Abstract
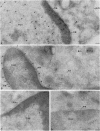
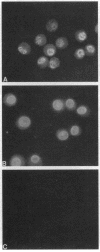
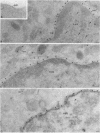
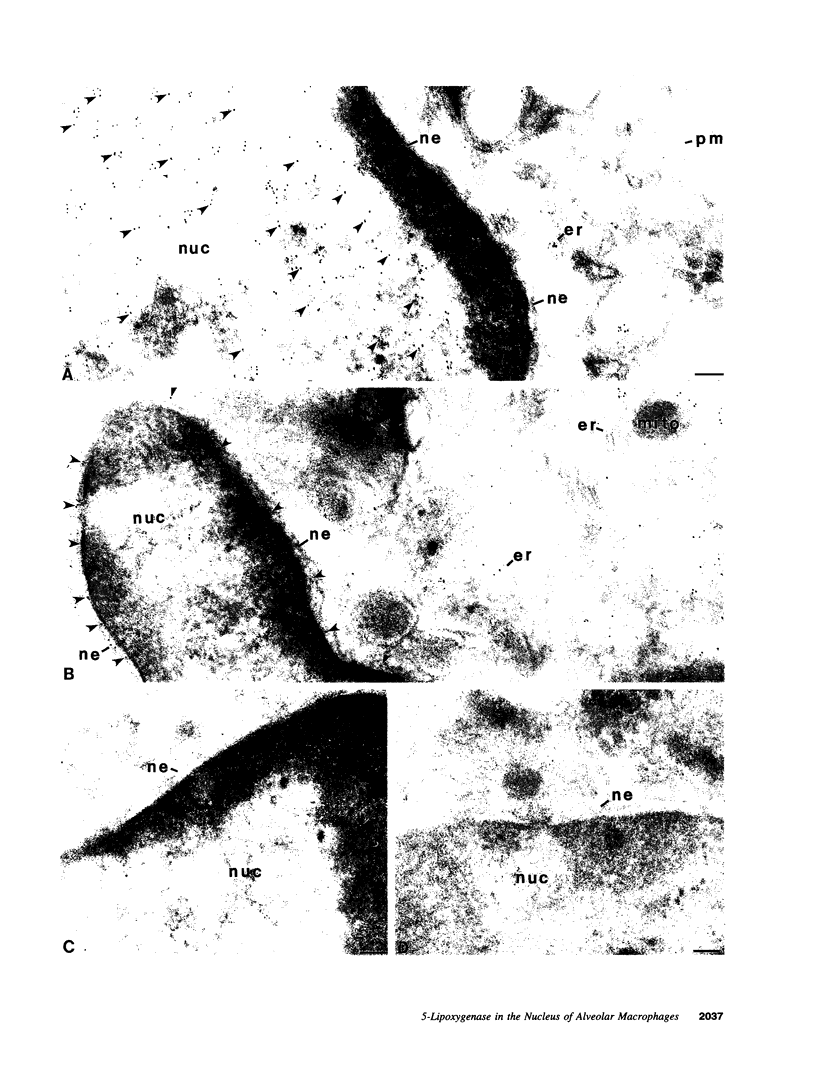
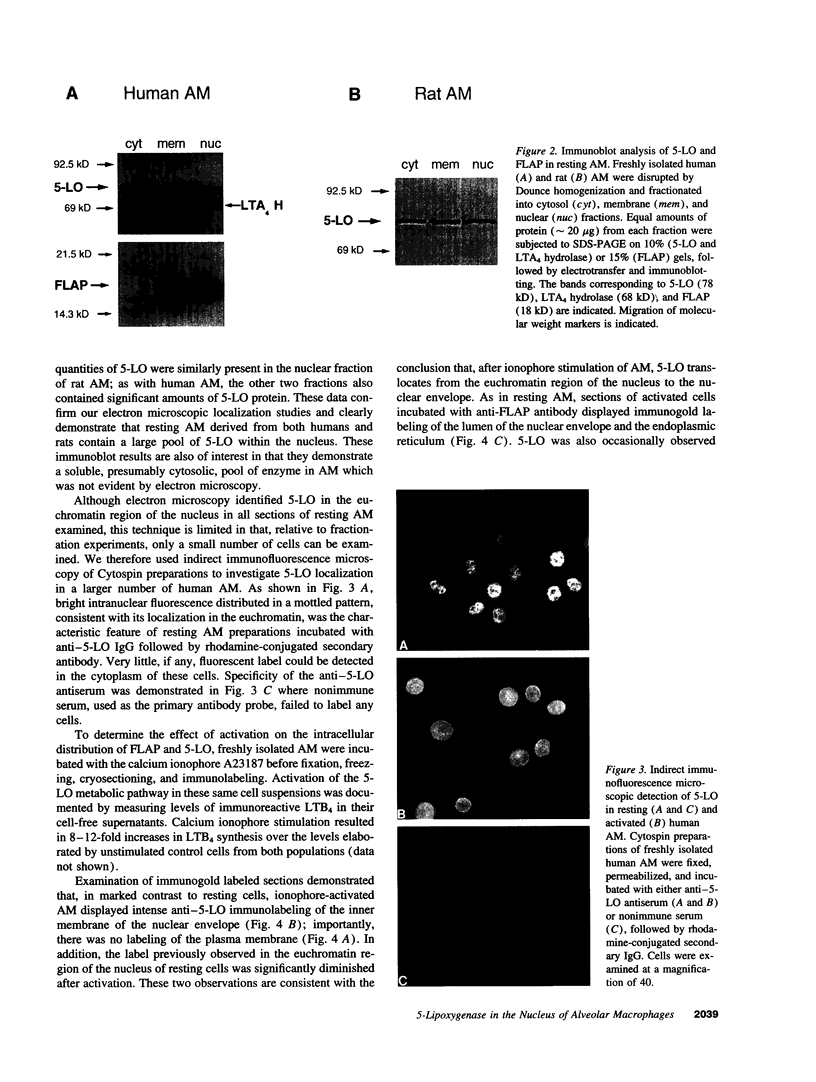
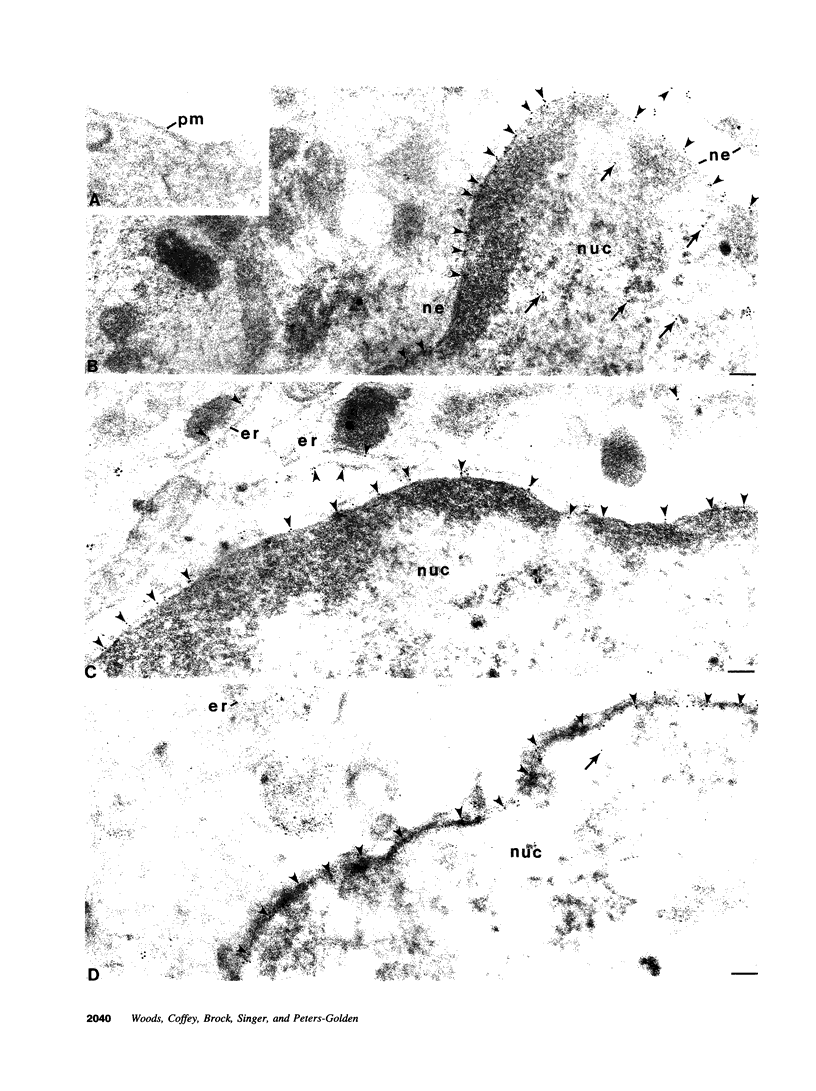
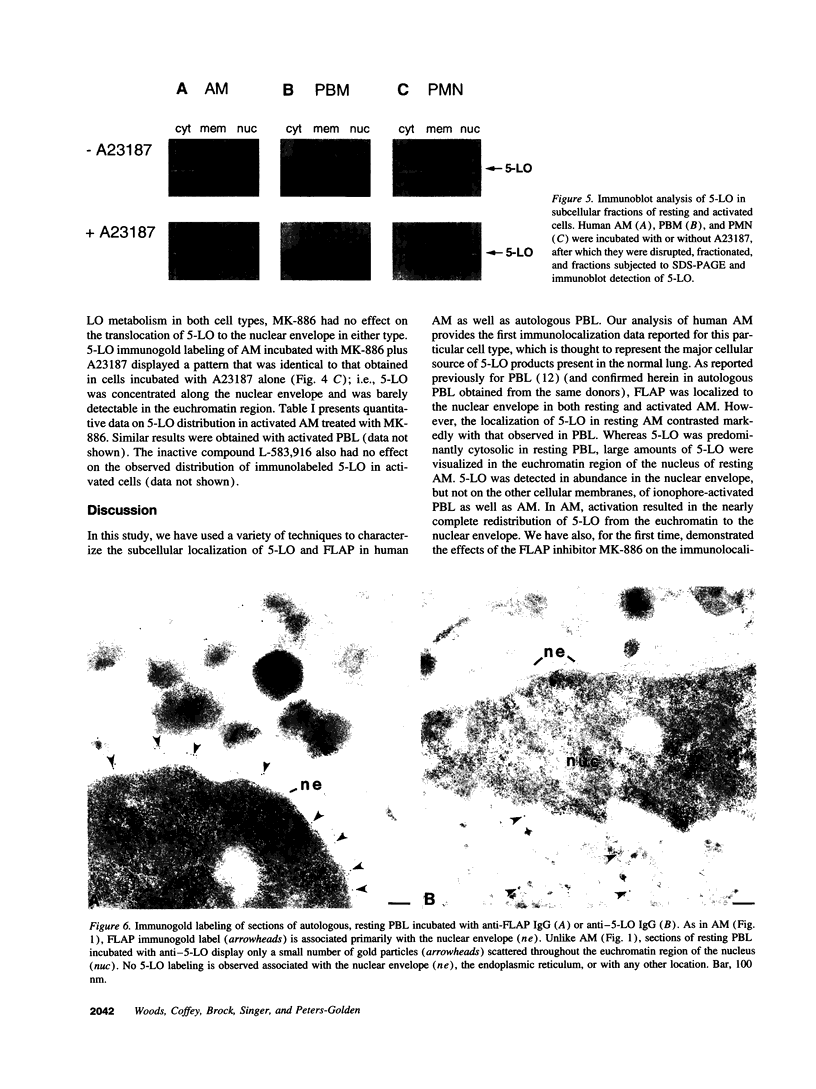
5-Lipoxygenase (5-LO) and 5-lipoxygenase-activating protein (FLAP) are two key proteins involved in the synthesis of leukotrienes (LT) from arachidonic acid. Although both alveolar macrophages (AM) and peripheral blood leukocytes (PBL) produce large amounts of LT after activation, 5-LO translocates from a soluble pool to a particulate fraction upon activation of PBL, but is contained in the particulate fraction in AM irrespective of activation. We have therefore examined the subcellular localization of 5-LO in autologous human AM and PBL collected from normal donors. While immunogold electron microscopy demonstrated little 5-LO in resting PBL, resting AM exhibited abundant 5-LO epitopes in the euchromatin region of the nucleus. The presence of substantial quantities of 5-LO in the nucleus of resting AM was verified by cell fractionation and immunoblot analysis and by indirect immunofluorescence microscopy. In both AM and PBL activated by A23187, all of the observable 5-LO immunogold labeling was found associated with the nuclear envelope. In resting cells of both types, FLAP was predominantly associated with the nuclear envelope, and its localization was not affected by activation with A23187. The effects of MK-886, which binds to FLAP, were examined in ionophore-stimulated AM and PBL. Although MK-886 inhibited LT synthesis in both cell types, it failed to prevent the translocation of 5-LO to the nuclear envelope. These results indicate that the nuclear envelope is the site at which 5-LO interacts with FLAP and arachidonic acid to catalyze LT synthesis in activated AM as well as PBL, and that in resting AM the euchromatin region of the nucleus is the predominant source of the translocated enzyme. In addition, LT synthesis is a two-step process consisting of FLAP-independent translocation of 5-LO to the nuclear envelope followed by the FLAP-dependent activation of the enzyme.