Abstract
This review emphasizes how translation from bench research to clinical knowledge and vice versa has resulted in considerable progress in understanding the immunopathogenesis of psoriasis. First, the journey in understanding the pathogenic mechanisms behind psoriasis is described. The roles of different components of the adaptive and innate immune systems involved in driving the inflammatory response are explained. Discovery of new immune pathways i.e. the IL23/Th17 axis and its subsequent impact on the development of novel biological therapies is highlighted. Identification of potential targets warranting further research for future therapeutic development are also discussed.
Keywords: psoriasis, immunopathogenesis, T cells, adaptive/innate immune response
Psoriasis is a common and stigmatising chronic inflammatory skin disease affecting about 2 % of the population worldwide 1. This condition may cause significant morbidity due to the possible co-existence of psoriatic arthritis and association with a large number of systemic diseases 2. A recent cohort study 3 has also shown that severe psoriasis (defined as psoriasis patients with a history of systemic therapy) is associated with an increased risk of mortality as male and female patients in the study died 3.5 and 4.4 years younger respectively than those without psoriasis (even after adjustment for classical risk factors of mortality). Hence psoriasis is a major public health problem. On an individual basis, it has a negative impact on patients' quality of life. Therefore, psoriasis poses a major social and economic burden on society. Current existing therapies only relieve symptoms but cannot cure disease. Therapeutic costs are expensive with treatments carrying substantial side effects. Therefore better understanding of the immunopathogenesis of psoriasis remains at the forefront of both basic and translational research as this is essential for the development of improved therapies. The immunological and inflammatory basis for psoriasis has been extensively reviewed in the literature 4-7. Although considerable progress has been made in understanding the immunopathogenesis of psoriasis 1, many fundamentally important questions regarding the functional roles of cells and molecules implicated in psoriasis remain unanswered. The aim of this review is to summarise the important findings from clinical studies and experimental models that have led to a better understanding of the immunopathogenesis of psoriasis with emphasis on how the transfer between knowledge acquired from basic research to clinical studies and vice versa is crucial for rapid progression in both areas. Important areas for future research directions will also be highlighted.
Clinical subtypes, histological features and associated comorbidities
Different clinical subtypes of psoriasis exist with psoriasis vulgaris, the commonest type of psoriasis, accounting for 90 % of all cases. About 50 % of psoriasis patients have psoriatic nail disease with nail pitting as the most common change. Psoriatic arthritis is a seronegative inflammatory arthritis with a prevalence of up to 25 % 8 and in the vast majority (90 %) of psoriasis patients with psoriatic arthritis, the skin manifestations precede arthritis.
Psoriasis is thought to be a complex condition resulting from a combination of genetic predisposition and environmental triggers. The acute forms of psoriasis, guttate and generalised pustular psoriasis (von Zumbusch psoriasis), are both associated with infections. The former usually occurs about 2 weeks after a b-haemolytic streptococcal infection such as tonsillitis or pharyngitis, or a viral infection. Apart from infection, other triggering factors which may elicit psoriasis in genetically predisposed individuals include trauma (Koebner phenomen) 9, HIV infection 10, hypocalcaemia in generalized pustular psoriasis 11, psychogenic stress 12,13 and certain drugs including lithium, beta-blockers, antimalarials, interferon, NSAIDs and rapid tapers of high dose corticosteroids 14. Increased alcohol consumption and smoking have been associated with psoriasis but neither has been shown to be a major risk factor.
The 3 main histological features of psoriasis are epidermal hyperplasia, dilatation and proliferation of dermal blood vessels and accumulation of inflammatory cells, particularly neutrophils and T lymphocytes in the dermis. It now transpires that the first two features are caused by inflammatory cell infiltrates. The increased vascularity in the dermis seen histologically is driven by angiogenic factors. One of these factors, vascular endothelial growth factor (VEGF), is found at high levels in psoriasis plaques 15. The interaction between VEGF and the angiopoietin/Tie signalling pathway is modulated by tumour necrosis factor a (TNFa), a key pro-inflammatory cytokine in psoriasis 16,17.
Psoriasis is also associated with a number of systemic disorders 2 including Crohn's disease, diabetes mellitus (notably type 2) 18, metabolic syndrome 19, obesity, cardiovascular disease 20, depression 21 and cancer. It is uncertain whether cancers particularly lymphoma and skin cancer, are related to psoriasis or to its treatment. Psoriasis has been identified as an independent risk factor for cardiovascular disease 22. Potential mechanisms may include the presence of circulating pro-inflammatory factors and endothelial activation 22.
Journey in understanding the immunopathogenesis of psoriasis
Genetics of psoriasis
A complete overview of the genetics of psoriasis is beyond the scope of the current article but has been extensively reviewed elsewhere 23,24. Twin and family studies have shown that psoriasis has a strong genetic component although the inheritance pattern is still unclear. 71 % of patients with childhood psoriasis have a positive family history 25. Siblings 26 and first-degree relatives 27 of psoriasis patients show a four-fold or more increased risk in developing psoriasis. Analysis of concordance rates in twin studies 28-31 show a threefold increased risk of psoriasis in monozygotic twins compared to dizygotic twins, being approximately 72 % and 15-23 % respectively for northern European individuals, and 35 % and 12 % respectively for Australian individuals. These data further support genetics as a major influence in psoriasis.
At least ten chromosomal loci have been identified showing statistically significant evidence for linkage to psoriasis (PSORS 1-10). However, the only region that has consistently been identified in genetic screens of families with psoriasis is the major-histocompatibility complex (MHC) region on chromosome 6 named PSORS1 32,33 and markers within this region have repeatedly been shown to have the greatest association with psoriasis in different genome wide association scans 34,35. This region is considered to be responsible for up to 50 % of genetic susceptibility to psoriasis. Within PSORS1 is the human leukocyte antigen-C (HLA-C) gene which is the strongest candidate gene for psoriasis identified to date 32, with its allele HLA-Cw6 (HLA-Cw*0602) shown to be the predominant risk allele. A new psoriasis susceptibility gene ZNF313/RNF114, which may regulate T cell activation through ubiquitin ligase activity, has been identified 34. This further supports the notion that multiple gene products share a role in the immune regulation of psoriasis, contributing to disease pathogenesis. Other novel psoriasis susceptibility genes that have been identified from genomewide association scans have been summarized in a recent review 1 and include the IL-23R, CDKAL1 and the IL-4/IL-13 gene cluster 1.
