Abstract
The proper coordination between DNA replication and mitosis during cell cycle progression is crucial for genomic stability. During G2 and mitosis, Set8 catalyzes monomethylation of histone H4 on lysine 20 (H4K20me1), which promotes chromatin compaction. Set8 levels decline in S phase, but why and how this occurs is unclear. Here, we show that Set8 is targeted for proteolysis in S phase and in response to DNA damage by the E3 ubiquitin ligase, CRL4Cdt2. Set8 ubiquitylation occurs on chromatin, and is coupled to DNA replication via a specific degron in Set8 that binds PCNA. Inactivation of CRL4Cdt2 leads to Set8 stabilization and aberrant H4K20me1 accumulation in replicating cells. Transient S phase expression of a Set8 mutant lacking the degron promotes premature H4K20me1 accumulation and chromatin compaction, and triggers a checkpoint-mediated G2 arrest. Thus, CRL4Cdt2-dependent destruction of Set8 in S phase preserves genome stability by preventing aberrant chromatin compaction during DNA synthesis.
Introduction
DNA replication and other cell-cycle events, such as replication origin licensing in G1 and chromatin condensation in mitosis, are carefully coordinated to maintain genomic stability. The process of DNA replication is coupled with several other events, including chromatin assembly, sister-chromatid cohesion, ubiquitylation of specific cell-cycle regulators, activation of the DNA replication checkpoint, and DNA repair. Recent studies showed that the CRL4Cdt2 E3 ubiquitin ligase, which functions in a replication-coupled manner through binding to PCNA, plays a critical role in coordinating origin licensing in G1 and DNA replication in S phase (Jin et al., 2006; Kim et al., 2008; Lovejoy et al., 2006; Sansam et al., 2006; Zhong et al., 2003). To understand whether CRL4Cdt2 has additional roles in coordinating DNA replication with other cell-cycle events, we sought to identify additional CRL4Cdt2 substrates.
The CRL4Cdt2 E3 ligase complex is comprised of the scaffold protein Cul4, the adaptor protein Ddb1, and the putative substrate receptor protein Cdt2 (Angers et al., 2006; Higa et al., 2006; Jin et al., 2006; Sansam et al., 2006). The best-characterized substrate of CRL4Cdt2 is the “licensing” factor Cdt1, which is required to recruit the MCM2-7 complex to replication origins in G1. During DNA replication, Cdt1 binds to PCNA through a PCNA interacting protein motif (PIP box), and is degraded on chromatin in a PCNA- and CRL4Cdt2-dependent manner (Arias and Walter, 2005; Arias and Walter, 2006; Jin et al., 2006; Nishitani et al., 2006; Sansam et al., 2006; Senga et al., 2006). This replication-coupled mechanism for Cdt1 degradation ensures that fired replication origins cannot be re-licensed in the same S phase. The CRL4Cdt2-mediated degradation of Cdt1 occurs not only in S phase, but also after DNA damage (Higa et al., 2006; Higa et al., 2003; Hu et al., 2004; Hu and Xiong, 2006; Jin et al., 2006; Sansam et al., 2006; Senga et al., 2006). When it is bound to PCNA on chromatin, the PIP box of Cdt1 is presented as a degron and recognized by CRL4Cdt2. Our analysis of the PIP degron of Cdt1 has identified three sequence elements critical for binding to PCNA and Cdt2, which are conserved among known CRL4Cdt2 substrates (Havens and Walter, 2009).
In a genome-wide search for PIP degron-containing proteins, we identified Set8 (KMT5A/PR-Set7/SETD8) as a potential substrate of CRL4Cdt2. Set8 is the methyltransferase that monomethylates histone H4 on lysine 20 (H4K20me1) (Fang et al., 2002; Nishioka et al., 2002). Loss of Set8 in human, mouse, or Drosophila cells results in massive DNA damage during S phase and improper chromosome condensation in mitosis (Houston et al., 2008; Huen et al., 2008; Jorgensen et al., 2007; Karachentsev et al., 2005; Oda et al., 2009; Paulsen et al., 2009; Sakaguchi and Steward, 2007; Tardat et al., 2007). During the cell cycle, Set8 is most abundant during G2 and mitosis, and low during S phase (Huen et al., 2008; Oda et al., 2009; Yin et al., 2008). Concomitant with the elevation of its abundance in the G2 and M phases, Set8 promotes a transient accumulation of H4K20me1 (Houston et al., 2008; Huen et al., 2008; Oda et al., 2009; Rice et al., 2002). H4K20me1, which promotes chromatin compaction, may contribute to proper mitosis and impact the subsequent S phase (Houston et al., 2008; Oda et al., 2009; Sakaguchi and Steward, 2007; Trojer et al., 2007). While Set8 has a clear role in methylating H4K20 during mitosis, why and how it is down regulated during S phase is not clear. Interestingly, in the presence of proteasome inhibitors, Set8 is readily detected in S-phase cells and it colocalizes with the DNA replication protein PCNA (Huen et al., 2008; Jorgensen et al., 2007; Tardat et al., 2007). Furthermore, Set8 contains two PIP boxes that contribute to its binding to PCNA (Huen et al., 2008; Jorgensen et al., 2007).
In this study, we show that Set8 is degraded by a CRL4Cdt2-mediated mechanism during S phase and in response to DNA damage. The degradation of Set8 relies on its PIP degron and its interactions with both PCNA and Cdt2. This mechanism of Set8 degradation is observed not only in human cells, but also in Xenopus egg extracts. In the cell-free Xenopus system, Set8 is ubiquitylated on chromatin and destroyed in a PCNA-, Cdt2-, and PIP degron-dependent manner. Ablation of CRL4Cdt2 in human cells leads to stabilization of endogenous Set8 and aberrant accumulation of H4K20me1 in S-phase cells. When a stabilized PIP box mutant of Set8 is expressed in S-phase cells, it induces premature H4K20me1 accumulation and chromatin compaction, and triggers a checkpoint-mediated G2 arrest. We propose that CRL4Cdt2-mediated degradation of Set8 prevents H4K20me1 accumulation during S phase, thereby preventing premature chromatin compaction that interferes with genome duplication. The replication-coupled down regulation of Set8 is a critical mechanism that defines the functional window of Set8 during the cell cycle, contributing to the orderly execution of DNA replication and mitosis.
Results
Set8 is down-regulated by CRL4Cdt2 in cycling cells and in response to DNA damage
Our recent studies revealed three sequence elements that are conserved in known CRL4Cdt2 substrates and together comprise a ‘PIP degron’ (Fig. 1A): one is a canonical PIP box that is essential for PCNA binding, another is a TD motif at positions 5 and 6 of the PIP box that confers high affinity binding to PCNA, and the third is a basic residue located 4 amino acids downstream of the PIP box that is important for Cdt2 recruitment to the substrate-PCNA complex on chromatin (Havens and Walter, 2009). To identify additional substrates of the CRL4Cdt2 E3 ligase, we searched the Swiss-Prot database using ExPASy ScanProsite with the PIP degron consensus motif (Q/N-x-x-L/V/I/M-T-D-F/Y-F/Y-x-x-x-K/R). Like Cdt1 and p21, two known CRL4Cdt2 substrates (Abbas et al., 2008; Kim et al., 2008; Nishitani et al., 2008), Set8 was identified in this screen. Notably, the PIP degron sequence in Set8 was perfectly conserved across a wide range of metazoan organisms (Fig. 1A). This finding prompted us to investigate whether the stabilities of Set8 and Cdt1 were similarly regulated in cells. Both Set8 and Cdt1 are down regulated during S phase, and Cdt1 is degraded in response to DNA damage (Higa et al., 2003; Hu et al., 2004). A previous study showed that Set8 levels were reduced after DNA damage due to transcription repression (Shi et al., 2007). We found that in U2OS cells treated with 50 J/m2 UV, the level of endogenous Set8 rapidly declined within 1 hr (Fig. 1B, lanes 1–3). Furthermore, the UV-induced reduction of Set8 was prevented by MG132, suggesting that Set8 is degraded by the proteasome in response to DNA damage (Fig. 1B). The DNA damage-induced degradation of Cdt1 is independent of the checkpoint kinases ATM and ATR (Higa et al., 2003). Analogously, wortmannin, an inhibitor of ATM and ATR, and UCN-01, an inhibitor of the ATR effector kinase Chk1, had no effect on the UV-induced degradation of Set8 (Fig. 1C). These results show that Set8, like Cdt1, is degraded in a DNA damage-induced manner independently of ATM and ATR.
