SUMMARY
The association between aging and cancer is well exemplified by bladder cancer: with advancing age, the risk of developing bladder cancer increases, and patients’ clinical presentation and outcomes worsen. Care for elderly patients with bladder cancer requires specific knowledge of many key geriatric clinical issues in order to determine optimal treatment plans. While numerous studies have tried to address the role of urologic intervention for elderly patients with bladder cancer, many studies fail to incorporate a component of true functional assessment. Evaluation tools that incorporate comorbidities, disabilities and functional status will need to be developed, as chronological age is a poor predictor of treatment outcomes. Additionally, further research is necessary to better understand the basic mechanisms that predispose elderly patients to develop this costly and life-threatening disease. This Review examines the current literature evaluating the clinical and mechanistic interactions between aging and bladder cancer, and suggests the formulation of a research agenda to address the issues raised.
Keywords: aging, bladder cancer, elderly, frailty, geriatric
INTRODUCTION
Cancer is a disease that occurs more frequently in later life, and the proportion of cancers that occur in the elderly is increasing relative to younger age groups. By 2030, over 70% of all cancers are expected to occur in people aged over 65 years.1 Proposed mechanisms for the increased incidence of cancer in the aging population include an accumulation of genetic and cellular damage, prolonged exposure to carcinogens, and fundamental changes in the host environment. Cancer and aging are intimately linked at the most basic level: convergent mechanisms protect against both aging and cancer (e.g. antioxidant defenses), while the pathways regulating cellular proliferation typically exert divergent or opposing effects; specifically, protecting from cancer but promoting aging.2 The presence of age-related physiological changes in elderly patients, plus common comorbid conditions, presents clinicians with challenges that require specific knowledge of geriatric oncology.3
Bladder cancer illustrates well the association between cancer and aging, and occurs most commonly in the elderly: the median age at diagnosis is 69 years for men and 71 years for women.4 Bladder cancer is also the fourth and eighth most common malignancy in men and women, respectively, and the number of diagnosed cases is increasing annually in the US (Figure 1).5 In 2008, a Californian tumor registry study showed that the incidence of bladder cancer peaks at the age of 85 years, with a rate of increase roughly 10-fold higher than that seen in younger age groups.6 Advanced age may be associated with worse outcome, but stage and grade at diagnosis remain key determinants of prognosis. Advanced age seems to increase the risk of higher-stage cancer (Table 1), and also of high-grade disease if the cancer is found to be superficial.7-9 High-grade or muscle-invasive tumors are much more likely to progress and metastasize than low-grade, low-stage cancers, and the 5-year survival rates in patients with high-grade or muscle-invasive tumors are as low as 6%.10 The overall probability of developing invasive disease increases with age, rising from 0.01–0.02% before age 40 years, to 1.2–3.7% for those aged over 70 years (Figure 2).5
Figure 1.
The number of new bladder cancer cases by year in the US (based on data from the American Cancer Society Cancer Facts & Figures 2002–2008).5
Table 1.
Figure 2.
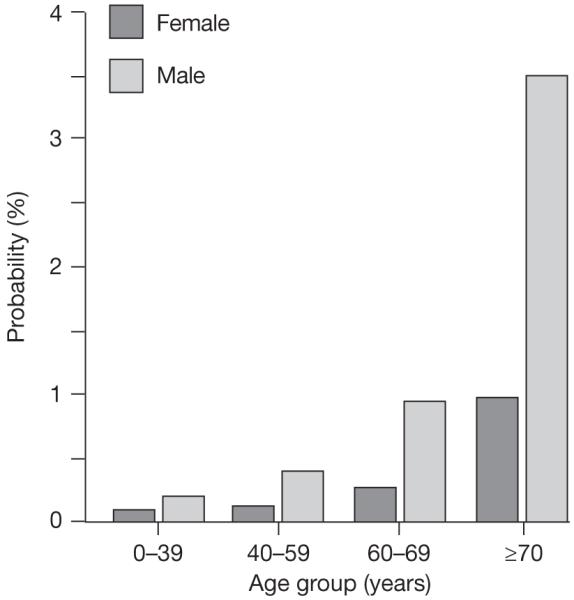
The probability of developing invasive bladder cancer by age group (based on data from the American Cancer Society Facts & Figures 2008).5
The management of high-grade disease in the elderly is costly and challenging. The current standard of care for superficial high-grade or recurrent low-grade disease remains intravesical immunotherapy with bacillus Calmette–Guérin (BCG). Cystectomy with reconstructive surgery is the current standard of care for invasive or recurrent superficial disease. Chemotherapy and radiation therapy can be used in conjunction with cystectomy, as either bladder-sparing therapy or as palliative treatment. The annual costs of caring for patients with muscle-invasive bladder cancer can exceed $35 million.11
The goal of this article is to review the current literature addressing both the clinical and mechanistic interactions between bladder cancer and aging, while also encouraging future progress in these fields through the formulation of a research agenda.