HLA-Cw6 and psoriasis
HLA-Cw6 is seen in up to 60 % of psoriasis patients compared to 15 % in the general population. Individuals carrying this allele have a 10-20-fold increased risk of developing psoriasis 36. A dosage effect of HLA-Cw*0602 has been observed, where homozygous individuals have a 2.5-fold increased risk of developing psoriasis compared to HLA-Cw6 heterozygous individuals 37. Homozygous individuals also experience an earlier onset but disease severity is not affected 37. The HLA-Cw6 allele is present in 90 % of patients with early onset psoriasis, in 50 % of those with late onset psoriasis, and only 7.4 % of a control population. HLA-Cw6 positive and negative psoriasis patients may exhibit distinctive clinical phenotypes. Some clinicians have designated patients with early onset psoriasis, a positive family history of psoriasis and the expression of HLA-Cw6 as having Type I psoriasis and those with late onset disease, no family history and a lack of expression of HLA-Cw6 as Type II psoriasis 38. Guttate psoriasis, both acute onset and persistent eruptive subtypes, are mostly confined to HLA-Cw6 positive patients. Meanwhile, psoriatic nail disease, palmoplantar pustulosis and psoriatic arthritis are more common in HLA-Cw6 negative patients 39,40. Apart from significantly earlier disease onset, HLA-Cw*0602 is associated with more widespread and recurrent psoriasis 39. Partial or total remission during pregnancy is much more frequent in HLA-Cw*0602 positive women whilst HLA-Cw*0602 negative women are more likely to have unchanged or worsening psoriasis during pregnancy 39. Koebner's phenomenon is also more common in HLA-Cw6 positive patients 39,40.
Gene to function perspective on HLA-Cw6
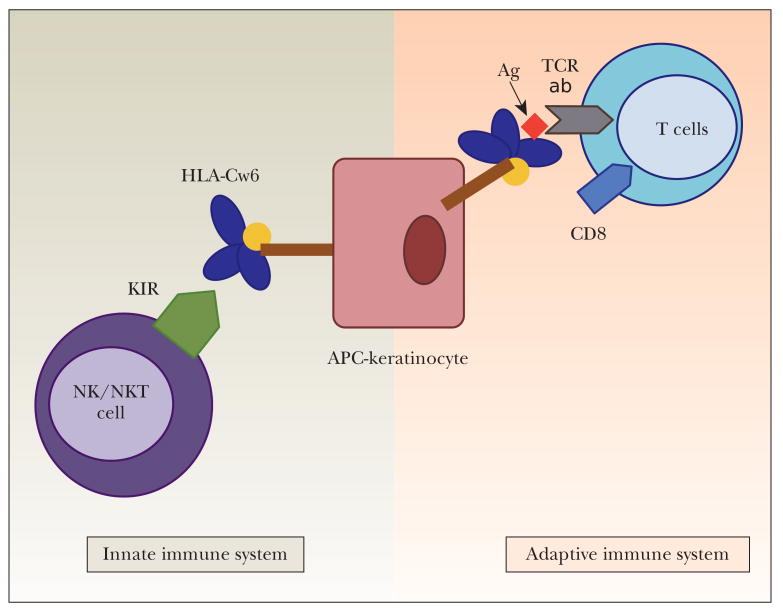
The strong association of HLA-Cw6 with psoriasis further supports psoriasis as an immune-mediated disorder. Despite this strong association, the functional role of HLA-Cw6 remains unknown. Both the adaptive and the innate immune systems are important in defence against pathogens, the former generates a highly specific response for a particular pathogen whilst the latter confers immediate non-specific defence against infection. Figure 1 illustrates that HLA-Cw6 may exert its effect via the adaptive or the innate immune system. HLA-Cw6 may act via the adaptive immune system by its antigen presenting capacity. This hypothesis is supported by the fact that guttate psoriasis, which is strongly associated with HLA-Cw6, is triggered by streptococcal pharyngitis 41,42. HLA-Cw6 may also exert an innate immune response via its interaction with a class of killer immunoglobulin-like receptors (KIRs) expressed on natural killer (NK) and natural killer T (NKT) cells, which are important components of the innate immune system and are implicated in the pathogenesis of psoriasis 43. NK cells produce predominantly IFN-g and may cause cytolysis of target cells. NK cell activity is up-regulated by IL-2, IL-12 and IL-15; which are all found at increased levels in psoriatic plaques. NK cells can mediate both activating and inhibitory immune responses as they express both activating and inhibitory killer immunoglobulin-like receptors. KIRs recognise different types of HLA-C molecules leading to either an overall activating or inhibitory immune response. KIRs have been associated with psoriasis and psoriatic arthritis 44. HLA-Cw6 is a natural ligand for KIR2DL1 (an inhibitory receptor) and it is possible that interaction between HLA-Cw6 and KIR2DL1 would lead to aberrant function of lymphoid cells in the immunopathogenesis of psoriasis. A better understanding of the functional role of HLA-Cw6 is important for both elucidating the immunopathogenesis of psoriasis and development of improved treatments for psoriasis.
Figure 1.
Potential of HLA-Cw6 to regulate adaptive as well as innate immune responses. HLA-Cw6 expressed on antigen presenting cells (APCs) such as dendritic cells can trigger adaptive immune responses via presentation of processed antigen to the TCR of CD8+ T cells. In addition, innate immune response can be elicited by interaction of HLA-Cw6 with its natural Killer immunoglobulin-like receptors expressed on NK and NKT cells.
