Abstract
Background
Phthalates are widely used chemicals, and human exposure is extensive. Recent studies have indicated that phthalates may have thyroid-disrupting properties.
Objective
We aimed to assess concentrations of phthalate metabolites in urine samples from Danish children and to investigate the associations with thyroid function, insulin-like growth factor I (IGF-I), and growth.
Methods
In 845 children 4–9 years of age, we determined urinary concentrations of 12 phthalate metabolites and serum levels of thyroid-stimulating hormone, thyroid hormones, and IGF-I.
Results
Phthalate metabolites were detected in all urine samples, of which monobutyl phthalate was present in highest concentration. Phthalate metabolites were negatively associated with serum levels of free and total triiodothyronine, although statistically significant primarily in girls. Metabolites of di(2-ethylhexyl) phthalate and diisononyl phthalate were negatively associated with IGF-I in boys. Most phthalate metabolites were negatively associated with height, weight, body surface, and height gain in both sexes.
Conclusions
Our study showed negative associations between urinary phthalate concentrations and thyroid hormones, IGF-I, and growth in children. Although our study was not designed to reveal the mechanism of action, the overall coherent negative associations between urine phthalate and thyroid and growth parameters may suggest causative negative roles of phthalate exposures for child health.
Keywords: growth, insulin-like growth factor I, phthalate, thyroid
A normal thyroid function is important for growth and neurological development in children, and hypothyroidism in childhood is accompanied by growth retardation. A growing number of reports have indicated that environmental chemicals can interfere with thyroid function [reviewed by Boas et al. (2006)]. Both experimental and observational studies of wildlife and humans have suggested specific chemicals to have thyroid-disrupting properties, including recent investigations of phthalates. Phthalates are widely used industrial chemicals that are applied in a large variety of commercial products—for example, as plasticizers in toys, personal care products, and building materials, including wall paint (Sathyanarayana et al. 2008; Schettler 2006; Wormuth et al. 2006). Thus, human exposure is extensive, as demonstrated in numerous studies by quantifying phthalate metabolites in urine samples from adults and children (Becker et al. 2004; Meeker et al. 2007; Silva et al. 2004). Most human studies have investigated reproductive effects of phthalate exposure, but recent studies also indicated thyroid-disrupting effects. Thus, serum levels of free thyroxine (T4) and total triiodothyronine (T3) in adult men were negatively associated with concentrations of metabolites of di(2-ethylhexyl) phthalate (DEHP) (Meeker et al. 2007). In pregnant women urinary concentrations of metabolites of di-n-butyl phthalate (DBP) were negatively correlated with serum levels of free and total T4 (Huang et al. 2007).
The mechanisms of thyroid-disruption may be multiple, because experimental studies have suggested that phthalates interfere with binding of T3 to transport proteins (Ishihara et al. 2003), interacting with the active T3 uptake at the plasma membrane (Shimada and Yamauchi 2004) or exerting antagonistic activity at the thyroid receptors (Shen et al. 2009).
Phthalate exposure in children is probably higher than in adults when relating intake to body weight (Koch et al. 2007). Furthermore, the adverse health outcomes due to environmental chemicals may be of greater significance in children because appropriate serum levels of thyroid hormone and insulin-like growth factor I (IGF-I) are significant for growth and neurological development. In a large cohort of children, we aimed to assess the exposure to six different diphthalates by measuring their metabolites in urine samples. Furthermore, we investigated the associations with growth and serum levels of thyroid hormones and IGF-I.
Materials and Methods
Study participants and design
A total of 845 children 4–9 years of age were submitted to thorough clinical examinations between January 2006 and August 2007. All children had previously participated in a longitudinal cohort study, for which 1,953 women were included consecutively at their first routine obstetric control early in pregnancy at three university hospitals in Copenhagen, Denmark. Information regarding pregnancy and maternal health was obtained from medical records and questionnaires answered by the women. Gestational age of the newborn child was based on sonography, last menstrual period, and clinical evaluation of the newborn child. In case of discrepancies, sonography measurements were used. Details of the study have previously been published (Chellakooty et al. 2006). The children were examined shortly after birth and at 3, 18, and 36 months of age by standardized examinations. The length of the newborn child was measured supine with a Kiddimeter (Raven Equipment Ltd., Essex, UK) to the nearest 0.1 cm. All children participating up to 3 years of age (n = 1,440) were asked to participate in a follow-up study, 902 of whom consented. Of these, all children delivering a spot urine sample (n = 845) composed the present study population. The present study comprised measurements of height, weight, clinical assessment of pubertal stage (Tanner stage), ultrasound of the thyroid gland, including calculation of the gland volume (n = 839; Boas et al. 2009), blood samples (n = 786), and spot urine samples. In addition, the parents filled in a questionnaire on health and lifestyle.
In the present study, we present information on phthalate exposure based on data from all children from whom urine samples had been collected. For the statistical analyses of associations between phthalate concentrations and growth or endocrine measures, we excluded all children suffering from diseases prone to affect growth or endocrine status as well as all children with clinical signs of puberty. Thus, a total of 26 children were excluded because of heart disease (n = 3), brain tumor (n = 1), Langerhans cell histiocytosis (n = 1), diabetes (n = 2), epilepsy (n = 3), cerebral palsy (n = 1), chronic gastrointestinal diseases (n = 2), juvenile arthritis (n = 1), pathological thyroid function tests [two with thyroid-stimulating hormone (TSH) > 3 SD, one with T4 < 3 SD], or puberty (n = 12).
Hormone analyses
Nonfasting peripheral venous blood samples were drawn from an antecubital vein between midmorning and late afternoon. Samples were separated by centrifugation and stored at −20°C until analyses. All analyses were carried out blinded for the technician and in random order.
TSH and thyroid hormones (T4, free T4, T3, and free T3) were measured with an electrochemiluminescence immunoassay (Modular Analytics E170; Roche GmbH, Mannheim, Germany). Total assay variations for TSH were 8.7% and 8.4% at concentrations of 0.9 and 4.9 mU/L, respectively; T4, 5.6% and 5.6% at 81 and 167 nmol/L; free T4, 6.0% and 8.1% at 12 and 30 pmol/L; T3, 6.7% and 6.6% at 3.2 and 6.0 nmol/L; and free T3, 6.4% and 6.4% at 5.3 and 15.0 pmol/L.