CLINICAL OUTCOMES IN ELDERLY PATIENTS WITH BLADDER CANCER
Superficial bladder cancer
Relatively little has been published regarding the efficacy of treating superficial disease in elderly patients (Table 2). A retrospective phase II database review from a multicenter trial found that overall response to either BCG or BCG plus interferon α was decreased in participants aged 80 years or over compared with younger patients.12 At 24 months’ follow-up, absolute response was reduced by 22% in patients aged 80 years or over compared with patients aged 61–70 years (39% vs 61%). Age remained a significant variable for decreased response to therapy after controlling for multiple other relevant variables.
Table 2.
Clinical summary of selected studies evaluating the role of aging in bladder cancer outcomes
| Study | Type of bladder cancer |
Treatment | Study design | Definition of old (years) |
Number of participants (% defined as old) |
Functional assessment |
Results |
|---|---|---|---|---|---|---|---|
| Joudi et al. (2006)12 |
Superficial | BCG or BCG plus IFN-α |
Retrospective phase II database review |
>80 | ~1,000 (~12%) |
None | The 2-year cancer-free survival rates in patients aged 61–70 years and >80 years were 61% and 39%, respectively. Age was an independent predictor of response |
| Herr et al. (2007)13 |
Superficial | BCG | Retrospective case series |
>70 | 805 (26% aged 70–79; 7% aged ≥80) |
None | A small but significant difference in the 5-year tumor-free recurrence rate was observed between patients older and younger than 70 years, favoring the younger group. On multivariate analysis, only tumor stage and grade were significant predictors of response |
| Chamie et al. (2008)18 |
Muscle- invasive |
Cystectomy (n = 8,034) or RT (n = 2,773) |
Retrospective database review (SEER) |
>79 | 10,807 (cystectomy 8%, RT 11%) |
None | In patients aged >79 years, there was no survival benefit associated with cystectomy (18 months with cystectomy vs 15 months without cystectomy), no survival benefit associated with cystectomy without pelvic LND, and no difference in survival between cystectomy and external beam RT |
| Nielsen et al. (2007)19 |
Muscle- invasive |
Cystectomy | Retrospective case series |
>80 | 888 (6%) | None | Increasing age was associated with an increased risk for adverse pathology and disease recurrence, and a decrease in disease-specific survival and use of adjuvant therapy |
| Figueroa et al. (1998)20 |
Muscle- invasive |
Cystectomy | Retrospective case series |
≥75 | 1,166 (35%) | None | The 3-year survival rates were 60% and 68% in participants aged ≥75 years and <70 years, respectively, and the 5-year survival rates were 53% and 63%, respectively (P = 0.001) |
| Soulié et al. (2001)22 |
Muscle- invasive |
Cystectomy | Retrospective case series |
≥75 | 565 (13%) | ASA physical status classification |
At a median follow-up period of 14.4 months, the overall mortality rate was 31.5%, median hospital stay was 34 days, median intensive care unit stay was 14 days, and early and late complication rates were 38.4% and 45.6%, respectively |
| Gamé et al. (2001)23 |
Muscle- invasive |
Cystectomy | Retrospective case series |
≥75 | 190 (13%) | ASA physical status classification |
Overall mortality rate was 32% at 14 months, median hospital stay was 24 days, and intraoperative, early and late complication rates were 60%, 64% and 24%, respectively |
| Zebic et al. (2005)24 |
Muscle- invasive |
Cystectomy | Retrospective case series |
≥75 | 53 (100%) | ASA physical status classification |
Median survival was 2 years, median hospital stay was 36 days, and early and late complication rates were 28% and 11%, respectively |
| Koppie et al. (2008)25 |
Muscle- invasive |
Cystectomy | Retrospective case series |
>75 | 1,121 (25%) | ACCI | Higher ACCI scores were associated with an increased likelihood of pT3+ tumors, lower overall survival and a lower likelihood of receiving adjuvant therapy |
| Stroumbakis et al. (1997)26 |
Muscle- invasive |
Cystectomy | Retrospective case series |
≥80 | 44 (100%) | KPS | Median survival was 25 months and the 6-month rehospitalization rate was 66%. KPS drop from 70% to 65% post surgery |
| Weizer et al. (2007)27 |
Muscle- invasive |
Cystectomy, external beam RT or chemotherapy |
Retrospective case series |
≥75 | 152 (100%) | KPS | Median survival was 22 months, and the 4-year disease-specific survival was 14% in patients with a KPS <80%, and 33% in patients with a KPS >80%; KPS was the only independent predictor of overall survival |
Abbreviations: ACCI, age-adjusted Charlson comorbidity index; ASA, American Society of Anesthesiologists; BCG, bacillus Calmette–Guérin; IFN-α, interferon α; KPS, Karnofsky performance status; LND, lymph node dissection; RT, radiation therapy; SEER, Surveillance, Epidemiology and End Results.