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balter M. S., Toews G. B., Peters-Golden M. Different patterns of arachidonate metabolism in autologous human blood monocytes and alveolar macrophages. J Immunol. 1989 Jan 15;142(2):602–608. [PubMed] [Google Scholar]
- Bigby T. D., Holtzman M. J. Enhanced 5-lipoxygenase activity in lung macrophages compared to monocytes from normal subjects. J Immunol. 1987 Mar 1;138(5):1546–1550. [PubMed] [Google Scholar]
- Brock T. G., Paine R., 3rd, Peters-Golden M. Localization of 5-lipoxygenase to the nucleus of unstimulated rat basophilic leukemia cells. J Biol Chem. 1994 Sep 2;269(35):22059–22066. [PubMed] [Google Scholar]
- Capriotti A. M., Furth E. E., Arrasmith M. E., Laposata M. Arachidonate released upon agonist stimulation preferentially originates from arachidonate most recently incorporated into nuclear membrane phospholipids. J Biol Chem. 1988 Jul 15;263(20):10029–10034. [PubMed] [Google Scholar]
- Charleson S., Prasit P., Léger S., Gillard J. W., Vickers P. J., Mancini J. A., Charleson P., Guay J., Ford-Hutchinson A. W., Evans J. F. Characterization of a 5-lipoxygenase-activating protein binding assay: correlation of affinity for 5-lipoxygenase-activating protein with leukotriene synthesis inhibition. Mol Pharmacol. 1992 May;41(5):873–879. [PubMed] [Google Scholar]
- Coffey M. J., Gyetko M., Peters-Golden M. 1,25-Dihydroxyvitamin D3 upregulates 5-lipoxygenase metabolism and 5-lipoxygenase activating protein in peripheral blood monocytes as they differentiate into mature macrophages. J Lipid Mediat. 1993 Mar-Apr;6(1-3):43–51. [PubMed] [Google Scholar]
- Coffey M. J., Wilcoxen S. E., Peters-Golden M. Increases in 5-lipoxygenase activating protein expression account for enhanced capacity for 5-lipoxygenase metabolism that accompanies differentiation of peripheral blood monocytes into alveolar macrophages. Am J Respir Cell Mol Biol. 1994 Aug;11(2):153–158. doi: 10.1165/ajrcmb.11.2.8049076. [DOI] [PubMed] [Google Scholar]