IGF-I and insulin-like growth factor binding protein 3 (IGFBP-3) were measured with solid-phase enzyme-labeled chemiluminescent immunometric assays (Immulite 2000; Diagnostic Products Corp., Los Angeles, CA, USA) using World Health Organization National Institute for Biological Standards and Control International Reference Reagent 87/518 and 93/560 standards, respectively. The limits of detection (LODs) were 20 and 0.1 μg/mL, respectively. Intraassay variations were < 2.1% and 4.4%, respectively; and interassay variations were < 8.9% and 5.6%, respectively.
Urinary phthalate metabolite analyses
Spot urine samples were collected in polyethylene cups and stored as 10-mL aliquots in 20-mL glass scintillation vials with tops packed with aluminum foil at −20°C. Urine samples were analyzed for concentrations of 12 different phthalate metabolites by liquid chromatography (LC) tandem mass spectrometry with preceding enzymatic deconjugation followed by solid phase extraction. The method for preparation of samples, standard solutions, and quality controls as well as the instrumental analysis was previously described (Janjua et al. 2008) and used with the following modifications. The solvents for LC separation were as follows: A, 0.1% acetic acid in water; B, 0.1% acetic acid in acetonitrile. Solvent programming was 0.0–1.5 min, 5% B; 1.6 min, 27% B; 6.0 min, 30% B; 6.1–10.0 min, 45% B; 10.1 min, 70% B; 12.0–15.5 min, 90% B; 15.6–17.0 min, 5% B. For all analytes, the retention time on column was 6.55–13.14 min, and a good separation was obtained. The precursor and product ions (mz) were as previously described (Silva et al. 2007). The calibration curve range was 0.5–500 ng/mL. Method accuracy and precision were validated by repeating (n = 5) intraday analysis of pooled urine samples spiked with native phthalate standards (5, 10, and 50 ng/mL) and by repeating interday analysis of control urine samples spiked with low and high concentrations (n = 24 over a 2-month period). Mean (± SD) recovery ranged from 88% ± 8.6% to 108% ± 4.5%, and interday variation was < 10% for most analytes. LODs were calculated as previously described (Blount et al. 2000) and are listed in Table 1. All urine samples with extremely high concentrations of phthalate metabolites were reanalyzed to confirm the values.
Table 1.
Sex-specific phthalate metabolite concentrations (μg/L and μg/g creatinine) in spot urine samples from 845 Danish children 4–9 years of age (examined 2006–2007).
| Phthalate metabolite | LOD | Percent > LOD | Male |
Female |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | GM | Min | 25% | 75% | Max | Median | GM | Min | 25% | 75% | Max | ||||
| Uncorrected concentration (μg/L) | |||||||||||||||
| MEP | 0.24 | 100 | 21 | 21 | 0.6 | 11 | 39 | 731 | 21 | 21 | 1.1 | 10 | 44 | 684 | |
| MBP | 3.94 | 100 | 130 | 124 | 12 | 75 | 207 | 6,457 | 121 | 114 | 9.0 | 63 | 216 | 1,217 | |
| MBzP | 1.26 | 86 | 17 | 2.8 | 0.0 | 6.2 | 37 | 4,548 | 12 | 0.5 | 0.0 | 3.3 | 31 | 272 | |
| MEHP | 0.31 | 99 | 4.5 | 4.1 | 0.0 | 2.5 | 7.7 | 78 | 3.6 | 3.6 | 0.0 | 1.8 | 7.2 | 231 | |
| MEHHP | 0.60 | 100 | 37 | 33.2 | 1.0 | 19 | 64 | 1,718 | 31 | 28 | 1.3 | 14 | 55 | 1,672 | |
| MEOHP | 0.14 | 100 | 19 | 17 | 0.5 | 9.6 | 32 | 656 | 16 | 15 | 0.6 | 7.8 | 29 | 734 | |
| MECPP | 0.43 | 100 | 30 | 29 | 1.0 | 16 | 52 | 676 | 27 | 27 | 1.7 | 14 | 49 | 1,755 | |
| MOP | 0.04 | 16 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 11 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 11 | |
| MiNP | 0.62 | 49 | 0.6 | 1.1 | 0.0 | 0.0 | 1.8 | 1,100 | 0.5 | 1.1 | 0.0 | 0.0 | 1.7 | 61 | |
| MHiNPa | 0.31 | 98 | 6.6 | 5.8 | 0.1 | 3.3 | 11 | 793 | 4.9 | 4.5 | 0.0 | 2.1 | 8.2 | 400 | |
| MOiNPa | 0.16 | 99 | 3.4 | 2.9 | 0.0 | 1.7 | 5.1 | 312 | 2.7 | 2.3 | 0.1 | 1.3 | 4.1 | 188 | |
| MCiOP | 0.08 | 100 | 7.2 | 7.3 | 0.3 | 4.1 | 12 | 2,063 | 6.5 | 6.3 | 0.3 | 3.5 | 12 | 598 | |
| Creatinine-corrected concentration (μg/g creatinine) | |||||||||||||||
| MEP | 31 | 34 | 5.7 | 20 | 52 | 791 | 36 | 40 | 7.6 | 22 | 65 | 526 | |||
| MBP | 191 | 199 | 47.5 | 140 | 276 | 4,940 | 227 | 221 | 39 | 157 | 312 | 1,365 | |||
| MBzP | 26 | 25 | 0.0 | 10 | 49 | 2,916 | 20 | 22 | 0.0 | 6.9 | 42 | 474 | |||
| MEHP | 6.8 | 6.9 | 0.0 | 4.1 | 11 | 210 | 6.7 | 7.2 | 0.0 | 4.1 | 12 | 186 | |||
| MEHHP | 52 | 53 | 4.9 | 33 | 84 | 1,818 | 52 | 55 | 7.3 | 36 | 81 | 1,220 | |||
| MEOHP | 26 | 27 | 2.6 | 17 | 42 | 794 | 28 | 29 | 3.5 | 18 | 41 | 536 | |||
| MECPP | 43 | 46 | 6.5 | 29 | 68 | 1,648 | 49 | 51 | 8.2 | 33 | 75 | 1,280 | |||
| MOP | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 11 | |||
| MiNP | 1.0 | 1.7 | 0.0 | 0.0 | 2.7 | 1,194 | 1.1 | 2.0 | 0.0 | 0.0 | 3.3 | 45 | |||
| MHiNPa | 8.4 | 8.9 | 1.6 | 5.5 | 14 | 754 | 7.4 | 7.6 | 0.0 | 4.9 | 9.9 | 292 | |||
| MOiNPa | 4.1 | 4.4 | 0.0 | 2.8 | 6.2 | 296 | 3.9 | 3.9 | 0.2 | 2.6 | 5.2 | 137 | |||
| MCiOP | 10 | 12 | 1.9 | 6.9 | 18 | 2,241 | 12 | 12 | 1.4 | 7.5 | 18 | 574 | |||
Abbreviations: 25%, 25th percentile; 75%, 75th percentile; GM, geometric mean; Max, maximum; Min, minimum.