The only other published study of elderly patients with superficial bladder cancer reported 20 years’ experience at a single institution.13 Outcome measures included initial response to BCG and tumor-free recurrence. When the series was stratified by an arbitrary age of 70 years, a small but significant difference was seen in tumor-free recurrence, favoring the younger group. Increased age seemed to confer a less-durable response to BCG, earlier recurrences and a shorter cancer-free survival time. Nonetheless, following multivariable analysis, only tumor stage and grade remained as significant predictors of response to therapy.
Muscle-invasive bladder cancer
Muscle invasion doubles the risk of complications in patients with bladder cancer, while adding roughly $700 per month to the cost of care.11 As patients with muscle-invasive disease age, the number of clinically relevant comorbidities dramatically increases. Prout et al.7 evaluated Surveillance, Epidemiology and End Results (SEER) data and found that individuals aged 75 years or over with muscle-invasive bladder cancer had a higher prevalence of cardiac disease, prior cancer diagnosis, chronic anemia, and poor American Society of Anesthesiologists (ASA) Physical Status Classification (Box 1) than patients aged under 75 years. As comorbidities could increase the complication rate, they may have a direct effect on the choice of treatment for bladder cancer.
Box 1 Assessment tools and the meaning of scores.
American Society of Anesthesiologists (ASA) physical status classificationa
A normal, healthy patient
A patient with mild systemic disease
A patient with severe systemic disease
A patient with severe systemic disease that is a constant threat to life
A moribund patient who is not expected to survive with or without the operation
A declared brain-dead patient whose organs are being removed for donor purposes
Age-adjusted Charlson comorbidity index (ACCI)
Weight 1 for the following comorbid conditions: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, ulcer disease, mild liver disease and diabetes
Weight 2 for the following comorbid conditions: hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, any tumor, leukemia and lymphoma
Weight 3 for moderate or severe liver disease
Weight 6 for metastatic solid tumor or AIDS
Weight 1 for each decade over age 40 years
Karnofsky performance status (KPS; %)
Able to carry on normal activity and to work; no special care needed:
100: Normal; no complaints; no evidence of disease
90: Able to carry on normal activity; minor signs or symptoms of disease
80: Normal activity with effort; some signs or symptoms of disease
Unable to work; able to live at home and care for most personal needs; varying amount of assistance needed:
70: Cares for self; unable to carry on normal activity or to do active work
60: Requires occasional assistance, but is able to care for most of his/her personal needs
50: Requires considerable assistance and frequent medical care
Unable to care for self; requires equivalent of institutional or hospital care; disease may be progressing rapidly:
40: Disabled; requires special care and assistance
30: Severely disabled; hospital admission is indicated although death not imminent
20: Very sick; hospital admission necessary; active supportive treatment necessary
10: Moribund; fatal processes progressing rapidly
0: Dead
aUsed for assessing a patient before surgery.
Typically, cancer patients over the age of 70 years do not receive the same care as their younger counterparts,14 and the literature has offered little guidance with regard to the optimum management of elderly patients. Despite an absence of clear outcome data, older patients with muscle-invasive disease are much less likely to undergo cystectomy than younger patients: among participants in the SEER database, 40–55% of participants less than 75 years of age underwent surgery, whereas surgical rates were only 24% and 16% in those aged 75–79 and 80–84 years, respectively.7 In a similar analysis based on SEER data, a significant decrease in the likelihood of undergoing cystectomy as a primary treatment was reported, stage for stage, in those aged over 75 years than in those aged 75 years or under.8 The development of evidence-based patient selection criteria for bladder cancer treatments represents an important goal for the fields of geriatric urologic oncology, hematology-oncology and radiation oncology.
Studies have now evaluated the use of radical surgery in elderly patients (Table 2). The majority of the studies, however, have been retrospective case series, or reports based on large database analyses. Furthermore, although age has been studied as a predictor of clinical outcome, very few participants aged 80 years or older have been included. Moreover, while such elderly individuals often carry a high burden of comorbid diseases and disability, most studies have failed to evaluate the specific contributions of these factors, and merely use chronological age when determining clinical outcomes. Some of the earliest reports simply define ‘elderly’ as those patients aged over 65, 70 or 80 years, without any additional clinical information used to help better define this particular subset of older adults.15-17
The largest case series that included contemporary data reported on 10,807 patients with invasive bladder cancer who were identified in the SEER database from 1992–2004.18 Patients were stratified by age groups of below 60, 60–69, 70–79 and over 79 years. A total of 8,034 patients underwent cystectomy, while 2,077 had radiation therapy as primary treatment. Patients in the oldest age group were least likely to undergo cystectomy, and those elderly patients who did had no significant overall survival advantage (18 months) compared with those who did not (15 months). Interestingly, when older patients were stratified by the extent of lymph node dissection, the minimal benefit of cystectomy was erased in those patients who had limited or no nodal dissection. The authors also found that the benefit of cystectomy over radiation therapy was negligible for those older than 79 years.