Immune response
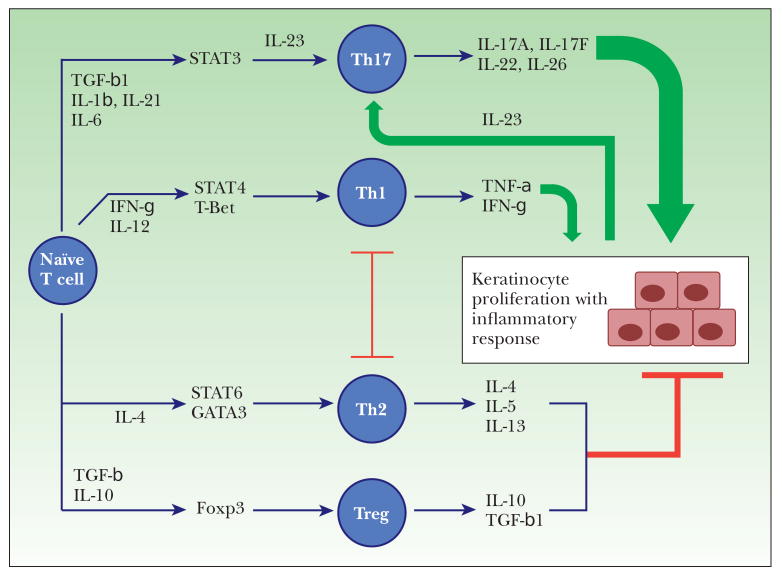
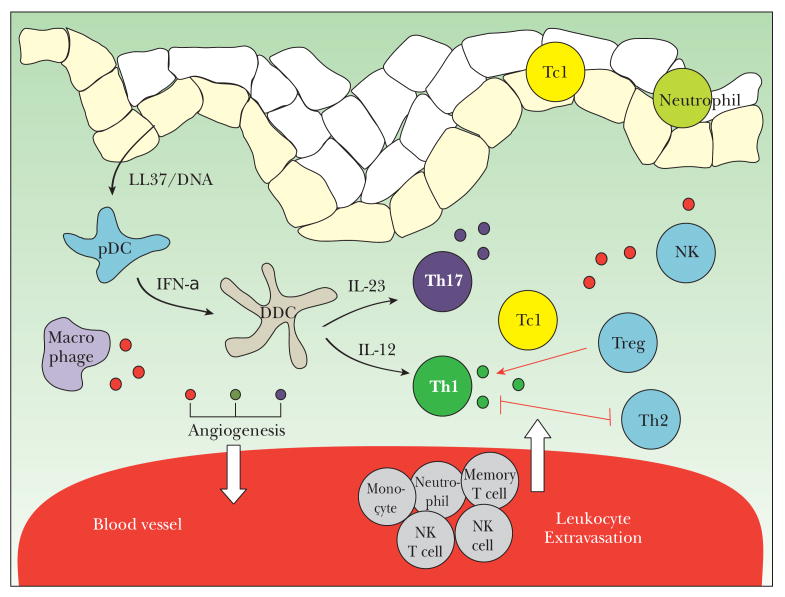
Epidermal keratinocytes are able to recruit and activate T cells. Most T cells infiltrating psoriatic skin are divided into T helper 1 (Th1; CD4 +) and T cytotoxic (Tc1; CD8 +) subsets 45. Two further T cell subtypes, Th17 cells 46 and regulatory T cells (Treg) 47 have been identified as important contributors to the pathogenesis of autoimmune diseases including psoriasis. Figure 2 illustrates the different T cell subtypes implicated in psoriasis. Involvement of both innate (keratinocytes, dendritic antigen-presenting cells, neutrophils, macrophages, natural killer (NK) and natural killer T (NKT) cells) and adaptive (CD4+ and CD8+ T lymphocytes) immune responses are important in mediating the inflammatory cascade 1,48 and this is summarized in figure 3.
Figure 2.
Role of CD4 T cell subtypes in psoriasis. Pro-inflammatory cytokines produced from Th1 and Th17 dominate the cytokine profile in psoriasis. They mediate keratinocyte hyperproliferation and trigger a ‘vicious cycle’ of inflammation. IL-23 released by psoriatic keratinocytes and sentinel cells such as dendritic cells and macrophages, is critical for maintenance of Th17 function. Low levels of anti-inflammatory cytokines released by Th2 and Treg cells potentially counteract but cannot balance the effects of Th1/Th17 cytokines. Green arrows denote stimulatory actions, red blocking lines denote inhibitory actions.
Figure 3.
Immune cell types and interactions implicated in psoriasis. Green dot denotes Th1 cytokines including IFNg and TNFa. Purple dot denotes cytokines produced by Th17 including IL17A, IL17F and IL22. Red dot denotes other inflammatory mediators such as IL-2 and IL-6. Low levels of anti-inflammatory cytokines released by Th2 and Treg cells potentially counteract but cannot balance the effects of Th1/Th17 cytokines. Th1 and Th2 cells have a mutually inhibitory effect as denoted by the red line. Treg inhibits Th1 actions as denoted by the red arrow.
Characterization of cells and cytokines showed that elevated levels of T helper (Th) 1 cytokines IFNg, T N F a and IL-12 are present in psoriasis, but not the T helper (Th) 2 cytokines IL-4, IL-5 or IL-10 which appeared to be protective against psoriasis. The use of Th2-type cytokines IL-4 49 and IL-10 50 in clinical trials as treatment for psoriasis suggest that a shift of the cytokine milieu from Th1 to Th2 response may reverse psoriasis inflammation. These observations led to the view that psoriasis is a Th 1-type disease 51. The crucial role of TNFa in psoriasis has become evident from three distinct TNFa inhibitors etanercept 52, infliximab 53 and adalimumab 54 which block the interaction of soluble TNFa with TNFa receptors on target cells and are highly effective in the treatment of psoriasis. By studying the progressive changes in inflammatory cytokines and chemokines induced by etanercept in psoriatic lesions, it has been suggested that TNFa may strongly regulate early cytokine production and support the inflammation driven by IFNg and the signal transducer and activator of transcription (STAT) pathways, thereby encouraging chemokine production that regulates T cells and DC interactions in the skin 7. TNFa has been linked to fatigue and depression 55 which are co-morbidities associated with psoriasis and TNFa receptors have been detected in the central nervous system. 55 Hence biological therapies targeting TNFa may also improve these psoriasis associated co-morbidities in the long term 56.
Role of T cells
Until the 1990s, psoriasis was thought to be a disease of disordered keratinoctye proliferation and differentiation 57 especially as epidermal hyperplasia was the most prominent clinical and histological feature. This initial view of psoriasis resulted in the use of antimetabolites including methotrexate which limit epidermal hyperproliferation. However, subsequent evidence from clinical studies, in vitro and in vivo experimental models support the concept that psoriasis is a T cell-mediated inflammatory skin disease 51 affecting genetically predisposed individuals and the observed epidermal hyperplasia is a result of cellular immune infiltration. The first piece of evidence resulting in psoriasis being widely considered as a T-cell mediated autoimmune disease came from the success of T-cell-targeted therapies such as cyclosporin 58,59 and tacrolimus 60 in the treatment of psoriasis 61. Monoclonal antibodies specific for CD3 and CD4, which are more selective biological T-cell antagonists, produced clinical improvement in some patients with severe psoriasis. A pivotal study involved the testing of interleukin-2 (IL-2)-diphtheria-toxin fusion protein in psoriasis patients 62. This agent was proposed to selectively deplete activated T cells expressing IL-2 receptors from psoriasis skin lesions and resulted in clinical and histopathological remission of psoriasis vulgaris. These studies stimulated further research into the development of T-cell-targeted drugs for psoriasis treatment. Subsequently, administration of another fusion protein, cytotoxic T-lymphocyte antigen 4 (CTLA4)-immunoglobulin, was shown to reverse the clinical and cellular features of psoriasis 63. This agent blocks T cell co-stimulation mediated by dendritic cells (DCs) but does not directly deplete T cells. Its effectiveness indicated that continuing T-cell co-stimulation is required to sustain psoriasis disease activity, including the excessive infiltration of T cells and DCs into the skin 64. These clinical studies all provided evidence that lesion-associated T cells are central to sustaining disease activity in psoriasis vulgaris. Further evidence highlighting the importance of T cells in psoriasis pathogenesis have been reviewed 1 including the appearance of clonal T cells in psoriatic lesions 65, the development of psoriasiform phenotype within symptomless (nonlesional) psoriatic skin after transplantion onto the xenotransplantation AGR 129 mouse model again highlights the importance of epidermal T cells in the development of psoriasis 66. The immune response involved in psoriasis pathogenesis is explained further below.
