans.
To determine whether the observed decrease in mouse SK3 channel expression during pregnancy represents a pattern shared with the human uterus, we isolated RNA from NP, late-gestation NL, or late-gestation L tissue (n = 3). Real-time PCR [quantitative PCR (qPCR)] analyses revealed that SK3 transcript was reduced in NL and L tissue compared with that in NP myometrium (Fig. 4A). To assess whether the levels of SK3 protein mimic transcript expression, we carried out immunoblotting with SK3-specfic antibodies. Total cell lysates extracted from NP, NL, and L myometrium showed that SK3 protein expression in humans decreases by late gestation (Fig. 4B). Quantification of the blots by densitometry verified that SK3 protein is downregulated in human uterine samples (n = 4; Fig. 4C). Thus, consistent with our findings in mice, SK3 channels in the human uterus are downregulated during late gestation.
Fig. 4.
SK3 channel expression in human uteri at term. A: real-time PCR analysis of SK3 transcript demonstrates a decline at term in both nonlaboring (NL) and laboring (L) tissue compared with NP uterine samples (n = 3). B: Western immunoblotting of lysates from human uteri confirms that SK3 protein is downregulated at term. C: quantification of SK3 protein levels normalized to those for GAPDH protein (n = 4). Means ± SE. *P < 0.05 vs. NP.
SK3 channel expression increases in estrogen-treated hMSMCs overexpressing Sp1.
Similar to the mouse KCNN3 promoter, the human promoter contains two Sp binding sites, and the expression of SK3 in the human uterus is diminished during pregnancy (Fig. 4). We sought to determine whether estrogen enhances SK3 channel expression in hMSMCs when Sp1 levels are elevated. hMSMCs isolated from eight patients were transfected with Sp1 or the control vector. The cells were then treated with vehicle or E2 (0.1–10 nM) for 48 h, and SK3 transcript was measured by qPCR. Due to variability between patient uterine samples, nonparametric data analysis was carried out by assigning a value of “0” for no change in transcript expression, “+” for an increase in transcript expression, and “−” for a decrease in transcript expression compared with levels produced in cells transfected with the control vector in the absence of E2 (0 nM E2) within each experiment. As shown in Table 1, cells transfected with Sp1 consistently exhibited an increase in SK3 transcript even in the absence of E2 (0 nM E2). Thus, Sp1 overexpression alone is sufficient to increase SK3 expression. Treatment with 10 and 5 nM E2 also enhanced SK3 transcript expression in hMSMCs that had been transfected with Sp1. This implies that although estrogen may lead to SK3 downregulation in MSMCs during pregnancy, it upregulates SK3 in these cells provided that Sp1 is abundant.
Table 1.
SK3 channel levels after Sp1 overexpression and 17β-estradiol treatment
| Vector, nM |
Sp1, nM |
|||||||
|---|---|---|---|---|---|---|---|---|
| Construct (Estradiol, nM) | 0 | 10 | 0* | 10* | 5* | 1 | 0.5 | 0.1 |
| Patient 1 | 0 | + | − | + | + | 0 | 0 | − |
| Patient 2 | 0 | 0 | + | + | + | + | + | − |
| Patient 3 | 0 | + | + | + | + | + | + | + |
| Patient 4 | 0 | 0 | + | − | + | 0 | − | − |
| Patient 5 | 0 | − | + | + | + | + | + | 0 |
| Patient 6 | 0 | 0 | + | + | 0 | 0 | 0 | + |
| Patient 7 | 0 | 0 | + | + | + | + | + | + |
| Patient 8 | 0 | + | + | + | + | + | + | + |
SK3, small-conductance calcium-activated potassium channel 3; Sp1, specificity protein 1. Cultured human myometrial smooth muscle cells were transfected with vector control or Sp1-containing vector. Cells were treated with 17β-estradiol (10–0.1 nM) or vehicle control. Cells were collected after 48 h, and transcript analysis was carried out using quantitative PCR and normalizing to GAPDH. Because of variability in human uterine samples from different patients, each experiment was assigned a nominal value with reference to control-treated cells to indicate a decrease of <10% (−), no change (0), or an increase of >10% (+) in the expression of SK3 transcript (n = 8).
P < 0.05 vs. control.