Another large study evaluated 888 patients from multiple centers, with data gathered from 1984 to 2003.19 The median patient age was 66 years, with 266 (30%) patients aged 70–80 years but only 51 (6%) patients aged over 80 years. Increasing age was associated with more-advanced pathologic stage and a lower likelihood of receiving adjuvant therapy, and was an independent predictor of adverse outcomes.
Another series of 404 patients aged over 70 years (352 aged 70–79 years, 52 aged ≥80 years; median age 74 years) was compared with data from 762 patients under 70 years of age: patients aged 70 years or older had a significant decrease in overall survival at 3 and 5 years following cystectomy compared with patients aged less than 70 years.20 Although cancer recurrence rates were not found to be different between the groups, cancer-specific survival rates were not reported. In this study, despite the decrease in overall survival in older patients, the authors concluded that properly selected elderly patients can not only undergo, but may also potentially benefit from, radical cystectomy. The investigators also included only patients deemed to be in “good health”, with little or no additional health or performance status information provided. How the findings from this study can be applied by future investigators and clinicians is not clear.
Several smaller studies have now included the ASA Physical Status Classification and/or age-adjusted Charlson comorbidity index (ACCI) (Box 1) in an attempt to better characterize those patients deemed elderly (Table 2); however, age was still the primary stratification tool used, and no attempt was made to correlate outcomes to physiologic measures (e.g. mobility performance), which have been shown to strongly predict frailty, future disability and adverse outcomes in older adults.21 Soulié et al.22 conducted one such study, and they reported on 73 patients aged over 75 years who underwent cystectomy; however, these elderly patients represented only 13% of the overall cohort. Although perioperative mortality was low, early and late complication rates were 38.4% and 45.6%, respectively, with a prolonged median hospital stay of 34 days. After a median follow-up period of 14.4 months, the overall mortality rate was 31.5%. These findings are similar to those reported by Gamé and colleagues,23 who reported results from their series of 25 patients aged 75 years or older who underwent cystectomy. Perioperative morbidity and mortality rates were lower than reported by Soulié and colleagues,22 but the median hospital stay was 24 days and the overall mortality rate at 14 months was 32%. Likewise, Zebic et al.24 reported a median hospital stay of 36 days with a median survival of 2 years in a series of 53 patients over 75 years of age who underwent cystectomy. Median survival stratified by ASA Physical Status Classification score was 2.2 years for ASA 2, 1.6 years for ASA 3 and 70 days for ASA 4. Koppie et al.25 reported the use of the ACCI as a stratification tool for assessing outcomes after cystectomy in the elderly. An institutional database review from 1990 to 2004 identified 1,121 patients who underwent cystectomy. The authors reported that, while overall survival after cystectomy decreased with increasing ACCI scores, no significant association was found between ACCI and progression-free survival.
To date, only two studies have addressed how functional geriatric assessment relates to radical cystectomy outcomes. In a retrospective case series of 44 patients aged over 80 years, Stroumbakis et al.26 reported a 6-month rehospitalization rate of 66%, a median survival of 25 months, and a drop in Karnofsky performance status (KPS; Box 1) score from 70% at baseline to 65% at both 1 and 3 months post surgery. The other study by Weizer et al.27 evaluated a contemporary series (1995–2004) of 152 patients aged 70 years or older, including 33 (22%) patients over 80 years, treated for muscle-invasive disease. In total, 106 patients underwent cystectomy either as primary or secondary treatment. KPS was assessed before definitive treatment, and was then correlated with outcomes. The authors found an overall 4-year survival rate of 14% for those with a KPS below 80%, compared with 33% for those with a KPS above 80%. Median survival for the entire cohort was 22 months. In a multivariable analysis that included age, KPS, marital status, treatment type, mobility and disease stage, the only independent predictor of overall survival was the KPS score. Patients with a KPS score below 80% had a 1.8-fold increase in the risk of death from any cause compared with those with a better performance status. This finding held true regardless of the definitive treatment chosen (cystectomy, external beam radiation therapy or chemotherapy) for patients with a KPS score above 90%. Interestingly, age as a categorical variable only approached, but did not reach, significance in predicting overall survival. Although a type II (false negative) error is possible, these findings demonstrate that functional status and related factors are at least as important, if not more important, than an individual’s birth date in predicting clinical outcome in patients with muscle-invasive bladder cancer.