Measured in only 250 randomly selected samples.
We analyzed the following metabolites: monoethyl phthalate (MEP) from diethyl phthalate (DEP); mono-n-butyl phthalate and monoisobutyl phthalate (MBP; analyzed as one compound) from di-n-butyl and DBP; monobenzyl phthalate (MBzP) from butyl benzyl phthalate; mono-(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) from DEHP; mono-n-octyl phthalate (MOP) from di-n-octyl phthalate; and monoisononyl phthalate (MiNP) and monocarboxyisooctyl phthalate (MCiOP) from diisononyl phthalate (DiNP). In 250 randomly selected samples (125 girls, 125 boys), two additional secondary metabolites of DiNP were measured: monohydroxyisononyl phthalate (MHiNP) and monooxoisononyl phthalate (MOiNP). In samples from 100 randomly selected children, the content of both free and total (sum of free and conjugated) phthalate metabolites was determined, and the ratio was calculated (concentration of free/concentration of total phthalate metabolites).
Urinary iodine and creatinine analyses
Because low or high serum levels of iodine are known to affect thyroid function, the overall iodine status of the population was estimated by quantifying the iodine (127I) concentration in 250 randomly selected urine samples using an inductively coupled plasma mass spectrometer (Agilent Technologies, Waldbron, Germany) after dilution by an aqueous solution containing tetramethylammonium hydroxide. LOD was 0.1 μg/L. Recovery of iodine (mean ±SD) was 103 ± 3% (n = 13). Creatinine was determined in all urine samples by colorimetric enzymatic assay.
Statistical analyses
Statistical analyses were performed with SPSS (version 17; SPSS, Inc., Chicago, IL, USA). Body surface area (BSA) was calculated using the DuBois formula: BSA (m2) = 0.007184 × height (cm)0.725 × weight (kg)0.425. Standard deviation scores (SDSs) of height, weight, body mass index (BMI), and BSA were calculated based on reference material from Danish children (Andersen et al. 1982; Nysom et al. 2001). Midparental height SDS (HSDSmidpar) was calculated as the mean height SDS of both parents. To estimate the difference between expected and observed height, we also calculated the difference between child and midparental height SDS (DiffHSDSmidpar), the change in height SDS between 0 and 3 years (ΔHSDS0–3) and between 1.5 years and the current examination (ΔHSDSchildhood).
Log transformation was applied to phthalate concentrations, phthalate ratios, TSH, IGF-I, IGFBP-3, and thyroid volume to improve the approximation of normal distribution. Statistical analyses included only phthalate metabolites measurable in more than 50% of children. For phthalate metabolite levels below the LOD, LOD divided by the square root of 2 was used. We calculated the sum of concentrations of DEHP metabolites (MEHP, MEHHP, MEOHP, and MECPP, corrected for molecular weights). To estimate associations with the combined phthalate exposure, we calculated a total phthalate score: Concentrations of each of the metabolites MEP, MBP, MBzP, MCiOP, and the sum of DEHP metabolites were divided into quartiles, and the total phthalate score was the sum of quartiles 0–3 (range, 0–15). Because evidence on dose–response relationships between phthalates and thyroid hormone levels is sparse (Hinton et al. 1986; O’Connor et al. 2002), the metabolites concerned were equally weighted in the calculation of the score.
We calculated the percentage of DEHP excreted as MEHP (MEHP%): MEHP concentration divided by the sum of all DEHP metabolite concentrations (MEHP, MEHHP, MEOHP, MECPP) × 100 (all concentrations were converted to nanomoles per milliliter). For samples with concentrations above LOD, the ratios between free and total metabolite concentrations were calculated.
We used parametric correlation analyses and the t-test to investigate associations between phthalate metabolite levels, age, body size, and SDS for anthropometric variables as well as sex differences. Multivariate linear regression was used to explore relationships between phthalate metabolite concentrations and serum hormone levels or growth estimates, including sex and age as covariates in analyses of hormone levels, and in addition HSDSmidpar and birth length in analyses of growth estimates.
Because urine was collected as spot samples, phthalate concentrations were adjusted for dilution by either dividing with the creatinine concentration or including the square root of creatinine concentrations in regression analyses. These two approaches yielded comparable estimates, so creatinine-corrected data represent phthalate divided by creatinine concentration. All statistical analyses were performed both with crude and creatinine-corrected phthalate concentrations. p-Values < 0.05 were considered statistically significant.
Ethical aspects
Parents gave informed consent for the participation of their child. The study was performed according to the Helsinki II Declaration and was approved by the local ethics committee and the Danish Registry Agency.