The IL-23/Th17 axis
The IL-23/Th17 axis is an exciting new area in psoriatic pathology because it has led to the development of promising new treatments for psoriasis which specifically target this axis. The development, characterization and function of Th17 cells and the role of IL-23 in Th17-cell-dependent chronic inflammation in psoriasis have been recently reviewed 67. Briefly, IL-23 is a heterodimeric cytokine 68 and consists of the protein IL-23p19 combined with IL-12p40, an IL-12 subunit. Intradermal injection of IL-23 in mice resulted in the development of a psoriasiform phenotype with histopathological features 69. IL-23 has been shown to mediate epidermal hyperplasia, acanthosis, hyperparakeratosis and orthohyperkeratosis via TNF-a, IL-20R2 and IL-22 69,70. These results are supported by findings in humans including an over-expression of IL-23p19 and IL-12p40 (precursors of IL-23) seen at the mRNA level in psoriatic skin lesions, compared to uninvolved skin. Further data indicate that production of IL-23 occurs at inflammatory skin sites and is mediated by tissue-resident and/or recruited immune cells, such as dendritic cells and possibly keratinocytes 71. The pathogenic role of IL-23 in psoriasis is strongly supported by the clinical findings that anti-TNF-a agents can reduce IL-23p19 and IL-12p40 mRNA levels, and reduction of IL-23 level caused by cyclosporin A, UV therapy and biological agents correlates to clinical improvements in psoriasis patients 72-74.
Transforming growth factor (TGF)-b1, IL-6 and IL-21 are all required to transform naïve T cells into cells expressing the unique lineage-specific transcription factor, RORC variant 2 and IL-23 receptors with subsequent binding of IL-23 resulting in differentiation into Th17 cells. Th17 cells in turn produce IL-17A, IL-17F, IL-22 and IL-26 which are proinflammatory cytokines 75. These cytokines activate keratinocytes leading to hyperproliferation and further production of proinflammatory cytokines, chemokines and antimicrobial peptides, which in turn recruit and activate other immune cells in the inflamed skin, leading to amplification of the inflammatory response and clinical features of the disease.
Support for a role of the IL-23/Th17 axis in psoriasis comes from whole genome studies showing that genetic variants of the IL-23 receptor and its ligand IL12B are associated with psoriasis 35.
Regarding the clinical relevance of the IL-23/Th17 pathway, targeting the common subunit p40 of IL-12 and IL-23 has demonstrated clinical improvement in psoriasis. Two anti-IL-12p40 monoclonal Abs, CNTO-1275/ustekinumab and ABT-874, have been recently developed as psoriasis treatments. Ustekinumab and ABT-874 are humanized IgG1 monoclonal antibodies that bind to the p40 subunit of human IL-12 and IL-23 and prevent interaction with IL-12Rb1. Phase I 76 and phase II 77,78 studies have supported the use of both antibodies as effective treatments for moderate to severe psoriasis. The safety profile of ustekinumab in psoriasis has been evaluated in 2 phase III studies. Of these, PHOENIX I assessed the efficacy and safety of ustekinumab 45 and 90 mg administered subcutaneously at weeks 0, 4, and then every 12 weeks over 76 weeks of treatment 79. 67.1 % and 66.4 % of patients who received ustekinumab 45mg and 90mg respectively, achieved PASI-75 (improvement of PASI score by 75 %) at week 12 compared to placebo control (3.1 %). Efficacy increased over time and maximum effect was seen at week 24 with PASI-75 achieved in 76.1 % of patients treated with 45mg and in 85 % of patients treated with 90mg. The observed adverse events were mild, non-life threatening and not significantly different from the placebo group. The most commonly reported adverse events were upper respiratory tract infections, nasopharyngitis, headache, and arthralgia. The PHOENIX II trial 80 was conducted to further assess if dosing intensification would increase the response to treatment in partial responder patients (between PASI-50 and PASI-75). It was found that dosing intensification resulted in increased clinical efficacy only in patients receiving 90mg, but not 45mg, of ustekinumab every 8 weeks (PASI-75 in 68.8 % of patients receiving 90mg every 8 weeks versus 33.3 % of patients receiving 90 mg every 12 weeks). The incidence and type of adverse events observed did not differ between PHOENIX I and II studies. Ustekinumab is also effective in the treatment of psoriatic arthritis 81 and this study again confirmed that ustekinumab is well tolerated 81.
Regulatory T cells
Regulatory T (Treg) cells are characterized by their ability to suppress the activation and proliferation of CD4+ and CD8+ effector T cells via mechanisms that either require direct contact with antigen presenting cells 82 or by releasing IL-10 83 or transforming growth factor beta 1 (TGF-b1) 84. Treg cells express CD4, CD25 and the specific transcription factor Foxp3. They account for between 1-5 % of the total population of peripheral CD4+ cells. Dysfunction of Treg cells has been implicated in the pathogenesis of autoimmune diseases such as multiple sclerosis, rheumatoid arthritis and autoimmune polyglandular syndrome type II. In psoriasis, Treg function and proliferation are both defective 47. This combination may result in a failure to constrain the activation and proliferation of pathogenic T cells, contributing to the ongoing inflammation seen in psoriasis. Hence strategies that correct Treg function or increase the Treg:pathogenic T cell ratio may be potential treatments for psoriasis 47. Phototherapy may induce Treg type suppressor cells as well as eliminate pathogenic T cells 85, supporting a possible role of Treg cells in protection against psoriasis.