MECHANISTIC INTERACTIONS BETWEEN BLADDER CANCER AND AGING
Several broad hypotheses have proposed potential mechanisms for the association between cancer and aging, whereby the biological processes of aging could influence the development and/or progression of cancer in older adults. The processes interact at multiple levels; for example, tumor protein 53 (p53)—a tumor suppressor—is involved in both cancer and aging: alteration of the p53 gene (TP53) is the most frequently encountered mutation in human cancers (including bladder cancer), and the efficiency of the response to p53 has been found to vary according to age. In a mouse study, Feng et al.28 reported that the efficiency of the p53 response was significantly reduced in older mice compared with their younger counterparts. The reduced response predominantly resulted from decreased transcriptional activity and p53-dependent apoptosis; decreased stabilization of p53 after stress was found to be the major factor in this decline.
Age-related changes in mutational frequency and epigenetics may also contribute to the develop ment of bladder cancer in later life. Stuart et al.29 used the Big Blue® lacI transgene in vivo mouse model (Stratagene, La Jolla, CA) to measure mutational frequency. They found that mutational frequency in the bladder increased with age at a rate greater than any other tissue examined, but that the spectrum of mutations in the bladder was identical to those found in younger animals. These findings indicate that the mutations seen in the older mice originated from the DNA lesions that occurred at an earlier time point, and accumulated during replication rather than with genetic damage over time. Furthermore, no remarkable age-related mutational changes resulting from oxidative damage, changes in the fidelity of DNA polymerase, or the efficiency of DNA repair were observed. Taken together, these data indicate that oxidative damage or accumulation of errors in nuclear DNA do not contribute to bladder aging or bladder cancer.
DNA hypermethylation of the promoter regions of tumor suppressor genes has been the most common epigenetic alteration studied. Typically, DNA hypermethylation leads to gene silencing, and, in the case of tumor suppressor genes, results in unchecked cellular replication. Cytosine–guanine linear nucleotide (CpG) island methylation has previously been shown to have a potential role in the pathogenesis of bladder cancer and patients’ outcomes.30-32 In 2007, Marsit et al.33 reported on the prevalence of epigenetic silencing of 16 tumor suppressor genes in a population-based incident case series of 331 patients with bladder transitional cell carcinomas. Age, male sex and smoking were significantly, positively associated with the methylation latent trait. In turn, this epigenetic trait was significantly associated with tumor stage and grade, and an increased risk of death. The protein encoded by the gene deleted in bladder cancer 1 (DBC1) is a putative tumor suppressor. Loss of heterozygosity on chromosome 9 is frequently found in bladder cancer, and DBC1 has been mapped to this region and noted to have multiple CpG islands in exon 1. Habuchi et al.34 reported that CpG hypermethylation of DBC1 was found in 52% of bladder cancer specimens examined, yet CpG hypermethylation did not correlate with tumor stage or grade. Weak methylation was found in normal urothelium, and increased with age. A possibility is that DBC1 hypermethylation is an early event, and accumulation over time may lead to an age-related, hypermethylation-based field defect in normal urothelium. Bornman et al.35 evaluated the role of CpG hypermethylation gene silencing in the decreased expression of E-cadherin, which has been reported in aggressive bladder cancer. The authors found hypermethylation in 43% of tumors evaluated, and they also noted low-level hypermethylation in normal urothelium in 3 out of 9 patients; all 3 patients were aged 70 years or older.
Finally, in a case–control model, Gu et al.36 explored the role of aging on the mRNA expression levels of the four major protectors of genomic integrity; p53, ataxia telangiectasia mutated, telomerase reverse transcriptase and telomeric repeat binding factor 2. When the study participants were divided by age (<57, 57–65 and >65 years), the expression levels were reported to decrease with advancing age in patients with bladder cancer, as well as relative to control cases. The authors suggested that attenuated genomic maintenance mechanisms might lead to increased risk for the development of bladder cancer in the elderly via the resultant increase in genomic instability. These studies are summarized in Table 3.
Table 3.