- Coffey M., Peters-Golden M., Fantone J. C., 3rd, Sporn P. H. Membrane association of active 5-lipoxygenase in resting cells. Evidence for novel regulation of the enzyme in the rat alveolar macrophage. J Biol Chem. 1992 Jan 5;267(1):570–576. [PubMed] [Google Scholar]
- Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., Miller D. K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990 Jan 18;343(6255):282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Jones R. E., Diehl R. E., Bennett C. D., Kargman S., Rouzer C. A. Cloning of the cDNA for human 5-lipoxygenase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):416–420. doi: 10.1073/pnas.85.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. F., Dupuis P., Ford-Hutchinson A. W. Purification and characterisation of leukotriene A4 hydrolase from rat neutrophils. Biochim Biophys Acta. 1985 May 29;840(1):43–50. doi: 10.1016/0304-4165(85)90160-6. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Cohn Z. A. The alveolar macrophage. J Appl Physiol (1985) 1986 Feb;60(2):353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W. FLAP: a novel drug target for inhibiting the synthesis of leukotrienes. Trends Pharmacol Sci. 1991 Feb;12(2):68–70. doi: 10.1016/0165-6147(91)90500-r. [DOI] [PubMed] [Google Scholar]
- Hatzelmann A., Fruchtmann R., Mohrs K. H., Raddatz S., Müller-Peddinghaus R. Mode of action of the new selective leukotriene synthesis inhibitor BAY X 1005 ((R)-2-[4-(quinolin-2-yl-methoxy)phenyl]-2-cyclopentyl acetic acid) and structurally related compounds. Biochem Pharmacol. 1993 Jan 7;45(1):101–111. doi: 10.1016/0006-2952(93)90382-7. [DOI] [PubMed] [Google Scholar]
- Kargman S., Prasit P., Evans J. F. Translocation of HL-60 cell 5-lipoxygenase. Inhibition of A23187- or N-formyl-methionyl-leucyl-phenylalanine-induced translocation by indole and quinoline leukotriene synthesis inhibitors. J Biol Chem. 1991 Dec 15;266(35):23745–23752. [PubMed] [Google Scholar]
- Kargman S., Rouzer C. A. Studies on the regulation, biosynthesis, and activation of 5-lipoxygenase in differentiated HL60 cells. J Biol Chem. 1989 Aug 5;264(22):13313–13320. [PubMed] [Google Scholar]