Contribution of animal models in understanding the roles of resident T cells in the skin and the importance of plasmacytoid dendritic cells (PDCs)
A major hindrance in studying the immunopathogenesis of psoriasis and subsequent development of new therapies is the lack of disease relevant animal models. No naturally occurring disorder in laboratory animals can mimic the complex phenotype of psoriasis. After decades of research, a number of psoriasis mouse models have been developed, each with specific advantages and shortcomings. A number of comprehensive and critical reviews of different psoriasis mouse models have been published recently 86-88. Criteria for an ideal psoriasis mouse model 86 should include:
Display of typical psoriasis clinical features and histopathology.
Feature the major role of T cells, due to overwhelming evidence in the literature.
Psoriatic lesions should clear with well established pharmacological treatments for the disease.
The pharmacologically validated model should reflect the clinical response in patients. There are two main approaches to the currently available mouse models: a) generation of mice with over-expression (transgenic) or deletion (knock out) of certain genes and gene products in specific tissue compartments such as the epidermis and b) xenotransplantation models involving transplantation of human skin onto immunosuppressed mice to avoid graft rejection. The latter approach may overcome the problem of species difference which exists in the former approach.
Although no single model is perfect, the xenotransplantation AGR 129 mouse model 89 has been instrumental in understanding the early events of the immunopathogenesis of psoriasis. AGR 129 mice are deficient in type I (A) and type II (G) IFN receptors, in addition to lacking T and B cells (RAG-2 –/–). In the AGR 129 mouse model, psoriatic phenotype develops spontaneously upon transplantation of symptomless non-lesional skin onto mice. No exogenous cells or factors are required to initiate the psoriatic lesions thereby allowing studies of early mechanisms in psoriasis immunopathogenesis. After transplantation of non-involved psoriatic skin onto the AGR 129 mouse, injection of the monoclonal anti-human CD3 antibody, which blocks T cell functions, results in inhibition of psoriasis development 89. Furthermore, application of a neutralizing anti-human TNF-a monoclonal antibody, or TNF receptor fusion protein, leads to significant reduction of T cell numbers in the graft and inhibition of psoriasis phenotype development 89. It was demonstrated that the source of TNF-a is local antigen presenting cells within the skin graft. Altogether, these data suggest that local TNF-a production is crucial for resident T cell proliferation and psoriasis development. These studies also provide evidence that local proliferation of effector T cells, rather than their recruitment, is important for induction of psoriasis.
Three types of DCs (Langerhans cells, dermal DCs and plasmacytoid DCs) play important roles in psoriasis. It has been demonstrated that plasmacytoid dendritic cells (PDCs) produce IFN-a, which results in the activation of many aspects of the innate immune system and helps drive the inflammatory process in psoriasis 90. A role for IFN-a in psoriasis is supported by three observations 90. First, an activated IFN-a signalling pathway is present in psoriatic skin lesion 91,92. Secondly, continuous excessive IFN-a/b signalling results in an inflammatory skin disease in a mouse model that resembles psoriasis. Thirdly, administration of recombinant IFN-a to psoriasis patients for treatment of viral infections or tumours may worsen psoriasis. It has been demonstrated that PDCs accumulate in the skin of psoriasis patients and are activated to produce IFN-a early in the development of psoriasis 90. PDCs are a rare cell population in peripheral blood and secondary lymphoid organs. They are the main effector cells in executing the antiviral activities of the innate immune system due to their unique ability to secrete large amounts of IFN-a in response to viral stimulation through their toll-like receptor (TLR)-7 and TLR-9. Through IFN-a production, PDCs drive the activation and expansion of the autoimmune T cell cascade leading to psoriasis 90, hence PDCs may provide a unique link between the innate and adaptive immune system in driving inflammation in psoriasis. In the same study, the number of PDCs were found to be increased in both plaque lesions and the nearby uninvolved skin of psoriasis patients but were completely absent in normal healthy skin or inflamed skin of atopic dermatitis. Activation of PDCs was demonstrated to occur locally in lesional psoriatic skin. IFN-a production by PDCs was increased early and transiently in the development of psoriasis and production declined as disease progressed. Intravenous ijection of an anti-BDCA-2 monoclonal antibody, which shared specific binding to human PDCs, led to a > 90 % reduction of IFN-a expression in the engrafted pre-psoriatic skin, resulting in complete inhibition of the subsequent pathogenic T cell activation and expansion and psoriatic phenotype development. Because human PDCs from the transplanted skin do not re-circulate in the AGR 129 mouse model, in vivo treatment with anti-BDCA-2 antibody selectively targeted human PDCs present in the engrafted pre-psoriatic skin, thereby confirming that PDCs represented the main IFN-a-producing cells. Administration of recombinant human IFN-a was found to be sufficient to reverse the anti-BDCA-2-mediated inhibition of T cell expansion and psoriasis development 90. Hence, activation of PDCs and their IFN-a production represents an important proximal event in the psoriasis immune axis, highlighting the contribution of innate immunity towards autoimmunity in psoriasis. The pathogenic role of PDCs in psoriasis is further supported by the observation that the topical ILR 7/8 ligand imiquimod which activates PDCs can exacerbate psoriasis 93. Hence therapies that target PDCs and PDC-derived IFN-a may be valuable for both prevention and early treatment of psoriasis. IFN-a targeted therapies may be advantageous over anti-TNF-a therapy in that its production is highly specific, both spatially and temporally i.e. IFN-a is mainly produced by dermal PDCs during the early stage of psoriasis development unlike TNF-a which is produced by a broad range of cells including myeloid DCs, T cells and keratinocytes throughout different stages of psoriasis. Hence, IFN-a targeted therapies may facilitate prevention and early treatment of psoriasis, minimizing the need for long term administration and monitoring of existing psoriasis therapies with a number of potential adverse side effects. PDCs and IFN-a have also been implicated in a number of other autoimmune diseases including lupus erythematosus, rheumatoid arthritis and insulin-dependent diabetes mellitus 94. Hence the role of PDC-induced innate activation via IFN-a production may also provide valuable insights into the pathogenesis of other autoimmune diseases and their treatment strategies.