Results
Clinical characteristics of the 845 participating children are shown in Table 2. All urine samples contained measurable amounts of metabolites of DEP, DBP, DEHP, and DiNP. Distributions of crude and creatinine-corrected concentrations of phthalate metabolites are presented in Table 1. Crude concentrations of all phthalate metabolites were positively associated with each other, and creatinine-corrected concentrations also were positively associated with each other (p < 0.01 in all cases). Samples from six children contained extremely high concentrations of MBP (one boy, 6,456 μg/L), MBzP (one boy, 4,547 μg/L), DEHP metabolites (MEHHP; one girl and one boy, 1,671–1,717 μg/L), or DiNP metabolites (two boys: MCiOP, 2,063 μg/L; MHiNP, 792 μg/L). Concentrations remained high after correcting for creatinine.
Table 2.
Clinical characteristics of the population [mean ± SD or n (%)].
| Characteristic | Total (n = 845) | Males (n = 503) | Females (n = 342) |
|---|---|---|---|
| Age (years) | 7.0 ± 1.3 | 6.9 ± 1.4 | 7.1 ± 1.1 |
| Birth weight (kg) | 3.5 ± 0.6 | 3.6 ± 0.6 | 3.5 ± 0.6 |
| Birth length (cm) | 50.8 ± 2.6 | 51.2 ± 2.6 | 50.4 ± 2.5 |
| Gestational age (days) | 278.8 ± 13.2 | 279.2 ± 12.6 | 278.3 ± 14.0 |
| WGA (%) | −0.6 ± 12.9 | −0.8 ± 12.7 | −0.2 ± 13.3 |
| Height (cm) | 124.5 ± 9.5 | 124.4 ± 10.1 | 124.7 ± 8.6 |
| Weight (kg) | 24.7 ± 5.4 | 24.6 ± 5.6 | 24.9 ± 4.9 |
| BMI (kg/m2) | 15.8 ± 1.7 | 15.7 ± 1.6 | 15.9 ± 1.8 |
| BSA (m2) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Thyroid volume (mL) | 3.1 ± 1.0 | 3.1 ± 1.1 | 3.1 ± 0.9 |
| Urinary creatinine (g/L) | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.4 |
| Urinary iodine (μg/L) | 216.4 ± 126.6 | 232.6 ± 131.5 | 200.4 ± 119.9 |
| Corrected urinary iodine (μg/g creatinine) | 328.7 ± 177.7 | 337.8 ± 178.2 | 319.7 ± 177.5 |
| Maternal parity [n (%)] | |||
| 1 | 524 (62) | 317 (63) | 207 (61) |
| 2 | 247 (29) | 143 (28) | 104 (31) |
| > 2 | 72 (9) | 43 (9) | 29 (8) |
| Outcome | |||
| Singletons | 803 (95) | 478 (95) | 325 (95) |
| Twins | 42 (5) | 25 (5) | 17 (5) |
WGA, weight for gestational age, given as percent deviation from expected mean weight for gestational age.
Boys presented significantly higher urine concentrations of MBzP, MEHP, MEHHP, MEOHP, and MCiOP (p < 0.05 in all cases) than did girls. However, when corrected for creatinine, concentrations of MEP, MBP, and MECPP were higher in girls (p < 0.05), whereas the concentrations of MBzP, MHiNP, and MCiOP remained highest in boys (p < 0.05 in all cases). In correlation analyses, all phthalate metabolites were negatively associated with absolute values of height, weight, BMI, and BSA, reaching significance for MEP, MEOHP, MECPP, sum of DEHP metabolites, and total phthalate score (r = −0.097 to −0.074; p < 0.05). Only MBzP was negatively (r = −0.077; p = 0.025) correlated with age. When corrected for creatinine, all phthalate metabolites were negatively correlated with age (r = −0.210 to −0.106; p < 0.005 in all cases).
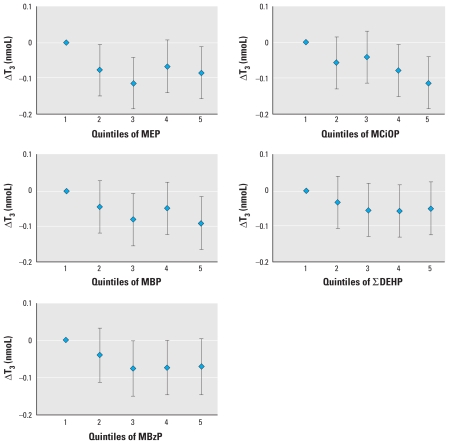
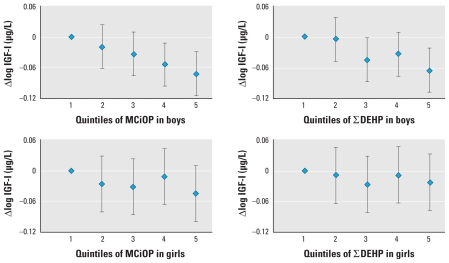
In the analyses of associations between phthalate concentrations and endocrine or growth measures, differences in results for boys and girls were generally within ranges consistent with random variation, but some estimates were statistically significant in only one group. In boys, phthalate metabolites were in general negatively associated with total and free T3, but few reached statistical significance (Table 3) [all confidence intervals are listed in Supplemental Material, Tables 1–3 (doi:10.1289/ehp.0901331)]. However, associations became nonsignificant when correcting phthalate concentrations with creatinine. We found no consistent associations with TSH, T4, or free T4 (see Supplemental Material, Tables 1 and 2). IGF-I was negatively associated with crude DEHP metabolites and MCiOP (Table 3, Figure 1), and IGFBP-3 with DEHP metabolites (p < 0.05). We found significantly negative associations between recent height gain (ΔHSDSchildhood) and both crude and creatinine-corrected DEHP metabolite concentrations (p < 0.05) (see Supplemental Material, Table 3). All other associations between ΔHSDSchildhood, IGF-I, IGFBP-3, and phthalate metabolites were similarly negative but not significantly. We found no consistent associations with current height (HSDS or DiffHSDSmidpar).
Table 3.