Figure 1.
Set8 is degraded by CRL4Cdt2 in response to DNA damage. (A) Sequence alignment of the PIP degrons of human Cdt1, p21, and Set8 from different species. Ψ is any moderately hydrophobic amino acid L, V, I, or M. ϑ is an aromatic residue, Y or F. B is a positively charged residue, R or K. Red residues are conserved in the PIP boxes, and the blue residues are only conserved in the PIP degrons. (B) UV-induced Set8 degradation is 26S proteasome-dependent. U2OS cells were treated with 50J/m2 UV, and the levels of endogenous Set8, Cdt1, and tubulin were monitored at the indicated times by Western blotting. Where indicated, 10 μM MG132 was added to cells 5 hr prior to irradiation. (C) UV-induced Set8 degradation is independent of ATM, ATR, and Chk1. U2OS cells were treated with 1 μM UCN-01 (Chk1 inhibitor) or 100 μM wortmannin (PI3KK inhibitor) for 1 hr prior to UV irradiation. Inhibition of Chk1 phosphorylation serves as a positive control for wortmannin activity. Note that UCN-01 does not inhibit the phosphorylation of Chk1 by ATR, but prevents the mobility change of Chk1 indicative of Chk1 autophosphorylation. (D) CRL4Cdt2 down regulates Set8 protein levels. U2OS cells were transfected with control siRNA or siRNAs targeting Ddb1, Cdt2 and Cul4A. The levels of the indicated proteins were monitored by Western blotting. (E) UV-induced Set8 degradation is Cdt2-dependent. U2OS cells transfected with Cdt2 siRNA or control siRNA were treated with UV followed by 100 μg/ml cycloheximide. The levels of the indicated proteins were analyzed in a time course. In all panels, * labels non-specific bands recognized by the indicated antibodies.
To address whether Set8 is a substrate of the CRL4Cdt2, we used siRNA to knock down the components of this E3 ubiquitin ligase. Compared to cells treated with control siRNA, cells treated with siRNAs targeting Cdt2, Ddb1, or Cul4A exhibited elevated levels of Set8 (Figs. 1D and S1A). In contrast, knockdown of Ddb2, a substrate receptor of the distinct CRL4Ddb2 E3 ligase, did not affect Set8 levels (Fig. S1B). Cdt2 siRNA did not affect the level of Set8 mRNA (Fig. S1C), excluding the possibility that the increase in Set8 protein is due to transcriptional changes. In cells treated with Cdt2 or Ddb1 siRNA, the UV-induced degradation of Set8 was significantly reduced as compared to that in control cells (Figs. 1E and S1D). Taken together, these results suggest that the CRL4Cdt2 E3 ligase down regulates the overall level of Set8 in an asynchronous cell population, and mediates its degradation in response to DNA damage.
UV-induced Set8 degradation is PIP degron-dependent
To assess whether the putative PIP degron of Set8 is required for its degradation, we disrupted the degron using point mutations. The two conserved aromatic residues in the PIP degron of Cdt1 are essential for its binding to PCNA (Arias and Walter, 2006; Havens and Walter, 2009). To determine whether the corresponding residues in the PIP degron of Set8 are functionally important, we generated a Set8 point mutant lacking these residues (F184A, Y185A; referred to as Set8ΔPIP) (Fig. 2A).
Figure 2.
UV-induced Set8 degradation is dependent on its PIP degron. (A) The Set8ΔPIP mutant lacks the two conserved aromatic residues in the putative PIP degron. (B) The Set8ΔPIP mutant is defective in binding to PCNA and Cdt2. Flag-tagged Set8WT and Set8ΔPIP were transiently expressed in U2OS cells and immunoprecipitated with anti-Flag antibody. The Set8, PCNA, and Cdt2 proteins in the immunoprecipitates and input extracts (2%) were analyzed by Western blotting. (C) Set8 ubiquitylation is induced by UV. HeLa cells stably expressing His/biotin-tagged ubiquitin and control HeLa cells were treated with UV or left untreated. Ubiquitylated proteins were captured with streptavidin beads under denaturing condition. Ubiquitylated Set8 was detected with Set8 antibody. * Nonspecific proteins bound to streptavidin beads and cross-reacted with Set8 antibody. (D) UV-induced Set8 ubiquitylation requires the PIP degron. Cells expressing Flag-tagged Set8WT or Set8ΔPIP were synchronized in G1, pretreated with MG132 for 3 hr, and irradiated with UV. The Set8 proteins were analyzed with anti-Flag antibody 2 hr post UV treatment. (E) The Set8ΔPIP mutant is more stable than Set8WT after UV damage. U2OS cells with induced Flag-tagged Set8WT or Set8ΔPIP were treated with UV, and cultured in cycloheximide-containing media for the indicated times. The levels of Flag-Set8 and tubulin were analyzed by Western blotting.
We first tested whether wild type and mutant Set8 proteins are able to associate with PCNA and Cdt2. Immunoprecipitation of Flag-tagged Set8WT captured both PCNA and Cdt2, showing that Set8 can interact with these proteins (Fig. 2B). Consistent with a previous report (Jorgensen et al., 2007), Set8ΔPIP failed to associate with PCNA (Fig. 2B), confirming that this PIP box of Set8 is critical for PCNA binding. Moreover, Cdt2 did not co-precipitate with Set8ΔPIP, suggesting that the PIP box is also needed for Cdt2 binding. These results suggest that the PIP degron of Set8, like that of Cdt1, is required for its interactions with both PCNA and Cdt2. It should be noted that Cdt1 interacts with Cdt2 only in the presence of DNA-bound PCNA (Havens and Walter, 2009). Thus, the interaction between Flag-Set8 and Cdt2 reported here might also be mediated by PCNA and DNA.
We next asked if the PIP degron of Set8 is required for its degradation in response to DNA damage. In cells expressing His/biotin-tagged ubiquitin, endogenous Set8 was ubiquitylated and captured by streptavidin beads in a UV-induced manner (Fig. 2C). Furthermore, Flag-tagged Set8WT, but not Set8ΔPIP, underwent enhanced ubiquitylation after UV treatment (Fig. 2D). When transiently expressed in cells, Set8WT was less stable than Set8ΔPIP after UV irradiation (Fig. S2). To more precisely measure the effects of PIP-box mutations on Set8 stability, we generated inducible cell lines that express Flag-tagged Set8WT or Set8ΔPIP. We induced the Set8 proteins to similar levels, treated cells with UV and cycloheximide, and monitored the stabilities of the Set8 proteins in a time course. Set8ΔPIP was more stable than Set8WT after UV irradiation (Fig. 2E). These data suggest that UV-induced Set8 ubiquitylation and degradation require the PIP degron of Set8.