- Kargman S., Vickers P. J., Evans J. F. A23187-induced translocation of 5-lipoxygenase in osteosarcoma cells. J Cell Biol. 1992 Dec;119(6):1701–1709. doi: 10.1083/jcb.119.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Maridor G., Nigg E. A. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992 Jan;116(1):43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Soberman R. J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990 Sep 6;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Login G. R., Dvorak A. M. Microwave energy fixation for electron microscopy. Am J Pathol. 1985 Aug;120(2):230–243. [PMC free article] [PubMed] [Google Scholar]
- Mancini J. A., Abramovitz M., Cox M. E., Wong E., Charleson S., Perrier H., Wang Z., Prasit P., Vickers P. J. 5-lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett. 1993 Mar 8;318(3):277–281. doi: 10.1016/0014-5793(93)80528-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Funk C. D., Rådmark O., Hög J. O., Jörnvall H., Samuelsson B. Molecular cloning and amino acid sequence of human 5-lipoxygenase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):26–30. doi: 10.1073/pnas.85.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Léveillé C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990 Jan 18;343(6255):278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Lei Z. M., Rao C. V. The enzymes in cyclooxygenase and lipoxygenase pathways of arachidonic acid metabolism in human corpora lutea: dependence on luteal phase, cellular and subcellular distribution. Prostaglandins Leukot Essent Fatty Acids. 1991 May;43(1):1–12. doi: 10.1016/0952-3278(91)90125-o. [DOI] [PubMed] [Google Scholar]
- Natsui K., Ueda N., Yamamoto S., Komatsu N., Watanabe K. Arachidonate 5-lipoxygenase of porcine pancreas: its localization in acinar cells. Biochim Biophys Acta. 1991 Sep 11;1085(2):241–247. doi: 10.1016/0005-2760(91)90100-v. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M., McNish R. W., Hyzy R., Shelly C., Toews G. B. Alterations in the pattern of arachidonate metabolism accompany rat macrophage differentiation in the lung. J Immunol. 1990 Jan 1;144(1):263–270. [PubMed] [Google Scholar]