Summary of the basic science studies assessing the mechanistic interactions between bladder cancer and aging
| Study | Study purpose | Study design | Results |
|---|---|---|---|
| Stuart et al. (2000)29 |
To assess how mutational frequency is affected by age |
Murine study using the Big Blue® lacI transgene in vivo mouse model (Stratagene, La Jolla, CA) |
Mutational frequency in the bladder increased with age, and seemed to accumulate as a result of replication and not accumulation of genetic damage over time. Aging-associated mutational changes were not due to oxidative damage, errors in DNA synthesis, or errors in DNA repair |
| Marsit et al. (2007)33 |
To evaluate tumor suppressor gene hypermethylation |
Population-based, incident case series of 331 patients with bladder transitional cell carcinoma |
Methylation latent trait associated with age, male sex, smoking, tumor stage and grade, and risk of death |
| Habuchi et al. (2001)34 |
To evaluate CpG island hypermethylation of DBC1 gene |
Case series | Hypermethylation of DBC1 was noted in 52% specimens, but was correlated with tumor stage or grade. Weak methylation was noted in normal urothelium and increases with age |
| Bornman et al. (2001)35 |
To evaluate gene silencing of E-cadherin by CpG island hypermethylation |
Case series | Hypermethylation was noted in 43% of tumors. Low-level hypermethylation was noted in normal urothelium in 33% of specimens; all these patients were aged >70 years |
| Gu et al. (2005)36 |
To evaluate the role of mRNA expression levels of the four major protectors of genomic integrity; p53, ATM, hTERT and TRF2 |
Case–control study | In patients with bladder cancer, expression levels of p53, ATM, hTERT and TRF2 decreased with advancing age, and relative to control cases |
Abbreviations: ATM, ataxia telangiectasia mutated; CpG, cytosine–guanine linear nucleotide; DBC1, deleted in bladder cancer 1; hTERT, telomerase reverse transcriptase; p53, tumor protein 53; TRF2, telomeric repeat binding factor 2.
FUTURE DIRECTIONS AND RESEARCH PRIORITIES
Elderly adults with bladder cancer are faced with difficult choices with regard to the optimum management of their condition. Health-care providers need to consider the unique needs of older patients with bladder cancer. When facing any potentially catastrophic illness, issues related to functional independence and quality of life must be considered, as these factors may assume an importance equal to or even greater than that of survival. Many studies fail to include clinical outcome measures that are truly meaningful for older adults; therefore, these studies are not able to appreciate the tremendous variability between individuals as they age. As the earlier discussion indicates, aging represents a very large risk factor for the development of bladder cancer, and may also increase the likelihood of muscle-invasive disease. Nevertheless, based on the available evidence, comorbidity, functional status and frailty may represent far better predictors of undesirable outcomes than chronological age alone. With these considerations in mind, future studies of elderly patients will need to incorporate these other dimensions of health status, as is normally done in the context of a geriatric assessment.37 Clinical domains that should be assessed include function, objective measures of physical performance, comorbidity, nutrition, social support, cognition and depression.
A number of the above domains have been incorporated into a geriatric condition called ‘frailty’.21 Frailty is a syndrome that reflects increased vulnerability and risk of future adverse outcomes such as disability, hospitalization and death. Frailty results from cumulative declines in multiple physiologic systems, and frail patients have a reduced capacity to maintain normal homeostasis in response to common challenges.38 A frailty score, based on measures of unintentional weight loss, sense of exhaustion, weakness, low physical activity and slowed walking speed,21 has been studied in a number of different contexts, and needs to be evaluated for its ability to predict the outcome of different types of treatment in older adults with bladder cancer. The nature of the relationship between frailty and cancer also needs to be defined, given the potential contribution of cancers or cancer treatments to frailty.39 Moreover, shared inflammatory mechanisms may contribute to the pathogenesis of frailty, aging-related changes involving the bladder, and cancer. Chronic bladder inflammation seems to be a significant risk factor for both bladder cancer and detrusor underactivity with urinary retention.40,41 Detrusor underactivity is known to increase with aging in both men and women, yet is more common in men, possibly due to outlet obstruction from the prostate.42 Chronic urinary retention resulting from detrusor underactivity could plausibly lead to increased inflammation in the setting of enhanced exposure to a carcinogen. Chronic inflammation in the form of elevated levels of circulating proinflammatory cytokines, such as interleukin 6, has been linked to frailty, and provides a biomarker predictive of future disability.43 Whether these conditions share common mechanisms that could ultimately be treated with targeted therapy, and whether frailty measures can help provide an evidence-based framework for improving the treatment of older adults with bladder cancer, remains to be seen.
CONCLUSIONS
The optimum management of bladder cancer in the elderly remains unknown. The literature to date indicates that aging may have an adverse role in the response to treatment of superficial disease, and that outcomes for cystectomy may be adversely affected as well. Although authors of numerous retrospective studies have suggested that aggressive treatment is safe, few have been able to demonstrate a clear survival advantage for the elderly patient. Furthermore, only two studies have assessed geriatric function,26,27 which could be as important as, or more important than, survival for some patients. The mechanisms behind the high risk of developing bladder cancer are only beginning to be explored at the basic science level. A research agenda that incorporates age-related changes in the bladder, as well as treatment outcomes based on measurable criteria of frailty, will begin to help shed light on this costly and lethal disease that is most commonly seen in the geriatric population.
REVIEW CRITERIA.
An extensive search of all English-language literature was performed using PubMed and OVID MEDLINE (1950–present) databases for the following key words: “geriatric”, “age”, “aging”, “frailty”, “elderly” and “bladder cancer”. All relevant manuscripts were reviewed for possible inclusion and selected articles cited.