CRL4Cdt2 mediates Set8 degradation in Xenopus egg extracts
To examine Set8 destruction in a biochemically tractable system, we turned to Xenopus egg extracts, which recapitulate the DNA replication and damage dependent degradation of Cdt1 by CRL4Cdt2 (Jin et al., 2006). As shown in Figure 3A, Cdt1 was rapidly destroyed in a high-speed supernatant (HSS) of Xenopus egg cytoplasm supplemented with plasmid DNA that had been damaged with methyl methanesulfonate (MMS) (Figure 3A, bottom panel) (Jin et al., 2006). Under the same experimental conditions, GST-Flag-tagged human Set8 was also rapidly degraded (Fig. 3A, top panel). As seen for Cdt1, Set8 degradation was induced by MMS-treated DNA, but not by undamaged DNA or in the absence of DNA (Fig. 3A), showing that this is a DNA damage-dependent process. Depletion of PCNA or Cdt2 efficiently prevented DNA damage-induced destruction of Set8 (Figs. 3B–C, and S3A). In each case, Set8 degradation was rescued by reconstitution of the extracts with the corresponding recombinant protein (Figs. 3B–C). Furthermore, Set8ΔPIP was completely stable in egg extracts (Fig. 3D, middle panel). Therefore, human Set8 is degraded in Xenopus egg extracts in a manner that depends on its PIP degron, PCNA, Cdt2, and DNA damage.
Figure 3.
CRL4 Cdt2-dependent and DNA damage-induced Set8 degradation in Xenopus egg extracts. (A) DNA damage and proteasome dependent destruction of Set8 in Xenopus egg extract. HSS was supplemented with human 50 nM GST-FLAG-Set8, as well as buffer (no DNA), undamaged plasmid, MMS-damaged plasmid, and MG132, as indicated, and at different times, samples were blotted for GST (top panel) or Cdt1 (bottom panel). (B) 50 nM recombinant GST-FLAG-tagged human Set8 was added to mock-depleted or PCNA-depleted HSS that was optionally supplemented with 5 μM recombinant human PCNA. At the different times, samples were blotted for GST. (C) 50 nM recombinant GST-Flag-tagged human Set8 was added to mock-depleted or Cdt2-depleted HSS that was optionally supplemented with 25 nM recombinant human Cdt2, as indicated. At different times, samples were blotted for GST. (D) HSS was supplemented with MMS-treated plasmid, as well as buffer, 50 nM GST-Flag-tagged Set8WT, or Set8ΔPIP. At different times, samples were blotted for the indicated proteins. (E) HSS was supplemented with 1 mg/ml Myc-Ubiquitin and MG132, as well as buffer or 50 nM GST-Flag-tagged Set8WT, as indicated. Immobilized, 1 kb MMS DNA was added and after 10 min, chromatin was recovered from the extract. Chromatin-bound proteins were denatured to release them from chromatin then diluted and immunoprecipitated with Flag antibody. The isolated material was blotted for Set8 (bottom panel), Ubiquitin (middle panel), or Myc (top panel). (F) Immobilized, 1 kb MMS DNA was isolated from mock-depleted or Cdt2-depleted HSS, each of which was supplemented with 2 mg/ml methyl-ubiquitin and 50 nM GST-Flag-tagged Set8 and incubated for 10 min. Samples were blotted for Cdt2, Set8, and PCNA. (G) Methyl-ubiquitin, GST-Flag-tagged Set8WT or Set8ΔPIP, and 5 ng/μl MMS plasmid DNA was added to HSS. Set8WT, Set8ΔPIP, and their associating proteins were immunoprecipitated from total extract with Flag antibody, and the indicated proteins were analyzed by Western blotting. * A protein nonspecifically recognized by PCNA antibody.
When Set8WT was added to HSS, it bound to chromatin and recruited CRL4Cdt2 (Fig. S3B). Moreover, Set8 was ubiquitylated on the chromatin (Figs. 3E), but this was reduced when Cdt2 was depleted from HSS (Fig. 3F). In contrast to Set8WT, Set8ΔPIP did not bind chromatin efficiently (Fig. S3B), failed to recruit Cdt2 or Ddb1 to chromatin above background levels (Figs. S3C), and was not efficiently ubiquitylated (Figs. 3G and S3C). Together, these results indicate that like Cdt1, Set8 docks onto chromatin-bound PCNA, recruits CRL4Cdt2, and then undergoes ubiquitylation. Interestingly, addition of Set8WT but not Set8ΔPIP to HSS resulted in decreased ubiquitylation of Cdt1 (Fig. S3C), suggesting a competition between the two CRL4Cdt2 substrates.
Set8 is degraded during S phase in a CRL4Cdt2-dependent manner
Having established that Set8 is a substrate of CRL4Cdt2 following DNA damage, we next investigated whether Set8 is targeted for destruction by CRL4Cdt2 during DNA replication, as suggested by the elevated Set8 levels observed after silencing of CRL4Cdt2 components in unperturbed populations of asynchronous cells (Fig. 1D). We first synchronized cells in S phase with hydroxyurea (HU), and then briefly induced expression of Flag-Set8WT. Once Flag-Set8WT became readily detectable, we stopped Set8 induction and released cells from HU. As cells resumed DNA replication after the release from HU, the levels of Flag-Set8WT rapidly declined (Fig. 4A; FACS profiles shown in Fig. S4A). This decline of Set8WT was inhibited by MG132 (Fig. 4A), suggesting that Set8 is actively degraded by the proteasome in replicating cells. Consistently, endogenous Set8 was rapidly degraded in S-phase cells synchronously released from a thymindine block (Fig. 4B). Furthermore, Set8WT was also rapidly degraded during chromosomal DNA replication in Xenopus egg extracts (Fig. 4C). Geminin, which blocks replication initiation via inhibition of Cdt1 function, prevented Set8WT destruction in this setting, demonstrating that Set8 is degraded in a replication-dependent manner in Xenopus egg extracts.
Figure 4.
PIP degron-mediated Set8 degradation during DNA replication. (A) Set8 is actively degraded by the proteasome in replicating cells. Cells harboring inducible Flag-Set8 were synchronized in S phase with 1 mM HU for 24 hr. During the last 4 hr of HU treatment, Flag-Set8 was induced, and 10 μM MG132 was added when indicated. Subsequently, cells were released into HU-free and cycloheximide-containing media with or without MG132. Set8 levels were analyzed in a time course by Western blotting. (B) Endogenous Set8 is stabilized by knockdown of Cdt2, but not Ddb2. Cells transfected with siRNAs targeting Cdt2 or Ddb2 and cells mock transfected were synchronized with thymidine and released into cycloheximide-containing media. The levels of endogenous Set8 were analyzed at the indicated time points by Western blotting. (C) Set8 is degraded in Xenopus egg extracts in a replication- and PIP degron-dependent manner. Sperm chromatin was incubated with HSS for 30 min to promote replication licensing. Subsequently, a highly concentrated nucleoplasmic extract (Walter et al., 1998) containing GST-Flag-tagged Set8WT or Set8ΔPIP was added, which stimulated efficient replication initiation (data not shown). At different times after NPE addition, the reactions were stopped and samples were blotted for GST and Cdt1. In lanes 1–3, HSS was incubated with 200 nM Geminin before addition of sperm chromatin.
To test if the degradation of Set8 during DNA replication was mediated by CRL4Cdt2, we monitored the effect of Cdt2 knockdown on the stability of endogenous Set8. Knockdown of Cdt2 but not Ddb2 significantly stabilized Set8 in replicating cells (Fig. 4B). To determine whether the PIP degron of Set8 is needed for its degradation in S phase, we compared the stabilities of Set8WT and Set8ΔPIP during DNA replication. In Xenopus egg extracts, the degradation of Set8 during DNA replication was dramatically inhibited by mutations in the PIP degron (Fig. 4C). In human cells synchronously released from HU, Set8ΔPIP was more stable than Set8WT (Fig. S4B). Nonetheless, we noted that in replicating human cells, Set8ΔPIP was still degraded at a slow rate even when Cdt2 was knocked down (Figs. S4B–S4C), suggesting that a PIP degron- and Cdt2-independent mechanism also contributes to Set8 destruction (see discussion). Taken together, these results suggest that CRL4Cdt2 plays an important role in repressing Set8 levels during S phase.