Role of bacterial superantigen
Of the many environmental factors implicated in the immunopathogenesis of psoriasis, throat infection with a-haemolytic streptococci is an external trigger that has been associated with initiation and exacerbation of guttate psoriasis 95. However, a specific antigen responsible for T cell activation in the skin has yet to be conclusively identified. It has been suggested that antigen presenting cells in the tonsillar tissue may ingest bacterial wall fragments and are then re-circulated into the skin where they may activate a T cell response, resulting in psoriatic lesions 96. Molecular mimicry is another hypothesis where T cell clones originally generated in response to streptococcal tonsillitis or pharyngitis are subsequently reactivated in the skin because of a cross-reactive epitope derived from hyperproliferative epidermal keratinocytes such as keratin 16 or keratin 1797. In a recent review98 the close correlation between global epidemiological variation in psoriasis prevalence with that of historical mortality from epidemics of invasive streptococcal infections was highlighted and the authors suggested that the psoriasis susceptibility genotype may confer protection from mortality in such epidemics. It is postulated that changes in immunological pathways including the adaptive and the innate immune response as well as the Th17 cell cytokine network all confer protection against mortality during epidemics of invasive streptococcal infections by increasing efficiency in internalizing streptococci but also increase predisposition to psoriasis development 98.
Psoriasis as an autoimmune disease
Although the presence of T-lymphocyte subsets in the early phase of the disease and the response to T-lymphocyte-targeting therapies strongly support the hypothesis of psoriasis as a T-lymphocyte-mediated autoimmune disease, there are still significant gaps in our understanding of this disease. Unlike a well characterized autoimmune skin disease such as pemphigus vulgaris, where the autoantigens and effector molecules (autoantibodies, complement) have been extensively defined using in vitro and in vivo model systems 99, no reproducible autoantigen has yet been defined in psoriasis.
Recent data suggest that LL37 may be a key activator of PDCs in psoriasis, converting self-DNA into an autoimmune trigger 100. The role of PDCs as key mediators in the immunopathogenesis of psoriasis has already been discussed. PDCs can recognise bacterial and viral DNA through Toll-like receptors (TLR7 and TLR9) and release type 1 IFNs like IFN-a. Under normal circumstances PDCs do not respond to self-DNA. However, in autoimmune disease, the endogenous antimicrobial peptide LL37 may combine with and convert self-DNA into a potent autoimmune trigger which activates PDCs into producing IFN-a. The antimicrobial peptide LL37 is mainly expressed by keratinocytes and is released by keratinocytes in response to injury or infection. LL37 is also released by migratory inflammatory cells including neutrophils and is present in the dermal compartment in association with PDCs. Increased expression of LL37 appears to be specific in psoriasis as it is found to be highly upregulated in psoriatic lesions but not normal skin, uninvolved skin of psoriasis patients or other skin disease lesions.
Data suggest that when coupled with DNA, LL37 is taken up by PDCs and these LL37:DNA complexes then activate PDCs through TLR9 and stimulate IFN-a production 100. This may explain how self-DNA may trigger autoimmunity in psoriasis.
a1b1 integrin and epidermal T cell accumulation
Based on the findings that expansion of skin resident T cells is important in psoriasis development in the xenotransplantation AGR mouse model, the role of tissue-specific factors in activation and expansion of resident T cells has been further explored 66. T cells need to pass through the dermo-epidermal junction in order to enter the epidermis and collagen fibrils are an essential part of the dermo-epidermal junction. The most important basement membrane collagen is collagen IV. Long-term activation of T cells results in the expression of a receptor for collagen IV, the heterodimeric integrin a1b1 (synonymous with CD49a-CD29 or very late antigen 1, VLA-1). It has been shown that epidermal accumulation of a1b1-positive Th1 and Tc1 cells correlate with psoriasis development. Blocking a1b1 with a neutralizing monoclonal antibody prevents epidermal T cell accumulation and subsequent psoriasis development in the xenotransplantation AGR mouse model. a1b1 expression may act as a checkpoint for entry of T cells into epidermis with a1b1-positive epidermal T cells potentially playing an important role in psoriatic lesion formation. Hence targeting of these integrins may offer new therapeutic approaches in psoriasis and possibly in other autoimmune disease.
Conclusion
Extensive progress has been made in understanding the immunopathogenesis of psoriasis, over the last 10 years. Transfer between knowledge acquired from bench research and clinical studies is crucial for rapid progression in both areas. The discovery of the IL23/Th17 pathway and the subsequent development of new treatments have been major breakthroughs and better insight into psoriasis immunopathogenesis does not only lead to improved treatments for psoriasis but may also provide better understanding of pathological mechanisms behind other autoimmune diseases such as Crohn's disease and better therapeutic treatments for these diseases.
Acknowledgments
We acknowledge support by the following grant funding bodies: Medical Research Council Clinical Research Training Fellowship, Wellcome Trust Programme GR078173MA, NIH RO1AR040065, NIHR Comprehensive Biomedical Research Centre Guy's and St. Thomas' Hospital and King's College London, Medical Research Council UK Programme G0601387, Dunhill Medical Trust. Dr. John Mee provided helpful discussions.
