Abstract
The examination of the catharanthine C16 substituent effects on the Fe(III)-promoted biomimetic coupling reaction with vindoline is detailed, confirming the importance of the presence of a C16 electron-withdrawing substituent, and establishing an unanticipated unique role (>10-fold) that the C16 methyl ester plays in the expression of the natural product properties. Thus, replacement of the vinblastine C16′ methyl ester with an ethyl ester (10-fold), a cyano group (100-fold), an aldehyde (100-fold), a hydroxymethyl group (1000-fold), or a primary carboxamide (>1000-fold) led to surprisingly large reductions in cytotoxic activity.
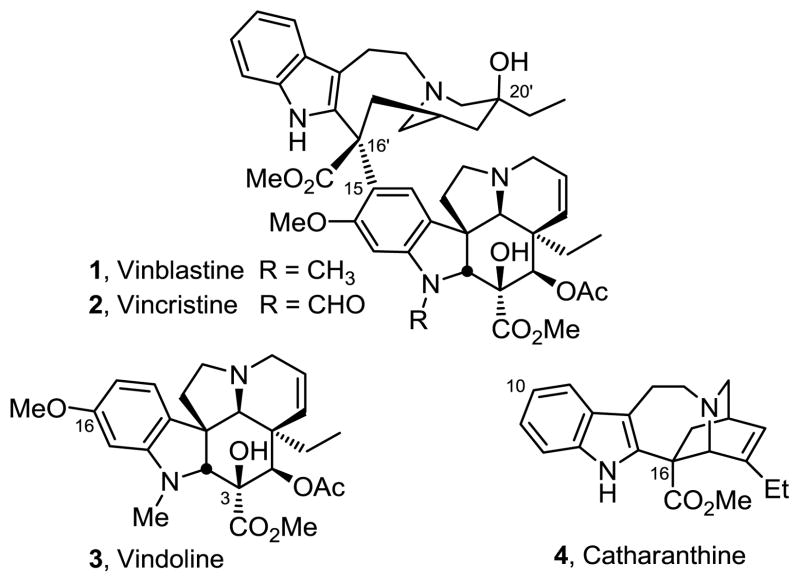
Vinblastine (1) and vincristine (2) represent the most widely recognized members of the vinca alkaloids as a result of their clinical use as antitumor drugs (Figure 1).1–3 Originally isolated in trace quantities from Catharanthus roseus (L.) G. Don,1 their biological properties were among the first to be shown to arise from perturbations in microtubule dynamics and inhibition of mitosis that today is still regarded as one of the more successful drug targets for the treatment of cancer.3–5
Figure 1.
Natural products.
We recently utilized a one-pot, two-step biomimetic Fe(III)-promoted coupling of vindoline (3) with catharanthine (4) in the total synthesis of vinblastine and reported its extension to the preparation of a series of related natural products including vincristine and key analogues.6,7 Although key mechanistic insights into this coupling6–9 and subsequent olefin oxidation7,10 have been disclosed in the studies to date, the unusual differences in the substrate scope and diastereoselectivity of the Fe(III)-promoted coupling (single natural C16′ diastereomer at 25 °C in aqueous buffer) and the more traditional Polonovski fragmentation11,12 (5:1 at −78 °C or 1:1 at 0 °C in CH2Cl2) or 3-chloroindolenine-based13 couplings that proceed through azabenzfulvene intermediates suggests that there are mechanistic features of the former reaction that are not yet well understood and that affect the resulting C16′ stereochemistry (equation 1). In the case of the Fe(III)-promoted coupling, the attack by vindoline formally occurs with clean inversion of the stereochemistry at the reacting C16 center of the catharanthine C16–C21 bond undergoing cleavage. It has been suggested that the reaction proceeds by two single electron oxidations of catharanthine with the initial radical cation formation occurring at the basic tertiary amine followed by a subsequent oxidative cleavage of the C16–C21 bond.8,9 Herein, we disclose the first report of the examination of catharanthine C16 substituent effects on the biomimetic coupling reaction, establishing and confirming the importance of the presence of a C16 electron-withdrawing substituent, and further disclose the unanticipated unique role that the C16 methyl ester plays in the expression of the natural product properties.