CME.
Medscape Continuing Medical Education online
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit. Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide CME for physicians. Medscape, LLC designates this educational activity for a maximum of 0.75 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To receive credit, please go to http://www.medscape.com/cme/ncp and complete the post-test.
Learning objectives.
Upon completion of this activity, participants should be able to:
Describe the epidemiology of bladder cancer.
Identify the outcomes of treatment of bladder cancer among older adults
Specify valuable predictors of poor treatment outcomes among elderly patients with bladder cancer.
Describe possible genetic mechanisms explaining the interaction between bladder cancer and age.
KEY POINTS.
The annual number of bladder cancer cases in the US is increasing, with the elderly representing the largest group at risk
While age seems to adversely affect treatment outcomes for patients with all stages of bladder cancer, the optimum management of geriatric patients remains unclear
The majority of the data for treatment outcomes in elderly patients come from retrospective data analyses that are limited by selection biases and lack of a true assessment of physiologic reserves in the elderly
Current research into the etiology of bladder cancer in the elderly indicates a potential role for epigenetic events that accumulate over time, as opposed to repeated genetic damage
Future research agendas should be established to study the biology and physiology of aging as it relates to the development and progression of bladder cancer in the elderly, from the perspective of both basic mechanistic and clinical outcomes trials
Acknowledgments
This work was supported by grants from the American Cancer Society MRSG-08-270-01-CCE (JAT), the National Institutes of Health R01-AG028657 (GAK, JAT), a Dennis W Jahnigen Career Development Award from the American Geriatrics Society (JAT) and the Travelers Chair in Geriatrics and Gerontology (GAK). Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Footnotes
Competing interests
The authors and the Journal Deputy Editor S Farley declared no competing interests. The CME questions author CP Vega declared that he has served as an advisor or consultant to Novartis, Inc.
References
- 1.Edwards BK, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on US cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 2.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman SM, et al. Geriatric oncology: a field coming of age. J Clin Oncol. 2007;25:1821–1823. doi: 10.1200/JCO.2007.10.6567. [DOI] [PubMed] [Google Scholar]
- 4.Lynch CF, Cohen MB. Urinary system. Cancer. 1995;75:316–329. doi: 10.1002/1097-0142(19950101)75:1+<316::aid-cncr2820751314>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society [accessed 21 January 2009];Cancer Facts & Figures 2008. 2008 online. [ http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf]
- 6.Schultzel M, et al. Late age (85 years or older) peak incidence of bladder cancer. J Urol. 2008;179:1302–1305. doi: 10.1016/j.juro.2007.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prout GR, Jr, et al. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: a population-based study. Cancer. 2005;104:1638–1647. doi: 10.1002/cncr.21354. [DOI] [PubMed] [Google Scholar]
- 8.Konety BR, Joslyn SA. Factors influencing aggressive therapy for bladder cancer: an analysis of data from the SEER program. J Urol. 2003;170:1765–1771. doi: 10.1097/01.ju.0000091620.86778.2e. [DOI] [PubMed] [Google Scholar]
- 9.Briggs NC, et al. Age as a predictor of an aggressive clinical course for superficial bladder cancer in men. Cancer. 1992;69:1445–1451. doi: 10.1002/1097-0142(19920315)69:6<1445::aid-cncr2820690623>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Ries LA, et al. [accessed 21 January 2009];SEER Cancer Statistics Review, 1975–2005. 2008 December 31; online. [ http://seer.cancer.gov/csr/1975_2005/]
- 11.Cooksley CD, et al. Clinical model of cost of bladder cancer in the elderly. Urology. 2008;71:519–525. doi: 10.1016/j.urology.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 12.Joudi FN, et al. The impact of age on the response of patients with superficial bladder cancer to intravesical immunotherapy. J Urol. 2006;175:1634–1639. doi: 10.1016/S0022-5347(05)00973-0. [DOI] [PubMed] [Google Scholar]
- 13.Herr HW. Age and outcome of superficial bladder cancer treated with bacille Calmette–Guérin therapy. Urology. 2007;70:65–68. doi: 10.1016/j.urology.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Rodin MB, Mohile SG. Assessing decisional capacity in the elderly. Semin Oncol. 2008;35:625–632. doi: 10.1053/j.seminoncol.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Wood DP, Jr, et al. Radical cystectomy for carcinoma of the bladder in the elderly patient. J Urol. 1987;138:46–48. doi: 10.1016/s0022-5347(17)42983-1. [DOI] [PubMed] [Google Scholar]