References
- 1.Nestle FO, Kaplan DH, Barker J. Mechanisms of Disease: Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–6. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 3.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493–9. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 4.Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- 5.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 7.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 8.Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol. 2003;4:441–7. doi: 10.2165/00128071-200304070-00001. [DOI] [PubMed] [Google Scholar]
- 9.Eyre RW, Krueger GG. The Koebner response in psoriasis. In: Roenigk HH, Maibach HI, editors. Psoriasis. New York: Marcel Dekker; 1984. pp. 105–16. [Google Scholar]
- 10.Reveille JD, Conant MA, Duvic M. Human immunodeficiency virus-associated psoriasis, psoriatic arthritis, and Reiter's syndrome: a disease continuum? Arthritis Rheum. 1990;33:1574–8. doi: 10.1002/art.1780331016. [DOI] [PubMed] [Google Scholar]
- 11.Stewart AF, Battaglini-Sabetta J, Millstone L. Hypocalcemia-induced pustular psoriasis of von Zumbusch. New experience with an old syndrome. Ann Intern Med. 1984;100:677–80. doi: 10.7326/0003-4819-100-5-677. [DOI] [PubMed] [Google Scholar]
- 12.Fortune DG, Richards HL, Main CJ, Griffiths CE. What patients with psoriasis believe about their condition. J Am Acad Dermatol. 1998;39:196–201. doi: 10.1016/s0190-9622(98)70074-x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta MA, Gupta AK, Kirkby S, Schork NJ, Gorr SK, Ellis CN, et al. A psychocutaneous profile of psoriasis patients who are stress reactors. A study of 127 patients. Gen Hosp Psychiatry. 1989;11:166–73. doi: 10.1016/0163-8343(89)90036-4. [DOI] [PubMed] [Google Scholar]
- 14.Abel EA, DiCicco LM, Orenberg EK, Fraki JE, Farber EM. Drugs in exacerbation of psoriasis. J Am Acad Dermatol. 1986;15:1007–22. doi: 10.1016/s0190-9622(86)70265-x. [DOI] [PubMed] [Google Scholar]
- 15.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–6. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda K, Sapadin A, Shoji T, Fleischmajer R, Lebwohl M. Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Invest Dermatol. 2001;116:713–20. doi: 10.1046/j.1523-1747.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- 18.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–8. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 20.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 21.Esposito M, Saraceno R, Giunta A, Maccarone M, Chimenti S. An Italian study on psoriasis and depression. Dermatology. 2006;212:123–7. doi: 10.1159/000090652. [DOI] [PubMed] [Google Scholar]
- 22.Mrowietz U, Elder JT, Barker J. The importance of disease associations and concomitant therapy for the long-term management of psoriasis patients. Arch Dermatol Res. 2006;298:309–19. doi: 10.1007/s00403-006-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64 2:ii37–9. doi: 10.1136/ard.2004.030775. discussion ii40-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capon F, Trembath RC, Barker JN. An update on the genetics of psoriasis. Dermatol Clin. 2004;22:339–47. vii. doi: 10.1016/S0733-8635(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 25.Morris A, Rogers M, Fischer G, Williams K. Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol. 2001;18:188–98. doi: 10.1046/j.1525-1470.2001.018003188.x. [DOI] [PubMed] [Google Scholar]
- 26.Watson W, Cann HM, Farber EM, Nall ML. The genetics of psoriasis. Arch Dermatol. 1972;105:197–207. [PubMed] [Google Scholar]
- 27.Theeuwes M, Leder RO. Hereditary insights in psoriasis. Eur J Dermatol. 1993;3:335–41. [Google Scholar]
- 28.Brandrup F, Hauge M, Henningsen K, Eriksen B. Psoriasis in an unselected series of twins. Arch Dermatol. 1978;114:874–8. [PubMed] [Google Scholar]
- 29.Duffy DL, Spelman LS, Martin NG. Psoriasis in Australian twins. J Am Acad Dermatol. 1993;29:428–34. doi: 10.1016/0190-9622(93)70206-9. [DOI] [PubMed] [Google Scholar]
- 30.Farber EM, Nall ML, Watson W. Natural history of psoriasis in 61 twin pairs. Arch Dermatol. 1974;109:207–11. [PubMed] [Google Scholar]
- 31.Pisani M, Ruocco V. ‘Twin’ psoriasis in monozygotic twins. Arch Dermatol. 1984;120:1418–9. [PubMed] [Google Scholar]
- 32.Nair RP, Stuart PE, Nistor I, Hiremagalore R, Chia NV, Jenisch S, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–51. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RD, Frodsham A, et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet. 1997;6:813–20. doi: 10.1093/hmg/6.5.813. [DOI] [PubMed] [Google Scholar]
- 34.Capon F, Bijlmakers MJ, Wolf N, Quaranta M, Huffmeier U, Allen M, et al. Identification of ZNF313/RNF114 as a novel psoriasis susceptibility gene. Hum Mol Genet. 2008;17:1938–45. doi: 10.1093/hmg/ddn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–6. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 36.Mallon E, Newson R, Bunker CB. HLA-Cw6 and the genetic predisposition to psoriasis: a meta-analysis of published serologic studies. J Invest Dermatol. 1999;113:693–5. doi: 10.1046/j.1523-1747.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- 37.Gudjonsson JE, Karason A, Antonsdottir A, Runarsdottir EH, Hauksson VB, Upmanyu R, et al. Psoriasis patients who are homozygous for the HLA-Cw*0602 allele have a 2.5-fold increased risk of developing psoriasis compared with Cw6 heterozygotes. Br J Dermatol. 2003;148:233–5. doi: 10.1046/j.1365-2133.2003.05115.x. [DOI] [PubMed] [Google Scholar]
- 38.Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13:450–6. doi: 10.1016/s0190-9622(85)70188-0. [DOI] [PubMed] [Google Scholar]
- 39.Gudjonsson JE, Karason A, Runarsdottir EH, Antonsdottir AA, Hauksson VB, Jonsson HH, et al. Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients–an analysis of 1019 HLA-C- and HLA-B-typed patients. J Invest Dermatol. 2006;126:740–5. doi: 10.1038/sj.jid.5700118. [DOI] [PubMed] [Google Scholar]
- 40.Fan X, Yang S, Sun LD, Liang YH, Gao M, Zhang KY, et al. Comparison of clinical features of HLA-Cw*0602-positive and -negative psoriasis patients in a Han Chinese population. Acta Derm Venereol. 2007;87:335–40. doi: 10.2340/00015555-0253. [DOI] [PubMed] [Google Scholar]
- 41.Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39–42. [PubMed] [Google Scholar]
- 42.Prinz JC. Psoriasis vulgaris–a sterile antibacterial skin reaction mediated by cross-reactive T cells? An immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326–32. doi: 10.1046/j.1365-2230.2001.00831.x. [DOI] [PubMed] [Google Scholar]
- 43.Nickoloff BJ. Skin innate immune system in psoriasis: friend or foe? J Clin Invest. 1999;104:1161–4. doi: 10.1172/JCI8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin MP, Nelson G, Lee JH, Pellett F, Gao X, Wade J, et al. Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol. 2002;169:2818–22. doi: 10.4049/jimmunol.169.6.2818. [DOI] [PubMed] [Google Scholar]
- 45.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46:1–23. doi: 10.1067/mjd.2002.120568. quiz -6. [DOI] [PubMed] [Google Scholar]
- 46.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+ CD25 high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54:S67–80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 49.Ghoreschi K, Thomas P, Breit S, Dugas M, Mailhammer R, van Eden W, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9:40–6. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 50.Asadullah K, Docke WD, Ebeling M, Friedrich M, Belbe G, Audring H, et al. Interleukin 10 treatment of psoriasis: clinical results of a phase 2 trial. Arch Dermatol. 1999;135:187–92. doi: 10.1001/archderm.135.2.187. [DOI] [PubMed] [Google Scholar]