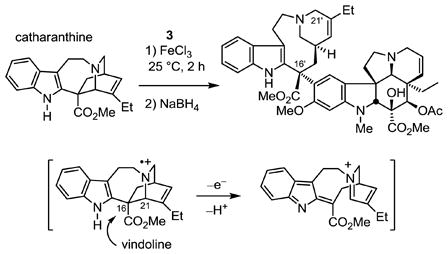 |
(1) |
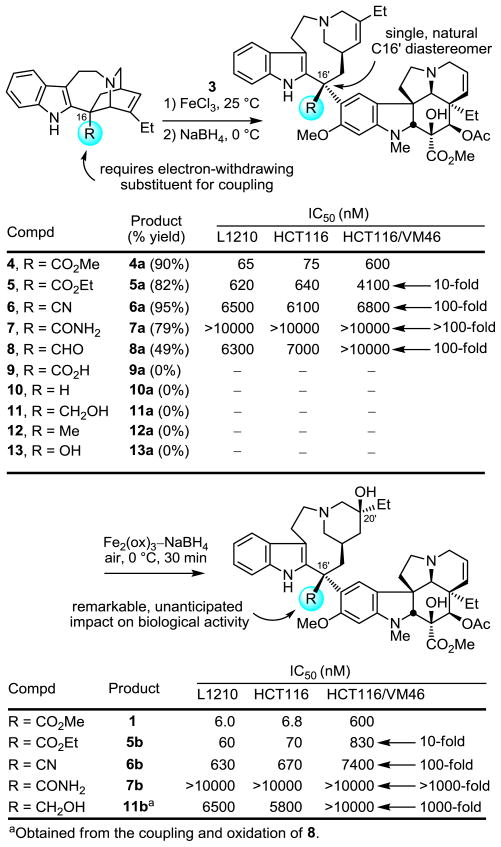
The electron-withdrawing properties of the C16 methyl ester group were anticipated to be key to the coupling of catharanthine with vindoline. Consequently, only a small series of alternative electron-withdrawing substituents were examined (R = CO2Et, CONH2, CN, CHO, CO2H) along with derivatives where it was removed (R = H), or replaced with an alkyl (R = CH2OH, CH3) or hydroxy (R = OH) group (Figure 2).14 As anticipated, the coupling (5 equiv FeCl3, 0.05 N aq HCl–CF3CH2OH 10:1, 25 °C, 2 h) required the presence of a C16 electron-withdrawing substituent with 4–7 providing comparable superb conversions (79–95% vs 90% for 4) to the corresponding anhydrovinblastine analogue, and the aldehyde 8 providing a perceptibly lower yield for the generation of product (49%) reflecting some competitive aldehyde reduction upon iminium ion reduction with NaBH4. Interestingly, the carboxylic acid derivative 9 failed to couple with 3, although this was not investigated in sufficient detail to define the origin of this observation, and the catharanthine analogues 10–13 that lack a C16 electron-withdrawing substituent also, but expectedly, did not couple with 3.
Figure 2.
C16 Substituent effects.
Since this vinblastine site has not been probed beyond reduction or methyl ester hydrolysis and subsequent decarboxylation,3,15 each derivative was also converted to the corresponding vinblastine analogue either by exposing the anhydrovinblastine analogue to the conditions developed for oxidation of the C15′–C20′ double bond with installation of the C20′ tertiary alcohol (Fe2(ox)3–NaBH4, air, 0 °C, 30 min),7,10 or more directly using the one-pot, two-step procedure of coupling and in situ oxidation.6,7 In turn, the cell growth inhibition or cytotoxic activity of both the anhydrovinblastine and vinblastine analogues were examined in three cytotoxic assays (72 h exposure) including the matched colon cancer cell lines HCT116 and HCT116/VM46, the latter of which is multidrug resistant by virtue of Pgp overexpression (Figure 2).16 Whereas the important role of the C16 methyl ester group in the coupling reaction could be anticipated, the remarkable sensitivity of the properties of the resulting anhydrovinblastine and vinblastine analogues to modifications at this site was unexpected. Each series exhibited the same striking sensitivity to the presence and nature of the C16′ substituent. Simply replacing the C16′ methyl ester group with the corresponding ethyl ester group resulted in a 10-fold loss in activity, a nitrile or aldehyde substitution resulted in a 100-fold loss in activity, incorporation of a hydroxymethyl group led to a 1000-fold loss in activity, and the primary carboxamide replacement produced a >1000-fold loss in activity. Although the importance of this site has been reported previously with more significant modifications (R = H, CH2OH, and COCH2CO2tBu),15 it is remarkable that even the more subtle changes detailed herein have such a profound impact on the biological properties of the natural products. Clearly, the role of the C16′ methyl ester extends well beyond facilitating the coupling reaction in the biosynthesis of the natural product; rather it plays an integral role in establishing the biological properties of the natural product presumably stabilizing the interaction of vinblastine with tubulin.17
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA115526 and CA042056). We wish to thank Dr. P. Hellier (P. Fabre) for the gift of catharanthine and vindoline, Professor A. Eschenmoser for insightful discussions, and NIH (GM087948, A.T.) and JSPS (H.G.) for fellowship support. W.M.R. is a Skaggs Fellow.