DISCUSSION
The uterus is a dynamic organ that switches from a relaxed state early in pregnancy to a powerful contractile state in late pregnancy to facilitate expulsion of the fetus. One determinant of uterine quiescence is the activity of myometrial K+ channels (17, 22). Generating both repolarizing and hyperpolarizing currents in MSMCs, these channels contribute significantly to uterine quiescence (7, 21). Accordingly, their activities must be regulated as pregnancy progresses so that myometrial quiescence is maintained until labor. As such, in the myometrium, the level of expression, the density, and the properties of K+ channels change dynamically throughout pregnancy (16, 22, 26). The K+ channels that have been most intensely studied during pregnancy and parturition are the ATP-sensitive K+ channel, Shaker-like voltage-gated K+ channels (Kv), the large-conductance calcium- and voltage-sensitive K+ channel (Kcnma1 gene-encoded maxi-K channel), and the small-conductance calcium-activated K+ channel (KCNN3 gene-encoded SK3).
To date, the SK3 channel is the only ion channel whose overexpression in transgenic mouse models has been reported to delay or impede parturition (2). Despite the interesting parturition phenotype of the SK3-overexpressing mice, little information has existed previously on the mechanisms by which SK3 channels are regulated during normal pregnancy or on how this leads to a modification of myometrial function. SK3 channel function can be altered through transcription from alternate promoters, resulting in the expression of dominant negative forms of the channel that can attenuate current and significantly enhance cell excitability (18, 30). Previous transcriptional studies using Cos7 cells demonstrated that Sp1 can augment SK3 expression (14). We were able to demonstrate that, whereas Sp1 activates the KCNN3 promoter, Sp3si reduces this KCNN3 promoter activation in a dose-dependent matter. In addition, we showed that Sp1 protein is diminished remarkably in pregnant myometrium, whereas Sp3si levels remain constant. This suggests that the physiological balance between Sp1 and Sp3 during pregnancy may underlie the regulation of SK3 channels and modulate the excitability of myometrial cells during pregnancy. Although studies in other tissues have provided some insight into how Sp factors are regulated (8, 23), further research is needed to determine how these transcription factor levels are altered during pregnancy. The results presented here suggest that the constant Sp3si protein levels seen during pregnancy may partially account for the reduction in the SK3 channel expression in mouse uterus, and this change in SK3 may aid in the transition to a laboring state. However, the mouse KCNN3 promoter contains multiple additional transcription factor binding sites that can potentially influence SK3 channel expression. For example, the cyclic AMP response elements (CRE), the Nkx-2 homeobox protein, and GATA transcription factors are all expressed in the uterus and may influence transcription of the SK3 channel. In particular, levels of the CRE-binding protein were reduced in both human laboring uterine fundal tissue and mouse uterine tissue at term (10), and estrogen enhanced expression of the CRE-binding protein (6). Further investigation is needed to understand the complex nature of transcriptional regulation of the KCNN3 promoter during pregnancy.
Hormones may contribute to SK3 changes during pregnancy. Hormones and other second messengers modulate the transcription of multiple ion channel genes, including those encoding the voltage-gated K+ channels Kv4.2 and Kv1.5 (12, 15, 31) and the BK channel-encoding mouse slo gene (20). Whereas many of these channel genes contain hormone response elements within the promoter, others contain transcription factor binding sites that either complement these elements or provide hormone responsiveness themselves. For example, many genes recognized as being regulated by progesterone do not contain response elements (24). For the SK3 channel in particular, injecting 17β-estradiol into the rostral hypothalamus of ovariectomized guinea pigs increases SK3 mRNA levels (3), indicating that estrogen may contribute to SK3 regulation in the myometrium during pregnancy. Previous studies showed that estrogen enhances SK3 channel expression despite the fact that the KCNN3 promoter does not contain an estrogen response element; thus Sp binding sites play an important role in estrogen-mediated regulation of the genome (14). Our laboratory has shown that SK3 channel expression decreases during pregnancy, during the period in which estrogen levels rise. In the mouse, estrogen levels range from 390 pg/ml in NP tissue to as high as 1,801 pg/ml during pregnancy (32). We hypothesized that if estrogen contributes to the downregulation of uterine SK3 channels during mouse pregnancy, 17β-estradiol treatment could mimic this response in NP animals. Our in vivo approach using ovariectomized mice implanted with a 17β-estradiol pellet revealed that estrogen levels could be elevated to levels similar to that characteristic of pregnancy and reduce SK3 channel expression. Immunohistochemistry demonstrated that SK3 channels are expressed throughout the uterus and that this pattern of expression is not altered with estrogen treatment. Nevertheless, it is likely that the level of the SK3 channel in the tissue changes. Thus, although estrogen can stimulate Sp1 activation of the KCNN3 gene, the physiological balance between the Sp factors likely regulates SK3 channel expression in vivo.