Regression analyses (adjusted for age and sex) of associations between phthalate metabolites and total T3, free T3, and IGF-I in prepubertal Danish children (n = 758).
| Crude analysis |
Creatinine-corrected analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All |
Boys |
Girls |
All |
Boys |
Girls |
|||||||
| Outcome | B | p-Value | B | p-Value | B | p-Value | B | p-Value | B | p-Value | B | p-Value |
| Total T3 | ||||||||||||
| MEP | −0.06 | 0.015* | −0.01 | 0.829 | −0.12 | 0.001* | −0.02 | 0.605 | 0.05 | 0.262 | −0.11 | 0.026* |
| MBP | −0.09 | 0.005* | −0.06 | 0.143 | −0.14 | 0.007* | −0.01 | 0.873 | −0.01 | 0.875 | −0.02 | 0.780 |
| MBzP | −0.05 | 0.016* | −0.04 | 0.150 | −0.06 | 0.041* | −0.03 | 0.266 | −0.03 | 0.412 | −0.03 | 0.436 |
| MCiOP | −0.07 | 0.017* | −0.07 | 0.060 | −0.06 | 0.142 | −0.01 | 0.837 | −0.04 | 0.347 | 0.06 | 0.345 |
| ∑DEHP | −0.04 | 0.170 | 0.00 | 0.950 | −0.10 | 0.022* | 0.06 | 0.153 | 0.09 | 0.082 | 0.00 | 0.965 |
| Phthalate score | −0.01 | 0.018* | 0.00 | 0.518 | −0.01 | 0.003* | 0.00 | 0.769 | 0.00 | 0.630 | 0.00 | 0.773 |
| Free T3 | ||||||||||||
| MEP | −0.13 | 0.013* | −0.08 | 0.253 | −0.18 | 0.013* | 0.00 | 0.986 | 0.10 | 0.310 | −0.14 | 0.179 |
| MBP | −0.21 | 0.002* | −0.26 | 0.004* | −0.15 | 0.141 | 0.03 | 0.785 | −0.09 | 0.517 | 0.15 | 0.325 |
| MBzP | −0.08 | 0.032* | −0.08 | 0.137 | −0.09 | 0.120 | −0.02 | 0.710 | −0.01 | 0.883 | −0.03 | 0.643 |
| MCiOP | −0.18 | 0.002* | −0.21 | 0.005* | −0.12 | 0.144 | −0.04 | 0.580 | −0.09 | 0.300 | 0.06 | 0.648 |
| ∑DEHP | −0.15 | 0.011* | −0.13 | 0.107 | −0.19 | 0.030* | 0.04 | 0.626 | 0.08 | 0.460 | −0.05 | 0.689 |
| Phthalate score | −0.01 | 0.006* | −0.01 | 0.061 | −0.02 | 0.038* | 0.00 | 0.562 | 0.00 | 0.844 | 0.00 | 0.662 |
| IGF-I | ||||||||||||
| MEP | −0.01 | 0.213 | −0.01 | 0.479 | −0.02 | 0.292 | −0.01 | 0.559 | 0.00 | 0.921 | −0.02 | 0.309 |
| MBP | −0.01 | 0.671 | −0.01 | 0.536 | 0.00 | 0.961 | 0.02 | 0.336 | 0.02 | 0.574 | 0.03 | 0.427 |
| MBzP | −0.01 | 0.383 | −0.02 | 0.170 | 0.00 | 0.796 | 0.00 | 0.737 | −0.01 | 0.321 | 0.01 | 0.510 |
| MCiOP | −0.04 | 0.003* | −0.04 | 0.006* | −0.03 | 0.153 | −0.04 | 0.006* | −0.05 | 0.020* | −0.04 | 0.137 |
| ∑DEHP | −0.03 | 0.014* | −0.05 | 0.002* | 0.00 | 0.816 | −0.04 | 0.034* | −0.07 | 0.003* | 0.01 | 0.798 |
| Phthalate score | 0.00 | 0.027* | 0.00 | 0.011* | 0.00 | 0.639 | 0.00 | 0.307 | 0.00 | 0.106 | 0.00 | 0.769 |
Abbreviations: B, regression coefficient; ∑DEHP, sum of concentrations of all measured DEHP metabolites corrected for molecular weights.
p < 0.05.
Figure 1.
Regression coefficients (95% confidence intervals) for a change in total T3 (ΔT3) associated with quintiles of MEP, MCiOP, MBP, MBzP, and sum of DEHP metabolite concentrations (∑DEHP) (adjusted for sex and age; n = 758).
In girls, all associations between peripheral thyroid hormones and unadjusted phthalate metabolites were negative, reaching significance for total T3 with most phthalate metabolites, for free T3 with MEP and DEHP metabolites (Table 3), and for total T4 with MEP (B = −5.19, p = 0.013) and total phthalate score (B = −0.48, p = 0.037) [Supplemental Material, Table 1 (doi:10.1289/ehp.0901331)]. IGF-I was not significantly associated with unadjusted phthalate metabolites (Table 3), but IGFBP-3 was significantly negatively associated with DEHP metabolites and MCiOP (p < 0.05) (see Supplemental Material, Table 2). We found significantly negative correlations between current height (HSDS or DiffHSDSmidpar) and most crude phthalate metabolites as well as the phthalate score (see Supplemental Material, Table 3). Early height gain (ΔHSDS0–3) was negatively associated with MCiOP. When correcting for creatinine, only a few associations with thyroid hormones remained significant. For growth estimates, most significant associations became nonsignificant, whereas several associations with recent height gain (ΔHSDSchildhood) became significant (see Supplemental Material, Table 3).
When analyzing boys and girls together, unadjusted phthalate metabolites showed significant negative associations with T3, free T3, and IGF-I (Figures 1 and 2, Table 3). The associations with IGF-I, but not with thyroid hormones, remained significant after correction for urinary creatinine [Supplemental Material, Figure 1 (doi:10.1289/ehp.0901331)]. Current growth measures (HSDS, DiffHSDSmidpar and ΔHSDSchildhood) were significantly negatively associated with most DEHP metabolites and their sum, for both crude and creatinine-corrected concentrations (see also Supplemental Material, Table 3). Thyroid volume ranged from 1.0 to 6.8 mL and demonstrated no statistically significant sex difference. In neither boys nor girls was thyroid volume SDS associated with concentrations of phthalate metabolites (data not shown).