Footnotes
Supporting Information Available: Full experimental details are provided. This material is available free of charge via the Internet at XXXXXXX.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.(a) Noble RL, Beer CT, Cutts JH. Ann N Y Acad Sci. 1958;76:882. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]; (b) Noble RL. Lloydia. 1964;27:280. [Google Scholar]; (c) Svoboda GH, Nuess N, Gorman M. J Am Pharm Assoc Sci Ed. 1959;48:659. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 2.Moncrief JW, Lipscomb WN. J Am Chem Soc. 1965;84:4963. doi: 10.1021/ja00949a056. [DOI] [PubMed] [Google Scholar]
- 3.Review: Neuss N, Neuss MN. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 229.
- 4.Reviews: Pearce HL. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 145.Borman LS, Kuehne ME. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 133.Fahy J. Curr Pharm Design. 2001;7:1181. doi: 10.2174/1381612013397483.
- 5.Reviews: Kuehne ME, Marko I. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 77.Potier P. J Nat Prod. 1980;43:72.Kutney JP. Nat Prod Rep. 1990;7:85.Kutney JP. Synlett. 1991. p. 11.Kutney JP. Acc Chem Res. 1993;26:559.For recent studies, see: Kuehne ME, Bornmann WG, Marko I, Qin Y, Le Boulluec KL, Frasier DA, Xu F, Malamba T, Ensinger CL, Borman LS, Huot AE, Exon C, Bizzarro FT, Cheung JB, Bane SL. Org Biomol Chem. 2003;1:2120. doi: 10.1039/b209990j.Miyazaki T, Yokoshima S, Simizu S, Osada H, Tokuyama H, Fukuyama T. Org Lett. 2007;9:4737. doi: 10.1021/ol702040y.Voss ME, Ralph JM, Xie D, Manning DD, Chen X, Frank AJ, Leyhane AJ, Liu L, Stevens JM, Budde C, Surman MD, Friedrich T, Peace D, Scott IL, Wolf M, Johnson R. Bioorg Med Chem Lett. 2009;19:1245. doi: 10.1016/j.bmcl.2008.12.077.Shao Y, Zhang H, Ding H, Quan H, Lou L, Hu L. J Nat Prod. 2009;72:1170. doi: 10.1021/np900157t.Sheng LX, Da YX, Long Y, Hong LZ, Cho TP.Bioorg Med Chem Lett 200818460218653334Passarella D, Giardini A, Peretto B, Fontana G, Sacchetti A, Silvani A, Ronchi C, Cappelletti G, Cartelli D, Borlak J, Daneili B. Bioorg Med Chem. 2008;16:6269. doi: 10.1016/j.bmc.2008.04.025.
- 6.Ishikawa H, Colby DA, Boger DL. J Am Chem Soc. 2008;130:420. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. J Am Chem Soc. 2009;131:4904. doi: 10.1021/ja809842b.Va P, Campbell EL, Robertson WM, Boger DL. J Am Chem Soc. 2010;132:8489. doi: 10.1021/ja1027748.Sasaki Y, Kato D, Boger DL. J Am Chem Soc. 2010:132. doi: 10.1021/ja106284s. in press.For supporting synthetic studies, see: Kato D, Sasaki Y, Boger DL. J Am Chem Soc. 2010;132:3685. doi: 10.1021/ja910695e.Ishikawa H, Elliott GI, Velcicky J, Choi Y, Boger DL. J Am Chem Soc. 2006;128:10596. doi: 10.1021/ja061256t.Elliott GI, Fuchs JR, Blagg BSJ, Ishikawa H, Tao H, Yuan Z, Boger DL. J Am Chem Soc. 2006;128:10589. doi: 10.1021/ja0612549.Elliott GI, Velcicky J, Ishikawa H, Li Y, Boger DL. Angew Chem Int Ed. 2006;45:620. doi: 10.1002/anie.200503024.Choi Y, Ishikawa H, Velcicky J, Elliott GI, Miller MM, Boger DL. Org Lett. 2005;7:4539. doi: 10.1021/ol051975x.Yuan Z, Ishikawa H, Boger DL. Org Lett. 2005;7:741. doi: 10.1021/ol050017s.Ishikawa H, Boger DL. Heterocycles. 2007;72:95.Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg SE, Soenen DB, Miller MM, Pollack S, Boger DL. J Am Chem Soc. 2002;124:11292. doi: 10.1021/ja027533n.Wolkenberg SE, Boger DL. J Org Chem. 2002;67:7361. doi: 10.1021/jo020437k.Campbell EL, Zuhl AM, Liu C, Boger DL. J Am Chem Soc. 2010;132:3009. doi: 10.1021/ja908819q.For reviews of related studies, see: Boger DL. Tetrahedron. 1983;39:2869.Boger DL. Chem Rev. 1986;86:781.