Although expression of the SK3 channel is known to play an important role in mouse parturition, its regulation in humans has not previously been explored. In this study we have shown that, as is the case in mice, in humans the SK3 transcript and protein are downregulated in the myometrium during late pregnancy (26). Although reduction of the transcript levels was greater than that of protein in the samples from nonlaboring women, it is possible that other mechanisms may stabilize the protein until the time of labor, at which point we saw a significant drop in both transcript and protein expression. In addition, both mouse and human contain two Sp binding sites in the KCNN3 promoter, suggesting that the regulatory mechanism is conserved. When Sp1 was overexpressed in human MSMCs that were then treated with 17β-estradiol, there was a trend toward an increase in SK3 channel expression. However, there was considerable variation in the magnitude of this effect. This variation in the human studies could potentially be a consequence of reduced expression of estrogen receptors in cultured myometrial cells or of variability between estrogen in the patient samples (9, 28). Changes in the density of the estrogen receptor in hMSMCs could potentially introduce significant variability to these experiments, since they involve indirect binding of the estrogen receptor to the promoter region of SK3. However, this dilemma was avoided by comparing expression levels within each primary culture experiment. We concluded that estrogen reduces SK3 expression in the uterus in vivo (Fig. 3) but can also promote SK3 channel expression in the presence of overexpressed Sp1 (Table 1), as found in previous studies (3, 14). Although direct evidence for specific binding of the Sp factors to the SK3 promoter with estradiol to enhance channel expression in the myometrium would be valuable, we presently lack good reagents for the chromatin immunoprecipitation experiments that would provide a definitive answer. However, we note that Jacobson et al. (14) demonstrated, using electrophoretic mobility shift assays, that Sp1 and Sp3 directly bind to the promoter region of SK3 and that the Sp1 and Sp3 binding sites are necessary for estrogen-mediated stimulation of SK3 transcription.
In conclusion, Sp1 levels are diminished in the uterus during mouse pregnancy, whereas those of Sp3si remain constant. With an abundance of Sp3si compared with Sp1, Sp3si is more likely to bind to Sp binding sites and reduce the expression of transcripts such as KCNN3. Estrogen enhanced the binding of Sp factors and contributed to Sp3si binding of the SK3 promoter during pregnancy to further diminish expression levels. Although multiple mechanisms may contribute to the regulation of SK3 channels during pregnancy, the reported studies demonstrate that an ion channel important to myometrial excitability during pregnancy is controlled via transcriptional regulation.
GRANTS
This work was supported by grants from the March of Dimes (21-FY08-566) and the National Institute of Child Health and Human Development (HD-037831) awarded to S. K. England. The National Center for Research Resources, Clinical and Translational Science Award (M01-RR-00059), supported the attainment of human tissue. S. L. Pierce is supported by a Predoctoral Fellowship from the American Heart Association (09PRE2280322).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Guntram Suske from the Institut fuer Molekularbiologie und Tumorforschung at Philipps-Universitaet in Marburg, Germany, for the generous gift of the Sp constructs, Katherine Walters of the University of Iowa Central Microscopy Research Facility for technical assistance with the immunohistochemistry, Kathryn Lamping of the Internal Medicine Department at the University of Iowa for critical review of the manuscript, Victoria Korovkina of the Obstetrics and Gynecology Department at the University of Iowa and Susan Stamnes and Rafael Cabeza of the Molecular Physiology and Biophysics Department at the University of Iowa for advice and assistance with the culture techniques, and Christine Blaumueller for editorial contributions to the manuscript.
REFERENCES
- 1. Benkusky NA, Fergus DJ, Zucchero TM, England SK. Regulation of the Ca2+-sensitive domains of the maxi-K channel in the mouse myometrium during gestation. J Biol Chem 275: 27712–27719, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, Knaus HG, Seeburg PH, Adelman JP. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science 289: 1942–1946, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bosch MA, Kelly MJ, Ronnekleiv OK. Distribution, neuronal colocalization, and 17beta-E2 modulation of small conductance calcium-activated K(+) channel (SK3) mRNA in the guinea pig brain. Endocrinology 143: 1097–1107, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Braun H, Koop R, Ertmer A, Nacht S, Suske G. Transcription factor Sp3 is regulated by acetylation. Nucleic Acids Res 29: 4994–5000, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics 118: 1566–1573, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Carlstrom L, Ke ZJ, Unnerstall JR, Cohen RS, Pandey SC. Estrogen modulation of the cyclic AMP response element-binding protein pathway. Effects of long-term and acute treatments. Neuroendocrinology 74: 227–243, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chanrachakul