Figure 2.
Regression coefficients (95% confidence intervals) for a change in IGF-I associated with quintiles of MCIOP and sum of DEHP metabolite (∑DEHP) concentrations in boys (n = 470) and girls (n = 297) (adjusted for age).
Mean ± SD MEHP% was 5.9 ± 2.7%. MEHP% was positively correlated with weight (r = 0.072, p = 0.039) and BSA (r = 0.072, p = 0.040) and negatively associated with T4 (r = −0.077, p = 0.034) and creatinine (r = −0.141, p < 0.001). Median ratios between free and total phthalate metabolite concentrations ranged from < 0.1 (MBP, MEHHP, MEOHP, MHiNP) to 0.6–0.8 (MEP, MECPP, MCiOP). Ratios correlated positively with each other (p ≤ 0.001 in all cases) and negatively with age (significant for MEP, MEOHP, and MHiNP) and body size.
Urinary concentration of creatinine was significantly positively associated with age, height, weight, and BSA (p ≤ 0.001 in all cases), and boys had higher levels of urinary creatinine than did girls (p = 0.001). Seventy-three urine samples were very dilute, with a creatinine concentration < 0.2 g/L. When excluding these samples from the statistical analyses, most associations between phthalate metabolites and total and free T3 became insignificant, although they remained negative (data not shown).
Median iodine concentration was 192.0 μg/L (282.3 μg I/g creatinine), and the 20th percentile was 115.9 μg/L. Thus, the subgroup of children tested was iodine sufficient according to World Health Organization criteria (World Health Organization et al. 2007). We repeated all the above analyses with inclusion of children with diseases or with exclusion of children born prematurely (n = 54) or small for gestational age (n = 40) and children with extremely high urinary concentrations of phthalate metabolites (n = 6). This did not change the overall pattern of associations. In extended versions of the regression analyses demonstrated in Table 3, we added interaction variables between phthalate metabolites and sex. In these analyses, associations between hormone levels and both phthalate levels and the interaction variable were generally nonsignificant, suggesting that estimates for boys and girls combined were valid estimates of effect and that the differences in estimates that we observed between boys and girls were generally consistent with random variation. However, we cannot rule out sex-specific effects in our study population because we had limited power to assess them. Phthalate concentrations in samples from the three excluded children with aberrant thyroid hormone concentrations were within the observed ranges in the rest of the children.
Discussion
In this comprehensive study of phthalate exposure and health effects in children, we determined urinary excretion of 12 phthalate metabolites in 845 iodine-sufficient Danish children 4–9 years of age and related phthalate exposure measures to thyroid function and growth. The concentrations of phthalate metabolites were largely comparable to levels previously reported from other studies of children (Becker et al. 2004; Koch et al. 2007; Sathyanarayana et al. 2008; Silva et al. 2004; Wolff et al. 2007), except for relatively low levels of MEP: The geometric mean of MEP in our study group was 21 μg/L, in contrast to 91 and 177 μg/L in American studies of children (Silva et al. 2004; Teitelbaum et al. 2008). Levels of MBP were higher in Danish children than in studies from the United States (Sathyanarayana et al. 2008; Silva et al. 2004; Wolff et al. 2007) but comparable to levels in young adults in Sweden (Jonsson et al. 2005) and children in Germany (Koch et al. 2007). This may indicate regional differences in exposure.
Overall, urinary phthalate concentrations were negatively associated with thyroid hormones and IGF-I as well as with childhood growth. In some cases results were statistically significant in girls but not in boys, or vice versa. In girls, crude concentrations of phthalate metabolites were negatively associated with total and free T3, thus potentially reflecting an effect of phthalates on thyroid function. In boys, these associations were not as consistent, although the overall trend was also negative. Adverse effects of phthalates on peripheral thyroid hormones are supported by results from two other epidemiological studies (Huang et al. 2007; Meeker et al. 2007). Thus, a study of pregnant women found an inverse association between MBP and T4 and free T4 (Huang et al. 2007), whereas a study of adult men reported negative associations between MEHP and free T4 and T3 (Meeker et al. 2007). Evidence from animal studies is sparse, but in rats exposed to DBP (O’Connor et al. 2002) and DEHP (Hinton et al. 1986) peripheral thyroid hormones were reduced, and several studies found reduced thyroid weight and histopathology indicating thyroid hyperactivity (smaller follicles, increased number, size, and iodine content of lysosomes) after phthalate exposure (Howarth et al. 2001; Poon et al. 1997). However, in view of the very complex and multifactorial regulation of thyroid size (Hansen et al. 2004), it is not surprising that the changes in phthalate concentrations were not reflected by thyroid size alterations in our population.
Our results also revealed associations of phthalates with IGF-I in children. Few previous studies have directly addressed the effect of endocrine-disrupting chemicals on the growth hormone (GH)/IGF-I axis. Studies of IGF-I effects indicated that prenatal exposure to DBP or DEHP may lead to induction of IGF-I mRNA in reproductive tissues (Bowman et al. 2005; Lin et al. 2008), reflecting a lowering of IGF-I levels. Additionally, phthalates may potentially interact with other endocrine pathways, such as the hypothalamic–pituitary axis or androgen biosynthesis. Such complex in vivo effects might be expected to contribute to differences in effects according to sex. Antiandrogenic drugs may reduce IGF-I (Juul et al. 1995), and because studies have shown phthalates to have antiandrogenic properties (Gray et al. 2000), phthalates may consequently interfere with IGF-I levels. Moreover, the GH/IGF-I axis is known to stimulate the activity of peripheral deiodinases, converting T4 to the biologically active T3 (Hussain et al. 1996; Jorgensen et al. 1989). Thus, an effect on IGF-I may indirectly reduce serum levels of T3.