- 8.Vukovic J, Goodbody AE, Kutney JP, Misawa M. Tetrahedron. 1988;44:325.For an analogous electrochemical coupling (0.6 V in buffer; NaBH4) to provide anhydrovinblastine, see: Gunic E, Tabakovic I, Gasic MJ. J Chem Soc, Chem Commun. 1993:1496.For an enzymatic coupling, see: Sagui F, Chirivi C, Fontana G, Nicotra S, Passarella D, Riva S, Danieli B. Tetrahedron. 2009;65:312.
- 9.For additional seminal studies on the Fe(III)-coupling to provide anhydrovinblastine, see: Szantay C, Jr, Balazs J, Bolcskei J, Szantay C. Tetrahedron. 1991;47:1265.Sundberg RJ, Hong J, Smith SQ, Sabato M, Tabakovic I. Tetrahedron. 1998;54:6259.
- 10.(a) Sakamoto N, Tan H, Hata E, Kihara N. JP 04164087. Chem Abstr. 1992;117:192139.; (b) Tan H, Sakamoto N, Hata E, Ishitoku T, Kihara N. US 5037977. Chem Abstr. 1990;113:6663.
- 11.(a) Potier P, Langlois N, Langlois Y, Gueritte F. J Chem Soc, Chem Commun. 1975:670. [Google Scholar]; (b) Langlois N, Gueritte F, Langlois Y, Potier P. J Am Chem Soc. 1976;98:7017. doi: 10.1021/ja00438a046. [DOI] [PubMed] [Google Scholar]; (c) Sundberg RJ, Gadamasetti KG, Hunt PJ. Tetrahedron. 1992;48:277. [Google Scholar]
- 12.(a) Kutney JP, Ratcliffe AH, Treasurywala AM, Wunderly S. Heterocycles. 1975;3:639. [Google Scholar]; (b) Kutney JP, Hibino T, Jahngen E, Okutani T, Ratcliffe AH, Treasurywala AM, Wunderly S. Helv Chim Acta. 1976;59:2858. doi: 10.1002/hlca.19760590824. [DOI] [PubMed] [Google Scholar]
- 13.For additional approaches to effecting analogous couplings, see: Magnus P, Stamford A, Ladlow M. J Am Chem Soc. 1990;112:8210.Magnus P, Mendoza JS, Stamford A, Ladlow M, Willis P. J Am Chem Soc. 1992;114:10232.Kuehne ME, Matson PA, Bornmann WG. J Org Chem. 1991;56:513.Bornmann WG, Kuehne ME. J Org Chem. 1992;57:1752.Kuehne ME, Zebovitz TC, Bornmann WG, Marko I. J Org Chem. 1987;52:4340.Schill G, Priester CU, Windhovel UF, Fritz H. Tetrahedron. 1987;43:3765.Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. J Am Chem Soc. 2002;124:2137. doi: 10.1021/ja0177049.Kuboyama T, Yokoshima S, Tokuyama H, Fukuyama T. Proc Natl Acad Sci USA. 2004;101:11966. doi: 10.1073/pnas.0401323101.
- 14.Details of the substrate preparations are provided in the Supporting Information.
- 15.(a) Potier P, Guenard D, Zavala F. Comp Rend. 1979;173:414. [PubMed] [Google Scholar]; (b) Barnett CJ, Cullinan GJ, Gerzon K, Hoying BC, Jones WE, Newlon WM, Poore GA, Robison RL, Sweeney MJ, Todd GC, Dyke RW, Nelson RL. J Med Chem. 1978;21:88. doi: 10.1021/jm00199a016. [DOI] [PubMed] [Google Scholar]
- 16.(a) Lampidis TJ, Kolonias D, Podona T, Israel M, Safa AR, Lothstein L, Savaraj N, Tapiero H, Priebe W. Biochemistry. 1997;36:2679. doi: 10.1021/bi9614489. [DOI] [PubMed] [Google Scholar]; (b) Perego P, De Cesare M, De Isabella P, Carenini N, Beggiolin G, Pezzoni G, Palumbo M, Tartaglia L, Prtesi G, Pisano C, Carminati P, Scheffer GL, Zunino F. Cancer Res. 2001;61:6034. [PubMed] [Google Scholar]
- 17.Gigant B, Wang C, Ravelli RBG, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M. Nature. 2005;435:519. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.