Forced expression of Set8 induces replication stress
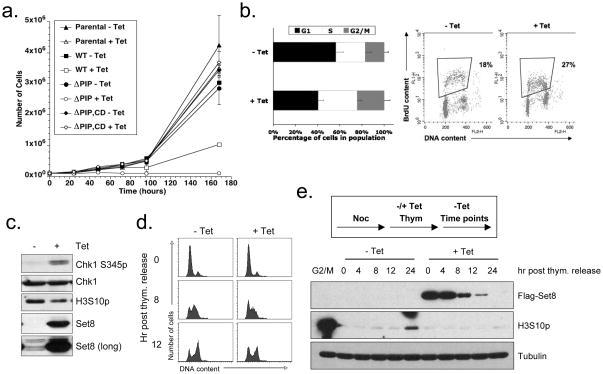
To understand why Set8 is down regulated during S phase, we sought to override this mechanism. When Set8WT was constitutively expressed at high levels, cell proliferation gradually slowed down (Fig. 5A). FACS analysis of cells expressing Set8WT revealed an increase in the population of S and G2/M phase cells (Fig. 5B, left panel). BrdU-labeling confirmed that the population of replicating cells was increased upon Set8WT induction (Fig. 5B, right panel). Moreover, for cells that were in S phase based on DNA content, the incorporation of BrdU was clearly less efficient when Set8WT was induced relative to control cells (Fig. 5B, right panel). These results suggest that constitutive Set8 expression interferes with DNA synthesis, leading to accumulation of cells in S phase and G2/M.
Figure 5.
Forced expression of Set8 interferes with the cell cycle. (A) Forced Set8 expression slows cell proliferation. U2OS cells harboring inducible Flag-tagged Set8WT, Set8ΔPIP, Set8ΔPIP,CD, or the parental cell line were cultured in the absence or presence of 0.1 μg/ml tetracycline (Tet). The total cell numbers at the indicated time points were plotted. Error bars indicate the standard deviation of three independent experiments. (B) Constitutive Set8 expression leads to reduced DNA synthesis and accumulation of cells in G2/M. Cells were cultured for 48 hr with or without Set8 induction. Cell cycle profiles (left panel) and BrdU incorporation (right panel) were analyzed by FACS. (C) Constitutive expression of Set8 elicits the ATR checkpoint. Cells were cultured for 48 hr with or without Set8 induction. The levels of Set8, phospho-Chk1 (Ser345), Chk1, and phospho-H3 (Ser10) were analyzed by Western blotting. (D–E) Overexpression of Set8 prior to S phase inhibits mitotic entry. Cells were synchronized in mitosis with 100 ng/ml nocodazole and then released into media containing 2 mM thymidine. Where indicated, Set8 was induced after the release from nocodazole. Cells synchronized at G1/S were then released from thymidine in the absence of Tet. Cell cycle profiles are shown in D, and protein levels of Flag-Set8, phospho-H3, and tubulin were analyzed by Western blotting in E.
The reduction in DNA synthesis and accumulation of cells in S and G2/M prompted us to investigate whether the ATR-mediated replication checkpoint was activated by forced Set8WT expression. Indeed, when Set8WT was induced for 48 hr in asynchronous cells, the level of phospho-Chk1 (Ser345), a marker of ATR activation, was significantly increased (Fig. 5C). Furthermore, consistent with compromised DNA replication and/or checkpoint-mediated cell cycle arrest prior to mitosis, the levels of phospho-H3 were reduced (Fig. 5C). These results provide further evidence that aberrant expression of Set8 interferes with proper DNA replication and activates the ATR checkpoint.
Since Set8 is known to function in mitosis, overexpression of Set8 may affect M phase and indirectly impact the subsequent S phase. To rule out this possibility, we synchronously released cells from a nocodazole block in mitosis, transiently induced Set8 expression, and arrested cells at the G1/S transition with thymidine. When cells were released into S phase with high levels of Set8, they progressed through S phase more slowly, as shown by FACS analysis (Figs. 5D). Furthermore, cells with induced Set8 did not accumulate phospho-H3 (Ser10), indicating a failure to enter mitosis (Fig. 5E). These results suggest that forced expression of Set8 prior to S phase interferes with DNA replication and prevents timely entry into mitosis.
The Set8ΔPIP mutant is a potent inhibitor of the cell cycle
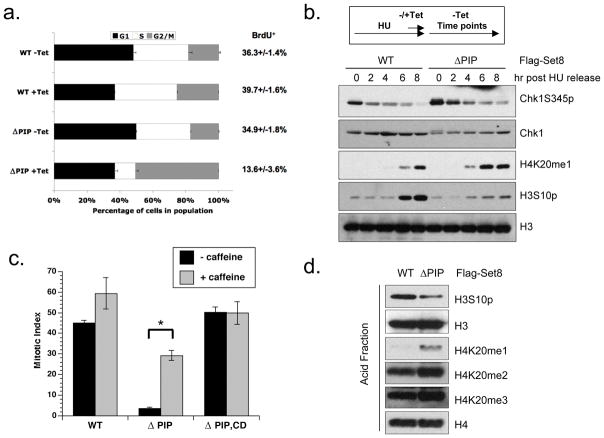
Although induction of wild type Set8 interferes with the cell cycle, this approach may not fully capture the effects of Set8 stabilization because even the induced Set8WT is rapidly degraded (Fig. 4). To more specifically address the role of CRL4Cdt2-mediated Set8 degradation, we induced expression of Set8WT and Set8ΔPIP, and compared their effects on the cell cycle. Compared to Set8WT, Set8ΔPIP induced a much more prominent increase in G2/M cells (Fig. 6A). Furthermore, expression of Set8ΔPIP dramatically reduced the BrdU-positive cells, suggesting that cells were trapped outside of S phase (Figs. 6A and S5A). Consistent with its effects on the cell cycle, Set8ΔPIP reduced cell proliferation even more dramatically than Set8WT (Fig. 5A). Thus, Set8 is a potent inhibitor of the cell cycle when the PIP degron is disrupted.
Figure 6.
Expression of the Set8ΔPIP mutant induces premature H4K20me1 accumulation. (A) Constitutive expression of the Set8ΔPIP mutant leads to a dramatic loss of S-phase cells and accumulation of G2/M cells. Cell cycle profiles and BrdU incorporation of cells expressing Set8WT or Set8ΔPIP for 48 hr. (B–D) Expression of the ΔPIP mutant prevents mitotic entry after release from HU arrest. In B, cells harboring inducible Set8WT or Set8ΔPIP were synchronized in S phase with HU for 24 hr. During the last 4 hr in HU, expression of Set8WT or Set8ΔPIP was induced. Subsequently, cells were released from HU in the absence of Tet, and the levels of the indicated proteins in whole-cell extracts were analyzed by Western blotting. In C, Set8ΔPIP,CD cells were included. Experiment was performed as in B, except that cells were released into media containing nocodazole to trap mitotic cells. Where indicated, caffeine was added at 5 mM to bypass ATM/ATR-mediated checkpoint response. Twenty-four hr after HU release, phospho-H3-positive mitotic cells were scored by FACS. Error bars indicate standard deviation and * indicates p < 0.005 by student’s t-test. In D, experiment was performed as in B, except that 4 hr after HU release, chromatin-bound histones were extracted with acid and analyzed by Western blotting.
To determine whether the effects of Set8ΔPIP on the cell cycle are dependent upon its catalytic activity, we generated a catalytically “dead” Set8ΔPIP, CD double mutant (Nishioka et al., 2002). In marked contrast to Set8ΔPIP, Set8ΔPIP, CD did not induce a G2/M arrest (Fig. 6C), nor did it inhibit cell proliferation (Fig. 5A). These results show that Set8ΔPIP remains catalytically active in cells, and that its activity is necessary for the induction of cell cycle arrest.