Growth rate and anthropometric measurements such as height, weight, and BSA showed overall negative associations with urinary concentrations of phthalate metabolites, consistent with an adverse effect of phthalates on growth. In support, animal studies have shown negative associations between prenatal phthalate exposure and birth weight (Tanaka 2005; Tyl et al. 2004) as well as gain of body weight (Fukuwatari et al. 2002), although conflicting data have been reported (Arcadi et al. 1998; Sharpe et al. 1995). In contrast to these studies, and to the common hypothesis of phthalates causing impaired fetal growth, Wolff et al. (2008) showed a positive association between phthalates of low molecular weight and duration of pregnancy and infant head circumference.
In our comprehensive study, we performed numerous association tests between a large number of outcome measures and 12 different phthalate metabolites. Clearly, such multiple significance testing implies a risk of obtaining “false-positive” results by chance. We therefore focused our interpretation of the results on overall trends and not on single significant associations. Thyroid hormones and growth factors are closely linked with each other and contribute significantly in the regulation of childhood growth. Thus, our observation of associations between phthalate exposure and growth supports the conclusion that phthalate exposure in this age group exerts an adverse biological effect.
However, words of caution appear necessary with regard to assessment of phthalate exposure. We collected spot urine samples, the concentrations of which will depend on recent fluid intake. Previous reports have attempted to correct, at least partly, for dilution by measuring urinary creatinine concentrations or specific gravity. However, the excretion of creatinine in children is strongly correlated with age and anthropometric variables as height, weight, and BSA (Skinner et al. 1996), as also seen in our study. In addition, there is a sex difference, with boys having higher excretion of creatinine compared with girls (Skinner et al. 1996). Thus, sex and anthropometric features per se may affect the level of the correction factor (creatinine). Consequently, when correcting phthalate concentrations by dividing by creatinine, corrected phthalate values will tend to decrease with age and body size, thus mimicking a negative association between phthalate levels and growth parameters. Such an interaction has also been demonstrated in a study of maternal urinary phthalate levels and the associations with infant outcome (Wolff et al. 2008). In contrast, associations with parameters declining with age, such as peripheral thyroid hormones in childhood, will tend to become more positive, which may be the case for the associations seen with thyroid hormones in our study.
Not only creatinine but also renal glomerular filtration rate and thus urinary volume are influenced by age, anthropometry, thyroid hormones (Iglesias and Diez 2009), and IGF-I (Feld and Hirschberg 1996). Thus, large and fast-growing children with high levels of thyroid hormones and IGF-I have a higher probability of large urinary volumes and consequently lower crude urinary concentrations of phthalate metabolites than do small children being exposed to the same amount of phthalates. Furthermore, small children may be exposed to higher levels of phthalates relative to body size (Wittassek and Angerer 2008), because small children have a higher food intake as well as a higher body surface per kilogram of body weight. Hence, the negative associations between urinary phthalate concentrations and body size or height gain may partly be explained by physiological mechanisms resulting in reverse causality. In order to adjust for the interaction between creatinine and outcome, other studies (Adibi et al. 2009) included the square root of creatinine. However, in our study, the application of this correction modus did not significantly change the statistical estimates and overall results.
Reservations should also be stated in relation to the fact that we collected only a single urine sample from each child, which may not be representative for their average exposure, although several studies concluded that a single urine sample could be moderately predictive of individual exposure over a couple of months (Hauser et al. 2004; Teitelbaum et al. 2008).
One previous study suggested that MEHP% may be a phenotypic marker of DEHP metabolism and excretion (Hauser 2008; Meeker et al. 2007). MEHP% was positively associated, and the fraction of free phthalate metabolites negatively associated, with age and anthropometric measurements, so it seems that age and body size may affect phthalate metabolism, both oxidation and glucuronidation. Other studies have found similar relations between hydrolyzed and oxidized metabolites (Becker et al. 2004). Interestingly, MEHP% was negatively associated with T4 in our material, which has also been reported in a previous study (Meeker et al. 2007). Thus, thyroid hormones may have an accelerating effect on the metabolism of phthalates.
Conclusions
Our study showed negative associations between urinary phthalate concentrations and thyroid hormones, IGF-I, and growth in healthy children. Although our study was not designed to reveal the mechanism of action, the overall coherent negative associations between urine phthalate and thyroid and growth parameters may suggest causative negative roles of phthalate exposures for child health.
Footnotes
Financial support was provided by the Novo Nordisk Foundation, the Velux Foundation, the Lundbeck Foundation (journal no. 124/05), the Danish Medical Research Council (9700909), and the Danish Ministry of Science Technology and Innovation (2107-05-0006).
Supplemental Material is available online (doi:10.1289/ehp.0901331 via http://dx.doi.org/).
We thank all participating families, and we acknowledge nurse H. Kelkeland; J. Angerer and M. Wittassek (University of Erlangen–Nuremberg, Germany) for some internal phthalate standards; and E. Huusfeldt Larsen (Technical University of Denmark) for iodine analyses.