- 16.Skinner EC, et al. Radical cystectomy in the elderly patient. J Urol. 1984;131:1065–1068. doi: 10.1016/s0022-5347(17)50808-3. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa A, et al. Treatment of bladder carcinoma in patients more than 80 years old. J Urol. 1985;134:889–891. doi: 10.1016/s0022-5347(17)47510-0. [DOI] [PubMed] [Google Scholar]
- 18.Chamie K, et al. Cystectomy in the elderly: does the survival benefit in younger patients translate to the octogenarians? BJU Int. 2008;102:284–290. doi: 10.1111/j.1464-410X.2008.07636.x. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen ME, et al. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol. 2007;51:699–706. doi: 10.1016/j.eururo.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Figueroa AJ, et al. Radical cystectomy for elderly patients with bladder carcinoma: an updated experience with 404 patients. Cancer. 1998;83:141–147. doi: 10.1002/(sici)1097-0142(19980701)83:1<141::aid-cncr19>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 22.Soulié M, et al. A multicenter study of the morbidity of radical cystectomy in select elderly patients with bladder cancer. J Urol. 2002;167:1325–1328. [PubMed] [Google Scholar]
- 23.Gamé X, et al. Radical cystectomy in patients older than 75 years: assessment of morbidity and mortality. Eur Urol. 2001;39:525–529. doi: 10.1159/000052498. [DOI] [PubMed] [Google Scholar]
- 24.Zebic N, et al. Radical cystectomy in patients aged ≥75 years: an updated review of patients treated with curative and palliative intent. BJU Int. 2005;95:1211–1214. doi: 10.1111/j.1464-410X.2005.05507.x. [DOI] [PubMed] [Google Scholar]
- 25.Koppie TM, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–2392. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 26.Stroumbakis N, et al. Radical cystectomy in the octogenarian. J Urol. 1997;158:2113–2117. doi: 10.1016/s0022-5347(01)68171-0. [DOI] [PubMed] [Google Scholar]
- 27.Weizer AZ, et al. Performance status is a predictor of overall survival of elderly patients with muscle invasive bladder cancer. J Urol. 2007;177:1287–1293. doi: 10.1016/j.juro.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 28.Feng Z, et al. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci USA. 2007;104:16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart GR, et al. Mutation frequency and specificity with age in liver, bladder and brain of lacI transgenic mice. Genetics. 2000;154:1291–1300. doi: 10.1093/genetics/154.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maruyama R, et al. Aberrant promoter methylation profile of bladder cancer and its relationship to clinicopathological features. Cancer Res. 2001;61:8659–8663. [PubMed] [Google Scholar]
- 31.Marsit CJ, et al. Carcinogen exposure and gene promoter hypermethylation in bladder cancer. Carcinogenesis. 2006;27:112–116. doi: 10.1093/carcin/bgi172. [DOI] [PubMed] [Google Scholar]
- 32.Jones PA, et al. DNA methylation in bladder cancer. Eur Urol. 1998;33(Suppl 4):7–8. doi: 10.1159/000052251. [DOI] [PubMed] [Google Scholar]
- 33.Marsit CJ, et al. Promoter hypermethylation is associated with current smoking, age, gender and survival in bladder cancer. Carcinogenesis. 2007;28:1745–1751. doi: 10.1093/carcin/bgm116. [DOI] [PubMed] [Google Scholar]
- 34.Habuchi T, et al. Hypermethylation at 9q32–33 tumour suppressor region is age-related in normal urothelium and an early and frequent alteration in bladder cancer. Oncogene. 2001;20:531–537. doi: 10.1038/sj.onc.1204122. [DOI] [PubMed] [Google Scholar]
- 35.Bornman DM, et al. Methylation of the E-cadherin gene in bladder neoplasia and in normal urothelial epithelium from elderly individuals. Am J Pathol. 2001;159:831–835. doi: 10.1016/S0002-9440(10)61758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu J, et al. Roles of tumor suppressor and telomere maintenance genes in cancer and aging—an epidemiological study. Carcinogenesis. 2005;26:1741–1747. doi: 10.1093/carcin/bgi126. [DOI] [PubMed] [Google Scholar]
- 37.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 38.Walston J, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 39.Monfardini S, Basso U. Oncological causes of frailty in older cancer patients. Eur J Cancer. 2007;43:1230–1231. doi: 10.1016/j.ejca.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Michaud DS. Chronic inflammation and bladder cancer. Urol Oncol. 2007;25:260–268. doi: 10.1016/j.urolonc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JA, et al. Null mutation in macrophage migration inhibitory factor prevents muscle cell loss and fibrosis in partial bladder outlet obstruction. Am J Physiol Renal Physiol. 2006;291:F1343–F1353. doi: 10.1152/ajprenal.00144.2006. [DOI] [PubMed] [Google Scholar]
- 42.Taylor JA, III, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54:1920–1932. doi: 10.1111/j.1532-5415.2006.00917.x. [DOI] [PubMed] [Google Scholar]
- 43.Leng SX, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]