- Pueringer R. J., Bahns C. C., Hunninghake G. W. Alveolar macrophages have greater amounts of the enzyme 5-lipoxygenase than do monocytes. J Appl Physiol (1985) 1992 Aug;73(2):781–786. doi: 10.1152/jappl.1992.73.2.781. [DOI] [PubMed] [Google Scholar]
- Rouzer C. A., Ford-Hutchinson A. W., Morton H. E., Gillard J. W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990 Jan 25;265(3):1436–1442. [PubMed] [Google Scholar]
- Rouzer C. A., Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J Biol Chem. 1988 Aug 5;263(22):10980–10988. [PubMed] [Google Scholar]
- Samuelsson B., Funk C. D. Enzymes involved in the biosynthesis of leukotriene B4. J Biol Chem. 1989 Nov 25;264(33):19469–19472. [PubMed] [Google Scholar]
- Tokuda H., Masuda S., Takakura Y., Sezaki H., Hashida M. Specific uptake of succinylated proteins via a scavenger receptor-mediated mechanism in cultured brain microvessel endothelial cells. Biochem Biophys Res Commun. 1993 Oct 15;196(1):18–24. doi: 10.1006/bbrc.1993.2210. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Vissers M. C., Jester S. A., Fantone J. C. Rapid purification of human peripheral blood monocytes by centrifugation through Ficoll-Hypaque and Sepracell-MN. J Immunol Methods. 1988 Jun 13;110(2):203–207. doi: 10.1016/0022-1759(88)90104-4. [DOI] [PubMed] [Google Scholar]
- Watkins J. D., Kent C. Immunolocalization of membrane-associated CTP:phosphocholine cytidylyltransferase in phosphatidylcholine-deficient Chinese hamster ovary cells. J Biol Chem. 1992 Mar 15;267(8):5686–5692. [PubMed] [Google Scholar]
- Wong A., Hwang S. M., Cook M. N., Hogaboom G. K., Crooke S. T. Interactions of 5-lipoxygenase with membranes: studies on the association of soluble enzyme with membranes and alterations in enzyme activity. Biochemistry. 1988 Sep 6;27(18):6763–6769. doi: 10.1021/bi00418a018. [DOI] [PubMed] [Google Scholar]
- Woods J. W., Evans J. F., Ethier D., Scott S., Vickers P. J., Hearn L., Heibein J. A., Charleson S., Singer I. I. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med. 1993 Dec 1;178(6):1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]