References
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169:1015–1024. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen E, Hutchings B, Jansen J, Nyholm M. H⊘jde og vægt hos danske b⊘rn [in Danish] Ugeskr Laeger. 1982;144(24):1760–1765. [PubMed] [Google Scholar]
- Arcadi FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, et al. Oral toxicity of bis(2-ethylhexyl) phthalate during pregnancy and suckling in the Long-Evans rat. Food Chem Toxicol. 1998;36:963–970. doi: 10.1016/s0278-6915(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, et al. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, et al. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72:4127–4134. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. Environmental chemicals and thyroid function. Eur J Endocrinol. 2006;154:599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- Boas M, Hegedus L, Feldt-Rasmussen U, Skakkebaek NE, Hilsted L, Main KM. Association of thyroid gland volume serum insulin-like growth factor-I and anthropometric variables in euthyroid prepubertal children. J Clin Endocrinol Metab. 2009;94:4031–4035. doi: 10.1210/jc.2009-0939. [DOI] [PubMed] [Google Scholar]
- Bowman CJ, Turner KJ, Sar M, Barlow NJ, Gaido KW, Foster PM. Altered gene expression during rat Wolffian duct development following di(n-butyl) phthalate exposure. Toxicol Sci. 2005;86:161–174. doi: 10.1093/toxsci/kfi172. [DOI] [PubMed] [Google Scholar]
- Chellakooty M, Juul A, Boisen KA, Damgaard IN, Kai CM, Schmidt IM, et al. A prospective study of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in 942 healthy infants: associations with birth weight, gender, growth velocity, and breastfeeding. J Clin Endocrinol Metab. 2006;91:820–826. doi: 10.1210/jc.2005-0950. [DOI] [PubMed] [Google Scholar]
- Feld S, Hirschberg R. Insulinlike growth factor I and the kidney. Trends Endocrinol Metab. 1996;7:85–93. doi: 10.1016/1043-2760(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Fukuwatari T, Suzuki Y, Sugimoto E, Shibata K. Elucidation of the toxic mechanism of the plasticizers, phthalic acid esters, putative endocrine disrupters: effects of dietary di(2-ethylhexyl)phthalate on the metabolism of tryptophan to niacin in rats. Biosci Biotechnol Biochem. 2002;66:705–710. doi: 10.1271/bbb.66.705. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Hansen PS, Brix TH, Bennedbaek FN, Bonnema SJ, Kyvik KO, Hegedus L. Genetic and environmental causes of individual differences in thyroid size: a study of healthy Danish twins. J Clin Endocrinol Metab. 2004;89:2071–2077. doi: 10.1210/jc.2003-031999. [DOI] [PubMed] [Google Scholar]
- Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl. 2008;31:112–117. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, Nunn A, et al. Effects of phthalic acid esters on the liver and thyroid. Environ Health Perspect. 1986;70:195–210. doi: 10.1289/ehp.8670195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth JA, Price SC, Dobrota M, Kentish PA, Hinton RH. Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol Lett. 2001;121:35–43. doi: 10.1016/s0378-4274(01)00313-7. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22:2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Schmitz O, Jorgensen JO, Christiansen JS, Weeke J, Schmid C, et al. Insulin-like growth factor I alters peripheral thyroid hormone metabolism in humans: comparison with growth hormone. Eur J Endocrinol. 1996;134:563–567. doi: 10.1530/eje.0.1340563. [DOI] [PubMed] [Google Scholar]
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160:503–515. doi: 10.1530/EJE-08-0837. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Sawatsubashi S, Yamauchi K. Endocrine disrupting chemicals: interference of thyroid hormone binding to transthyretins and to thyroid hormone receptors. Mol Cell Endocrinol. 2003;199:105–117. doi: 10.1016/s0303-7207(02)00302-7. [DOI] [PubMed] [Google Scholar]
- Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson AM. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl. 2008;31:118–130. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16:487–493. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS. Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J Clin Endocrinol Metab. 1989;69:1127–1132. doi: 10.1210/jcem-69-6-1127. [DOI] [PubMed] [Google Scholar]
- Juul A, Scheike T, Nielsen CT, Krabbe S, Muller J, Skakkebaek NE. Serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 levels are increased in central precocious puberty: effects of two different treatment regimens with gonadotropin-releasing hormone agonists, without or in combination with an antiandrogen (cyproterone acetate) J Clin Endocrinol Metab. 1995;80:3059–3067. doi: 10.1210/jcem.80.10.7559897. [DOI] [PubMed] [Google Scholar]
- Koch HM, Becker K, Wittassek M, Seiwert M, Angerer J, Kolossa-Gehring M. Di-n-butylphthalate and butylbenzylphthalate—urinary metabolite levels and estimated daily intakes: pilot study for the German Environmental Survey on children. J Expo Sci Environ Epidemiol. 2007;17:378–387. doi: 10.1038/sj.jes.7500526. [DOI] [PubMed] [Google Scholar]
- Lin H, Ge RS, Chen GR, Hu GX, Dong L, Lian QQ, et al. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc Natl Acad Sci USA. 2008;105:7218–7222. doi: 10.1073/pnas.0709260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nysom K, Molgaard C, Hutchings B, Michaelsen KF. Body mass index of 0 to 45-y-old Danes: reference values and comparison with published European reference values. Int J Obes Relat Metab Disord. 2001;25:177–184. doi: 10.1038/sj.ijo.0801515. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Frame SR, Ladics GS. Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicol Sci. 2002;69:92–108. doi: 10.1093/toxsci/69.1.92. [DOI] [PubMed] [Google Scholar]
- Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, Chu I. Subchronic oral toxicity of di-n-octyl phthalate and di(2-ethylhexyl) phthalate in the rat. Food Chem Toxicol. 1997;35:225–239. doi: 10.1016/s0278-6915(96)00064-6. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, et al. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260–e268. doi: 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect. 1995;103:1136–1143. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen O, Du G, Sun H, Wu W, Jiang Y, Song L, et al. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol Lett. 2009;191:9–14. doi: 10.1016/j.toxlet.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Shimada N, Yamauchi K. Characteristics of 3,5,3′-triiodothyronine (T3)-uptake system of tadpole red blood cells: effect of endocrine-disrupting chemicals on cellular T3 response. J Endocrinol. 2004;183:627–637. doi: 10.1677/joe.1.05893. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Skinner AM, Addison GM, Price DA. Changes in the urinary excretion of creatinine, albumin and N-acetyl-beta-d-glucosaminidase with increasing age and maturity in healthy schoolchildren. Eur J Pediatr. 1996;155:596–602. doi: 10.1007/BF01957912. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioural effects of bis(2-ethylhexyl) phthalate (DEHP) in a cross-mating toxicity study of mice. Food Chem Toxicol. 2005;43:581–589. doi: 10.1016/j.fct.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Fail PA, Seely JC, Brine DR, et al. Reproductive toxicity evaluation of dietary butyl benzyl phthalate (BBP) in rats. Reprod Toxicol. 2004;18:241–264. doi: 10.1016/j.reprotox.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Angerer J. Phthalates: metabolism and exposure. Int J Androl. 2008;31:131–138. doi: 10.1111/j.1365-2605.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. 3rd ed. Geneva: World Health Organization; 2007. [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]