The Set8ΔPIP mutant triggers a checkpoint-mediated G2 arrest
The loss of cells in S phase and accumulation of cells in G2/M indicates that Set8ΔPIP may interfere with DNA replication and/or the G2/M transition. To assess this possibility, we synchronized cells in S phase with double-thymidine block or HU, briefly induced Set8WT or Set8ΔPIP, and then terminated the induction and released the cells. As cells resumed DNA replication, the levels of phospho-Chk1 gradually declined (Figs. 6B and S5B). In cells with Set8ΔPIP, Chk1 was phosphorylated to higher levels in thymidine and HU, and phospho-Chk1 persisted for longer after cells resumed replication (Figs. 6B and S5B). Despite the persistent Chk1 phosphorylation, cells with Set8ΔPIP progressed through S phase without obvious delay (data not shown). However, both FACS and Western blotting analyses showed that while cells with Set8WT gradually entered mitosis and accumulated phospho-H3 over time, cells with Set8ΔPIP failed to accumulate significant levels of this mitotic mark (Figs. 6B and S5B–S5C). Thus, when briefly expressed in S-phase cells, Set8ΔPIP does not arrest replication but leads to a robust G2 arrest.
The modest but persistent Chk1 phosphorylation induced by Set8ΔPIP during S phase suggests that although DNA replication can proceed, the process is not normal. Although the effects of Set8ΔPIP are not sufficient to halt replication, they may contribute to the subsequent G2 arrest. Indeed, in the presence of caffeine, an inhibitor of ATM and ATR, the G2 arrest induced by Set8ΔPIP was significantly bypassed (Fig. 6C). Importantly, the catalytically inactive Set8ΔPIP,CD mutant did not induce a G2 arrest (Fig. 6C), showing that the catalytic activity of Set8ΔPIP is needed to trigger the checkpoint.
The Set8ΔPIP mutant leads to aberrant H4K20me1 accumulation during DNA replication
As cells resumed DNA replication after the release from HU or thymidine, H4K20me1 accumulated significantly faster in cells expressing Set8ΔPIP than in cells expressing Set8WT (Figs. 6B, 6D, and S5B–C). In cells expressing Set8WT, the levels of H4K20me1 rose shortly before mitosis, and the levels of H4K20me2/me3 increased modestly following H4K20me1 accumulation (Figs. S5B–C). In cells expressing Set8ΔPIP, the levels of H4K20me1 rose in S phase, whereas the levels of H4K20me2/me3 did not change significantly even after cells were arrested in G2 (Figs. 6D and S5B–C). These results suggest that H4K20me1 is the primary, if not the only, form of H4K20 methylation that is rapidly induced by Set8ΔPIP during S phase.
We also examined the accumulation of H4K20me1 by immunostaining in cells expressing Set8WT or Set8ΔPIP. In cells expressing Set8WT, PCNA staining and H4K20me1 staining were mutually exclusive (Figs. 7A and S6A), consistent with the degradation of Set8 in replicating cells and its accumulation in G2/M. In marked contrast, a significant fraction of S-phase cells expressing Set8ΔPIP were positive for both PCNA and H4K20me1 (Figs. 7A and S6A). In addition, a fraction of the Set8ΔPIP cells labeled with EdU were positive for H4K20me1, showing that this histone mark is aberrantly established in cells undergoing replication (Fig. S6B). Furthermore, PCNA and H4K20me1 significantly colocalized with each other in cells containing Set8ΔPIP (Fig. 7B), indicating that H4K20me1 prematurely accumulated at or around replication forks. In contrast to Set8ΔPIP, Set8ΔPIP,CD did not promote H4K20me1 accumulation in PCNA-positive cells (Figs. 7A and S6A), indicating that the ability of Set8ΔPIP to induce H4K20me1 in replicating cells is dependent upon its catalytic activity.
Figure 7.
The Set8ΔPIP mutant induces aberrant chromatin compaction in replicating cells. (A) Transient expression of the ΔPIP mutant in S-phase cells leads to aberrant co-staining of PCNA and H4K20me1. Cells were arrested in S phase with HU for 24 hr, and Set8WT or Set8ΔPIP was transiently induced during the last 4 hr. Cells were subsequently released from HU, and were stained with antibodies to PCNA and H4K20me1 4 hr after HU release. (B) Co-localization of PCNA and H4K20me1 in the replicating cells that express Set8ΔPIP. (C) Knockdown of Cdt2, but not Ddb2, leads to accumulation of H4K20me1 in PCNA-positive cells. Cells were transfected with siRNAs targeting Cdt2 and Ddb2, or mock treated. The levels of H4K20me1 and PCNA were analyzed by immunostaining. (D–E) The Set8ΔPIP mutant reduces the distance between two loci on chromosome 16 in replicating cells. Cells were synchronized and induced to express Set8WT, Set8ΔPIP, or Set8ΔPIP,CD as in A, and released for 6 hours. Dual colored FISH probes were used to visualize 16q22 and 16p13. Representative images of the two loci are shown in D. The distance between the two loci in the indicated cell populations were measured using confocal microscopy (see Materials and Methods). Distances were normalized to the uninduced population of the same cell line, and the average of three independent experiments is plotted in E. Error bars indicate standard deviation. * indicates p < 0.05 and ** indicates p < 0.001 by student’s t-test. (F) The Set8ΔPIP mutant promotes premature binding of condensin II to chromatin in S phase. Cells expressing Set8WT or Set8ΔPIP were synchronized and released as in A, and were subjected to chromatin fractionation 6 hr after HU release. The levels of CAP-D3, CAP-G2, Set8, and PCNA in chromatin fractions were analyzed by Western blotting.
To determine whether stabilization of endogenous Set8 in S phase promotes premature H4K20me1 accumulation, we monitored the effects of Cdt2 knockdown on the levels of H4K20me1 in PCNA-positive cells. Knockdown of Cdt2 led to a significant increase in the PCNA-positive cells with high levels of H4K20me1 (Figs. 7C and S6C), suggesting that stabilization of endogenous Set8 is sufficient to promote H4K20me1 accumulation in otherwise unperturbed, replicating cells. Similar premature H4K20me1 accumulation was induced by Ddb1 knockdown, but not by Ddb2 knockdown (Figs. 7C and S6C). In addition, a significant fraction of the Cdt2 knockdown cells with high H4K20me1 displayed phospho-RPA32 foci, indicating a link between aberrant H4K20me1 accumulation and activation of the ATM and/or ATR checkpoint kinases (Figs. S6D–E).
Set8ΔPIP induces premature chromatin compaction in replicating cells
H4K20me1 is a histone mark important for chromatin compaction, and its accumulation in mitosis is associated with chromatin condensation (Oda et al., 2009; Rice et al., 2002; Trojer et al., 2007). The premature accumulation of H4K20me1 in cells expressing Set8ΔPIP suggested that chromatin may be aberrantly compacted during S phase. To assess this possibility, we first used dual color FISH and confocal microscopy to measure the distance between two loci on chromosome 16 (16q22 and 16p13). In synchronously replicating cells, the average distance between the two loci was significantly reduced by Set8ΔPIP (Figs. 7D–E). Compared to Set8ΔPIP, Set8WT reduced the distance to a lesser extent, and Set8ΔPIP,CD did not affect the distance at all (Figs. 7D–E). These results suggest that the presence of Set8 activity during S phase promotes chromatin compaction.
In a second approach to monitor the effects of Set8 stabilization on chromatin compaction, we analyzed the association of linker histone H1 with chromatin by salt extraction. Coincident with the premature accumulation of H4K20me1 in Set8ΔPIP cells 6 hr after the release from HU (Fig. 6B), H1 was less extractable in Set8ΔPIP cells than in Set8WT cells (Fig. S6F, left panel, lanes 4–5, 9–10), indicating that H1 is more tightly bound to chromatin in the replicating cells with Set8ΔPIP.