- 51.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–22. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 53.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357:1842–7. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- 54.Patel T, Gordon KB. Adalimumab: efficacy and safety in psoriasis and rheumatoid arthritis. Dermatol Ther. 2004;17:427–31. doi: 10.1111/j.1396-0296.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- 55.Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–85. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 56.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 57.Krueger GG, Bergstresser PR, Lowe NJ, Voorhees JJ, Weinstein GD. Psoriasis. J Am Acad Dermatol. 1984;11:937–47. doi: 10.1016/s0190-9622(84)80018-3. [DOI] [PubMed] [Google Scholar]
- 58.Ellis CN, Gorsulowsky DC, Hamilton TA, Billings JK, Brown MD, Headington JT, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110–6. [PubMed] [Google Scholar]
- 59.Baker BS, Griffiths CE, Lambert S, Powles AV, Leonard JN, Valdimarsson H, et al. The effects of cyclosporin A on T lymphocyte and dendritic cell sub-populations in psoriasis. Br J Dermatol. 1987;116:503–10. doi: 10.1111/j.1365-2133.1987.tb05869.x. [DOI] [PubMed] [Google Scholar]
- 60.Jegasothy BV, Ackerman CD, Todo S, Fung JJ, Abu-Elmagd K, Starzl TE. Tacrolimus (FK 506)–a new therapeutic agent for severe recalcitrant psoriasis. Arch Dermatol. 1992;128:781–5. [PMC free article] [PubMed] [Google Scholar]
- 61.Prinz J, Braun-Falco O, Meurer M, Daddona P, Reiter C, Rieber P, et al. Chimaeric CD4 monoclonal antibody in treatment of generalised pustular psoriasis. Lancet. 1991;338:320–1. doi: 10.1016/0140-6736(91)90464-z. [DOI] [PubMed] [Google Scholar]
- 62.Gottlieb SL, Gilleaudeau P, Johnson R, Estes L, Woodworth TG, Gottlieb AB, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442–7. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- 63.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–52. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abrams JR, Kelley SL, Hayes E, Kikuchi T, Brown MJ, Kang S, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med. 2000;192:681–94. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menssen A, Trommler P, Vollmer S, Schendel D, Albert E, Gurtler L, et al. Evidence for an antigen-specific cellular immune response in skin lesions of patients with psoriasis vulgaris. J Immunol. 1995;155:4078–83. [PubMed] [Google Scholar]
- 66.Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–42. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 67.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 Axis in the Immunopathogenesis of Psoriasis. J Invest Dermatol. 2009;129:1339–50. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 68.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 69.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 71.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–15. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 72.Piskin G, Tursen U, Sylva-Steenland RM, Bos JD, Teunissen MB. Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers – IL-12, IL-18 and IL-23. Exp Dermatol. 2004;13:764–72. doi: 10.1111/j.0906-6705.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 73.Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–9. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- 74.Haider AS, Lowes MA, Suárez-Farinas M, Zaba LC, Cardinale I, Khatcherian A, et al. Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J Immunol. 2008;180:1913–20. doi: 10.4049/jimmunol.180.3.1913. [DOI] [PubMed] [Google Scholar]
- 75.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kauffman CL, Aria N, Toichi E, McCormick TS, Cooper KD, Gottlieb AB, et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J Invest Dermatol. 2004;123:1037–44. doi: 10.1111/j.0022-202X.2004.23448.x. [DOI] [PubMed] [Google Scholar]
- 77.Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–92. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 78.Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol. 2008;144:200–7. doi: 10.1001/archdermatol.2007.63. [DOI] [PubMed] [Google Scholar]
- 79.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–74. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 80.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–84. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 81.Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–40. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- 82.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–6. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 83.Annacker O, Asseman C, Read S, Powrie F. Interleukin-10 in the regulation of T cell-induced colitis. J Autoimmun. 2003;20:277–9. doi: 10.1016/s0896-8411(03)00045-3. [DOI] [PubMed] [Google Scholar]
- 84.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 85.Baadsgaard O, Salvo B, Mannie A, Dass B, Fox DA, Cooper KD. In vivo ultraviolet-exposed human epidermal cells activate T suppressor cell pathways that involve CD4+CD45RA+ suppressor-inducer T cells. J Immunol. 1990;145:2854–61. [PubMed] [Google Scholar]
- 86.Nestle FO, Nickoloff BJ. From classical mouse models of psoriasis to a spontaneous xenograft model featuring use of AGR mice. Ernst Schering Res Found Workshop. 2005:203–12. doi: 10.1007/3-540-26811-1_11. [DOI] [PubMed] [Google Scholar]
- 87.Schon MP. Animal models of psoriasis: a critical appraisal. Exp Dermatol. 2008;17:703–12. doi: 10.1111/j.1600-0625.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- 88.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127:1292–308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- 89.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. 2004;199:731–6. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmid P, Itin P, Cox D, McMaster GK, Horisberger MA. The type I interferon system is locally activated in psoriatic lesions. J Interferon Res. 1994;14:229–34. doi: 10.1089/jir.1994.14.229. [DOI] [PubMed] [Google Scholar]
- 92.van der Fits L, van der Wel LI, Laman JD, Prens EP, Verschuren MC. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-alpha sensitivity is unaltered. J Invest Dermatol. 2004;122:51–60. doi: 10.1046/j.0022-202X.2003.22113.x. [DOI] [PubMed] [Google Scholar]
- 93.Gilliet M, Conrad C, Geiges M, Cozzio A, Thurlimann W, Burg G, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490–5. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 94.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 95.Fry L. Psoriasis. Br J Dermatol. 1988;119:445–61. doi: 10.1111/j.1365-2133.1988.tb03248.x. [DOI] [PubMed] [Google Scholar]
- 96.Baker BS, Laman JD, Powles A, van der Fits L, Voerman JS, Melief MJ, et al. Peptidoglycan and peptidoglycan-specific Th1 cells in psoriatic skin lesions. J Pathol. 2006;209:174–81. doi: 10.1002/path.1954. [DOI] [PubMed] [Google Scholar]
- 97.Johnston A, Gudjonsson JE, Sigmundsdottir H, Love TJ, Valdimarsson H. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin Exp Immunol. 2004;138:83–93. doi: 10.1111/j.1365-2249.2004.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McFadden JP, Baker BS, Powles AV, Fry L. Psoriasis and streptococci: the natural selection of psoriasis revisited. Br J Dermatol. 2009;160:929–37. doi: 10.1111/j.1365-2133.2009.09102.x. [DOI] [PubMed] [Google Scholar]
- 99.Pias EK, Hilario-Vargas J, Li N, Diaz LA. Humoral autoimmunity in pemphigus. Autoimmunity. 2004;37:283–6. doi: 10.1080/08916930410001710848. [DOI] [PubMed] [Google Scholar]
- 100.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]