B, Pipkin FB, Khan RN. Contribution of coupling between human myometrial β2-adrenoreceptor and the BKCa channel to uterine quiescence. Am J Physiol Cell Physiol 287: C1747–C1752, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cheng YH, Imir A, Suzuki T, Fenkci V, Yilmaz B, Sasano H, Bulun SE. SP1 and SP3 mediate progesterone-dependent induction of the 17beta hydroxysteroid dehydrogenase type 2 gene in human endometrium. Biol Reprod 75: 605–614, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. Telomerase immortalization of human myometrial cells. Biol Reprod 67: 506–514, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA 100: 9518–9523, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dennig J, Beato M, Suske G. An inhibitor domain in Sp3 regulates its glutamine-rich activation domains. EMBO J 15: 5659–5667, 1996 [PMC free article] [PubMed] [Google Scholar]
- 12. Fountain SJ, Cheong A, Li J, Dondas NY, Zeng F, Wood IC, Beech DJ. Kv1.5 potassium channel gene regulation by Sp1 transcription factor and oxidative stress. Am J Physiol Heart Circ Physiol 293: H2719–H2725, 2007 [DOI] [PubMed] [Google Scholar]
- 13. García-López MJ, Martínez-Martos JM, Mayas MD, Carrera MP, Ramírez-Expósito MJ. Influence of hormonal status on enkephalin-degrading aminopeptidase activity in the HPA axis of female mice. Gen Comp Endocrinol 141: 135–140, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun 303: 660–668, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Jia Y, Takimoto K. GATA and FOG2 transcription factors differentially regulate the promoter for Kv4.2 K(+) channel gene in cardiac myocytes and PC12 cells. Cardiovasc Res 60: 278–287, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Khan RN, Smith SK, Morrison JJ, Ashford ML. Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proc Biol Sci 251: 9–15, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Kimura T, Ogita K, Kusui C, Ohashi K, Azuma C, Murata Y. What knockout mice can tell us about parturition. Rev Reprod 4: 73–80, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kolski-Andreaco A, Tomita H, Shakkottai VG, Gutman GA, Cahalan MD, Gargus JJ, Chandy KG. SK3-1C, a dominant-negative suppressor of SKCa and IKCa channels. J Biol Chem 279: 6893–6904, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Korovkina VP, Brainard AM, England SK. Translocation of an endoproteolytically cleaved maxi-K channel isoform: mechanisms to induce human myometrial cell repolarization. J Physiol 573: 329–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kundu P, Alioua A, Stefani E, Toro L. Regulation of mouse Slo gene expression: multiple promoters, transcription start sites, and genomic action of estrogen. J Biol Chem 282: 27478–27492, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Liu B, Arulkumaran S, Hill SJ, Khan RN. Comparison of potassium currents in human decidua before and after the onset of labor. Biol Reprod 68: 2281–2288, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lundgren DW, Moore JJ, Chang SM, Collins PL, Chang AS. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med 216: 57–64, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Ma W, Horvath GC, Kistler MK, Kistler WS. Expression patterns of SP1 and SP3 during mouse spermatogenesis: SP1 down-regulation correlates with two successive promoter changes and translationally compromised transcripts. Biol Reprod 79: 289–300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 297: R525–R545, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Phillips RJ, Tyson-Capper Née Pollard AJ, Bailey J, Robson SC, Europe-Finner GN. Regulation of expression of the chorionic gonadotropin/luteinizing hormone receptor gene in the human myometrium: involvement of specificity protein-1 (Sp1), Sp3, Sp4, Sp-like proteins, and histone deacetylases. J Clin Endocrinol Metab 90: 3479–3490, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Pierce SL, Kresowik JD, Lamping KG, England SK. Overexpression of SK3 channels dampens uterine contractility to prevent preterm labor in mice. Biol Reprod 78: 1058–1063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sapetschnig A, Koch F, Rischitor G, Mennenga T, Suske G. Complexity of translationally controlled transcription factor Sp3 isoform expression. J Biol Chem 279: 42095–42105, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Severino MF, Murray MJ, Brandon DD, Clinton GM, Burry KA, Novy MJ. Rapid loss of oestrogen and progesterone receptors in human leiomyoma and myometrial explant cultures. Mol Hum Reprod 2: 823–828, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Stielow B, Sapetschnig A, Wink C, Kruger I, Suske G. SUMO-modified Sp3 represses transcription by provoking local heterochromatic gene silencing. EMBO Rep 9: 899–906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci 24: 3537–3542, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu Y, Platoshyn O, Zhang J, Krick S, Zhao Y, Rubin LJ, Rothman A, Yuan JX. c-Jun decreases voltage-gated K(+) channel activity in pulmonary artery smooth muscle cells. Circulation 104: 1557–1563, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Fishman MC, Huang PL. Estrogen mediates the protective effects of pregnancy and chorionic gonadotropin in a mouse model of vascular injury. Arterioscler Thromb Vasc Biol 19: 2059–2065, 1999 [DOI] [PubMed] [Google Scholar]