To further confirm the effects of Set8 stabilization on chromatin compaction, we analyzed chromatin using micrococcal nuclease (MNase). When Set8ΔPIP was induced in S-phase cells, it reduced the levels of mononucleosomes generated by partial MNase digestion (Fig. S6G left panel, compare mononucleosomes in lanes 2–3 versus lanes 6–7). The ratio of mono- to di-nucleosomes was lower in Set8ΔPIP expressing cells than in control cells after 15 min of MNase digestion (Fig. S6G left panel, lanes 2 and 6, and lower panel). Furthermore, after a longer MNase digestion, more nucleosomes remained in the di- and tri-nucleosome states in Set8ΔPIP expressing cells (Fig. S6G left panel, lanes 4 and 8). These results suggest that Set8ΔPIP renders chromatin more compacted than Set8WT. In contrast to Set8ΔPIP, Set8ΔPIP,CD had no effect on MNase cleavage (Fig. S6G right panel), suggesting that the ability of Set8ΔPIP to promote chromatin compaction is dependent on its catalytic activity.
To understand how Set8 stabilization promotes aberrant chromatin compaction in S-phase cells, we tested the possibility that condensin was prematurely recruited to chromatin during replication. The binding of condensin II to chromatin in prophase is the initial event that triggers normal mitotic chromatin condensation (Hirota et al., 2004). Expression of Set8ΔPIP in S phase led to increased binding of CAP-D3 and CAP-G2, two specific components of condensin II, to chromatin in replicating cells (Fig. 7F), consistent with a recent report that CAP-D3 binds H4K20me1 (Liu et al., 2010).
Together, the experiments above present four independent lines of evidence that Set8 stabilization during S phase leads to premature chromatin compaction.
Discussion
Role of CRL4Cdt2 as a coordinator of DNA replication and chromatin compaction
The CRL4Cdt2 complex is a unique E3 ubiquitin ligase that specifically recognizes targets bound to PCNA on chromatin. This unique property of CRL4Cdt2 confers upon it the ability to ubiquitylate substrates and target them for degradation in a replication-coupled manner, suppressing cell-cycle events that are incompatible with ongoing DNA synthesis. During S phase, CRL4Cdt2 degrades the licensing factor Cdt1, and promotes the nuclear export of another licensing factor Cdc6 by degrading the CDK inhibitor p21 (Abbas et al., 2008; Arias and Walter, 2006; Jin et al., 2006; Kim et al., 2007; Kim et al., 2008; Nishitani et al., 2008; Nishitani et al., 2006; Sansam et al., 2006; Zhong et al., 2003). Thus, by restricting origin licensing to G1, CRL4Cdt2 plays a key role in coordinating G1 and S phases. Our results suggest that CRL4Cdt2 has another important role in coordinating S phase and mitosis. By suppressing Set8 function during S phase, CRL4Cdt2 ensures the orderly execution of DNA replication and H4K20me1-mediated chromatin compaction (Fig. S6H). Failure to degrade Set8 in S phase leads to premature chromatin compaction in replicating cells, which appears to interfere with DNA synthesis and prevents proper entry into mitosis. Together with the previous studies on CRL4Cdt2, our findings suggest that CRL4Cdt2 is a key factor that coordinates DNA replication with other cell-cycle events before and after S phase.
CRL4Cdt2- and PCNA-mediated degradation of Set8
The mechanism by which Set8 is ubiquitylated is remarkably similar to that of Cdt1. In S phase and after DNA damage, destruction of both Set8 and Cdt1 is promoted by CRL4Cdt2. Moreover, the PIP degron of Set8 closely resembles that of Cdt1. Finally, as seen for the interaction between Cdt1 and CRL4Cdt2, Set8 and CRL4Cdt2 appear to assemble into a complex only in the context of chromatin-bound PCNA. In support of this model, we find that in egg extracts, the addition of Set8 leads to PIP box-dependent recruitment of CRL4Cdt2 onto chromatin. In addition, in mammalian cells, coimmuno-precipitation of Cdt2 and Set8 is dependent on the Set8 PIP box, and in egg extracts, the interaction between Set8 and Cdt2 requires the presence of DNA (data not shown). In summary, both Cdt1 and Set8 are degraded via a mechanism that involves docking of their PIP degrons onto chromatin-bound PCNA, followed by degron-dependent recruitment of CRL4Cdt2 and ubiquitylation.
Although Set8ΔPIP is significantly stabilized after DNA damage, its degradation in S-phase cells is delayed but not abolished. These features of Set8 degradation in human cells also resemble those of Cdt1 (Nishitani et al., 2006; Senga et al., 2006), and they suggest that during S phase, Set8 can be degraded by a PIP degron-independent mechanism. In addition to CRL4Cdt2, the CRL1Skp2 is implicated in the down regulation of Cdt1 (Kondo et al., 2004; Li et al., 2003), and it may play a secondary role in suppressing Cdt1 during S phase (Nishitani et al., 2006; Senga et al., 2006; Takeda et al., 2005). Interestingly, knockdown of Skp2 leads to cell cycle arrest at the G1/S transition and elevated levels of Set8 (Yin et al., 2008). In our immunostaining analysis of unperturbed cycling cells, we noticed that a fraction of the PCNA-negative cells contained low levels of H4K20me1 (data not shown), suggesting that a PCNA-independent mechanism may suppress Set8 function outside of S phase. Since both Set8 and H4K20me1 accumulate during G2 and M phases, it is possible that Set8 is suppressed in G1. Consistent with this possibility, the levels of Set8 start to decline in G1 following nocodazole release (Huen et al., 2008; Yin et al., 2008). Our results suggest that while the CRL4Cdt2-mediated degradation of Set8 is a critical mechanism that defines the functional window of Set8 during the cell cycle, additional mechanisms may exist.
The need for Set8 degradation during S phase
Set8 promotes the accumulation of H4K20me1 during G2 and mitosis and is important for proper chromatin condensation (Houston et al., 2008; Oda et al., 2009; Sakaguchi and Steward, 2007). As cells exit from mitosis, the level of H4K20me1 rapidly declines and reaches its lowest point in early S phase (Huen et al., 2008; Oda et al., 2009). We found several lines of evidence that Set8 activity is incompatible with DNA replication. First, constitutive expression of Set8WT reduces DNA synthesis and triggers the ATR checkpoint response. Second, prolonged expression of the stabilized Set8ΔPIP mutant leads to a dramatic loss of replicating cells. Third, even when transiently expressed in S-phase cells, the Set8ΔPIP mutant induces premature H4K20me1 accumulation near DNA replication forks and triggers a checkpoint-mediated G2 arrest. Importantly, the ability of Set8ΔPIP to induce aberrant H4K20me1 accumulation and to activate the checkpoint is dependent upon its catalytic activity. Thus, if Set8 is not degraded by CRL4Cdt2 during S phase, it interferes with DNA replication by monomethylating H4K20 in a PIP box-independent manner. Set8 has affinity for the N terminal tail of H4 with acetylated K5, K8, and K12 (Yin et al., 2008). Because newly synthesized H4 is hyperacetylated at K5 and K12, Set8, if not degraded during ongoing DNA synthesis, could be recruited to the newly assembled chromatin at replication forks. Consistent with the idea that aberrant H4K20me1 accumulation compromises DNA replication, loss of Suv4-20h1/2, the methyltransferases that convert H4K20me1 to H4K20me2/3, leads to genomic instability and defects in S phase (Schotta et al., 2008). Similarly, ablation of PHF8, an H4K20me1 demethylase, leads to a reduction of cells in S phase and accumulation of cells at G2/M (Liu et al., 2010).
Why is aberrant H4K20me1 accumulation in S phase a problem for the cell cycle? H4K20me1 is specifically recognized by the chromatin compaction factor L3MBTL1 and condensin II component CAP-D3 (Liu et al., 2010; Trojer et al., 2007). The aberrant H4K20me1 accumulation around replication forks may lead to local chromatin compaction that interferes with fork progression and/or other replication-coupled cellular events. Consistent with this possibility, we found multiple lines of evidence of premature chromatin compaction in S-phase cells expressing the stabilized Set8ΔPIP mutant. Coincident with premature chromatin compaction, Set8ΔPIP elicited Chk1 phosphorylation during S phase, and triggered a checkpoint-mediated G2 arrest. Furthermore, phospho-RPA foci were detected in Cdt2 knockdown cells with high H4K20me1, indicating that RPA-coated single-stranded DNA, a key structure for ATR activation, was induced by aberrant chromatin compaction (Zou and Elledge, 2003). These findings suggest that aberrant H4K20me1 accumulation not only compromises genome duplication, but also impedes mitotic entry. In addition to its immediate impact on S phase and mitotic entry, aberrant H4K20me1 accumulation may have additional effects on the cell cycle through transcription repression (Congdon et al., 2010; Liu et al., 2010; Trojer et al., 2007). It remains possible that Set8 has substrates other than H4K20 whose aberrant methylation during S phase contributes to the faulty mitotic entry.
To ensure orderly cell cycle progression, mitotic events must not occur prematurely during the cell cycle. Thus, the key mitotic regulator Cdk1-Cyclin B is fully activated only after S phase is completed (O’Farrell, 2001). In addition, the ATR-mediated checkpoint pathway monitors DNA replication and prevents premature chromatin condensation (Brown and Baltimore, 2000; Nghiem et al., 2001). This study shows that the process of DNA replication itself, via the PCNA and CRL4Cdt2-mediated degradation of Set8, plays an active role in delaying chromatin compaction until S phase is completed. We expect that in the future, other substrates of CRL4Cdt2 will emerge whose presence in S phase is incompatible with the proper execution of DNA replication.
Materials and Methods
Cell culture, cell synchronization, and drug treatments
U2OS, HeLa, and 293T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum. U2OS-TR, RCZ11, RCZ23, and RCZ29 cells were cultured in DMEM with 10% serum and 50 μg/mL hygromycin (Invitrogen). Protein expression from stable cell lines was induced with 0.1 μg/mL tetracycline (Sigma). Zeocin (Invitrogen) was used for clonal selection at 100 μg/mL. To synchronize cells in S phase, 1 mM hydroxyurea (Sigma) was added for 20–24 hours. For G1/S synchronization, cells were arrested in mitosis with 100 ng/mL nocodazole (Sigma), washed thoroughly with PBS, and released into media containing 2 mM thymidine (Sigma). Alternatively, cells were synchronized by standard double thymidine block. To measure protein stability, cycloheximide and MG132 (Sigma) were used at 100 μg/mL and 10 μM, respectively. Kinase inhibitors UCN-01 and wortmannin (Sigma) were used at 1 μM and 100 μM, respectively. For all experiments involving ultraviolet radiation exposure, 50 J/m2 UV was used.
Antibodies and immunological techniques
Antibodies used for Western blotting were from Millipore (Set8), Upstate (phospho-H3), Abcam (H4K20me1, H3, CAP-D3, CAP-G2), Active Motif (H1, H4, H4K20me2, H4K20me3), Cell Signaling Technologies (Cul4A, Tubulin, phospho-Chk1), Santa Cruz (Chk1), Bethyl (Cdt1, DDB1, Cdt2), Novus (Cdt2), Sigma (Flag), Chemicon (PCNA). Horseraddish peroxidease-conjugated secondary antibodies were from Jackson ImmunoResearch. For immunofluorescence studies, antibodies used were from Abcam (PCNA), Bethyl (phospho-RPA32), and Active Motif (H4K20me1). Flag immunoprecipitations were performed with Flag M2-conjugated agarose beads (Sigma) in NETN buffer containing 20 mM Tris-HCl (pH 8.0), 120 mM NaCl, 1 mM EDTA, 0.5% NP-40, and protease inhibitor cocktail (Sigma).
Dual color FISH
Cells were harvested and incubated in hypotonic buffer (0.59% KC1) for 30min at 37 degrees, fixed in ice cold 3:1 MeOH: Acetic acid, and spread on glass slides. Slides were prepared for FISH using fluorescently labeled probes specific for the arms of chromosome 16 (16q22:red; 16p13:green) according to the manufacturer’s instructions (Cytocell; Cat# LPH 022). Coverslips were mounted and DNA was detected with 0.2 μg/ml DAPI/antifade solution (Cytocell). Fluorescent images were captured with a Hamamatsu Orca AG cooled CCD camera mounted on a Nikon TI/Yokagawa CSU-10 spinning disk confocal microscope with a 100x, 1.4 NA objective. A series of 0.25 μm optical sections were collected in the z-axis for each channel (DAPI, fluorescein, and Texas red). Inter- and intra-chromosome distances under each condition were measured with Slidebook analysis software. Approximately 25 to 40 intra-chromosome distances were measured for each condition for each of 3 biological replicates.
Supplementary Material
Acknowledgments
We thank Drs. Michelle Longworth, James Rocco, William Michaud, Ronald Lebofsky, and Puck Knipscheer for reagents; Dr. Marie Classon and members of the Zou lab for helpful discussions; Dr. Anindya Dutta for communicating unpublished results. This work is supported by NIH grants GM076388 to L. Z., GM080676 to J. C. W., and GM081607 to N. J. D. J. J. is a Pew Scholar and supported by a grant (AU-1711) from the Welch Foundation. R. C. C. and C. G. H. are supported by the NIH fellowships F32-GM089150 and F32-GM082014, respectively. A. L. M. and R. L. F. are supported by postdoctoral fellowships from the American Cancer Society.
Footnotes
Additional information can be found in Supplemental Materials and Methods
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008;22:2496–2506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- Arias EE, Walter JC. Replication-dependent destruction of Cdt1 limits DNA replication to a single round per cell cycle in Xenopus egg extracts. Genes Dev. 2005;19:114–126. doi: 10.1101/gad.1255805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias EE, Walter JC. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat Cell Biol. 2006;8:84–90. doi: 10.1038/ncb1346. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- Congdon LM, Houston SI, Veerappan CS, Spektor TM, Rice JC. PR-Set7-mediated monomethylation of histone H4 lysine 20 at specific genomic regions induces transcriptional repression. J Cell Biochem. 2010;110:609–619. doi: 10.1002/jcb.22570. [DOI] [PubMed] [Google Scholar]
- Fang J, Feng Q, Ketel CS, Wang H, Cao R, Xia L, Erdjument-Bromage H, Tempst P, Simon JA, Zhang Y. Purification and functional characterization of SET8, a nucleosomal histone H4-lysine 20-specific methyltransferase. Curr Biol. 2002;12:1086–1099. doi: 10.1016/s0960-9822(02)00924-7. [DOI] [PubMed] [Google Scholar]
- Havens CG, Walter JC. Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol Cell. 2009;35:93–104. doi: 10.1016/j.molcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa LA, Banks D, Wu M, Kobayashi R, Sun H, Zhang H. L2DTL/CDT2 interacts with the CUL4/DDB1 complex and PCNA and regulates CDT1 proteolysis in response to DNA damage. Cell Cycle. 2006;5:1675–1680. doi: 10.4161/cc.5.15.3149. [DOI] [PubMed] [Google Scholar]
- Higa LA, Mihaylov IS, Banks DP, Zheng J, Zhang H. Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat Cell Biol. 2003;5:1008–1015. doi: 10.1038/ncb1061. [DOI] [PubMed] [Google Scholar]
- Hirota T, Gerlich D, Koch B, Ellenberg J, Peters JM. Distinct functions of condensin I and II in mitotic chromosome assembly. J Cell Sci. 2004;117:6435–6445. doi: 10.1242/jcs.01604. [DOI] [PubMed] [Google Scholar]
- Houston SI, McManus KJ, Adams MM, Sims JK, Carpenter PB, Hendzel MJ, Rice JC. Catalytic function of the PR-Set7 histone H4 lysine 20 monomethyltransferase is essential for mitotic entry and genomic stability. J Biol Chem. 2008;283:19478–19488. doi: 10.1074/jbc.M710579200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, McCall CM, Ohta T, Xiong Y. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol. 2004;6:1003–1009. doi: 10.1038/ncb1172. [DOI] [PubMed] [Google Scholar]
- Hu J, Xiong Y. An evolutionarily conserved function of proliferating cell nuclear antigen for Cdt1 degradation by the Cul4-Ddb1 ubiquitin ligase in response to DNA damage. J Biol Chem. 2006;281:3753–3756. doi: 10.1074/jbc.C500464200. [DOI] [PubMed] [Google Scholar]
- Huen MS, Sy SM, van Deursen JM, Chen J. Direct interaction between SET8 and proliferating cell nuclear antigen couples H4-K20 methylation with DNA replication. J Biol Chem. 2008;283:11073–11077. doi: 10.1074/jbc.C700242200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Arias EE, Chen J, Harper JW, Walter JC. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Jorgensen S, Elvers I, Trelle MB, Menzel T, Eskildsen M, Jensen ON, Helleday T, Helin K, Sorensen CS. The histone methyltransferase SET8 is required for S-phase progression. J Cell Biol. 2007;179:1337–1345. doi: 10.1083/jcb.200706150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachentsev D, Sarma K, Reinberg D, Steward R. PR-Set7-dependent methylation of histone H4 Lys 20 functions in repression of gene expression and is essential for mitosis. Genes Dev. 2005;19:431–435. doi: 10.1101/gad.1263005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Feng H, Kipreos ET. C. elegans CUL-4 prevents rereplication by promoting the nuclear export of CDC-6 via a CKI-1-dependent pathway. Curr Biol. 2007;17:966–972. doi: 10.1016/j.cub.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Starostina NG, Kipreos ET. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 2008;22:2507–2519. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kobayashi M, Tanaka J, Yokoyama A, Suzuki S, Kato N, Onozawa M, Chiba K, Hashino S, Imamura M, et al. Rapid degradation of Cdt1 upon UV-induced DNA damage is mediated by SCFSkp2 complex. J Biol Chem. 2004;279:27315–27319. doi: 10.1074/jbc.M314023200. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Q, Liao R, Sun P, Wu X. The SCF(Skp2) ubiquitin ligase complex interacts with the human replication licensing factor Cdt1 and regulates Cdt1 degradation. J Biol Chem. 2003;278:30854–30858. doi: 10.1074/jbc.C300251200. [DOI] [PubMed] [Google Scholar]
- Liu W, Tanasa B, Tyurina OV, Zhou TY, Gassmann R, Liu WT, Ohgi KA, Benner C, Garcia-Bassets I, Aggarwal AK, et al. PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature. 2010;466:508–512. doi: 10.1038/nature09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy CA, Lock K, Yenamandra A, Cortez D. DDB1 maintains genome integrity through regulation of Cdt1. Mol Cell Biol. 2006;26:7977–7990. doi: 10.1128/MCB.00819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc Natl Acad Sci U S A. 2001;98:9092–9097. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Rice JC, Sarma K, Erdjument-Bromage H, Werner J, Wang Y, Chuikov S, Valenzuela P, Tempst P, Steward R, et al. PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell. 2002;9:1201–1213. doi: 10.1016/s1097-2765(02)00548-8. [DOI] [PubMed] [Google Scholar]
- Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T. CDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiation. J Biol Chem. 2008;283:29045–29052. doi: 10.1074/jbc.M806045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H, Sugimoto N, Roukos V, Nakanishi Y, Saijo M, Obuse C, Tsurimoto T, Nakayama KI, Nakayama K, Fujita M, et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. Embo J. 2006;25:1126–1136. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell PH. Triggering the all-or-nothing switch into mitosis. Trends Cell Biol. 2001;11:512–519. doi: 10.1016/s0962-8924(01)02142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Okamoto I, Murphy N, Chu J, Price SM, Shen MM, Torres-Padilla ME, Heard E, Reinberg D. Monomethylation of histone H4-lysine 20 is involved in chromosome structure and stability and is essential for mouse development. Mol Cell Biol. 2009;29:2278–2295. doi: 10.1128/MCB.01768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RD, Soni DV, Wollman R, Hahn AT, Yee MC, Guan A, Hesley JA, Miller SC, Cromwell EF, Solow-Cordero DE, et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell. 2009;35:228–239. doi: 10.1016/j.molcel.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JC, Nishioka K, Sarma K, Steward R, Reinberg D, Allis CD. Mitotic-specific methylation of histone H4 Lys 20 follows increased PR-Set7 expression and its localization to mitotic chromosomes. Genes Dev. 2002;16:2225–2230. doi: 10.1101/gad.1014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi A, Steward R. Aberrant monomethylation of histone H4 lysine 20 activates the DNA damage checkpoint in Drosophila melanogaster. J Cell Biol. 2007;176:155–162. doi: 10.1083/jcb.200607178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansam CL, Shepard JL, Lai K, Ianari A, Danielian PS, Amsterdam A, Hopkins N, Lees JA. DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint. Genes Dev. 2006;20:3117–3129. doi: 10.1101/gad.1482106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta G, Sengupta R, Kubicek S, Malin S, Kauer M, Callen E, Celeste A, Pagani M, Opravil S, De La Rosa-Velazquez IA, et al. A chromatin-wide transition to H4K20 monomethylation impairs genome integrity and programmed DNA rearrangements in the mouse. Genes Dev. 2008;22:2048–2061. doi: 10.1101/gad.476008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senga T, Sivaprasad U, Zhu W, Park JH, Arias EE, Walter JC, Dutta A. PCNA is a cofactor for Cdt1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J Biol Chem. 2006;281:6246–6252. doi: 10.1074/jbc.M512705200. [DOI] [PubMed] [Google Scholar]
- Shi X, Kachirskaia I, Yamaguchi H, West LE, Wen H, Wang EW, Dutta S, Appella E, Gozani O. Modulation of p53 function by SET8-mediated methylation at lysine 382. Mol Cell. 2007;27:636–646. doi: 10.1016/j.molcel.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda DY, Parvin JD, Dutta A. Degradation of Cdt1 during S phase is Skp2-independent and is required for efficient progression of mammalian cells through S phase. J Biol Chem. 2005;280:23416–23423. doi: 10.1074/jbc.M501208200. [DOI] [PubMed] [Google Scholar]
- Tardat M, Murr R, Herceg Z, Sardet C, Julien E. PR-Set7-dependent lysine methylation ensures genome replication and stability through S phase. J Cell Biol. 2007;179:1413–1426. doi: 10.1083/jcb.200706179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojer P, Li G, Sims RJ, 3rd, Vaquero A, Kalakonda N, Boccuni P, Lee D, Erdjument-Bromage H, Tempst P, Nimer SD, et al. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell. 2007;129:915–928. doi: 10.1016/j.cell.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Walter J, Sun L, Newport J. Regulated chromosomal DNA replication in the absence of a nucleus. Mol Cell. 1998;1:519–529. doi: 10.1016/s1097-2765(00)80052-0. [DOI] [PubMed] [Google Scholar]
- Yin Y, Yu VC, Zhu G, Chang DC. SET8 plays a role in controlling G1/S transition by blocking lysine acetylation in histone through binding to H4 N-terminal tail. Cell Cycle. 2008;7:1423–1432. doi: 10.4161/cc.7.10.5867. [DOI] [PubMed] [Google Scholar]
- Zhong W, Feng H, Santiago FE, Kipreos ET. CUL-4 ubiquitin ligase maintains genome stability by restraining DNA-replication licensing. Nature. 2003;423:885–889. doi: 10.1038/nature01747. [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.