Abstract
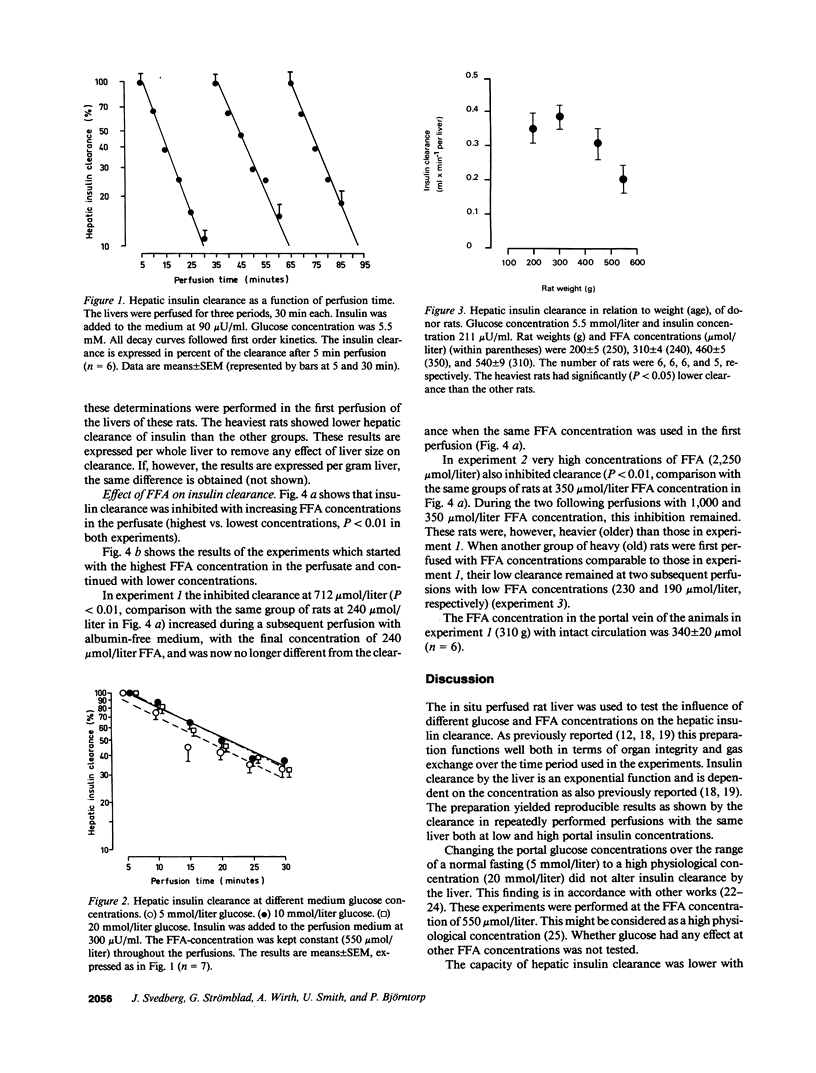
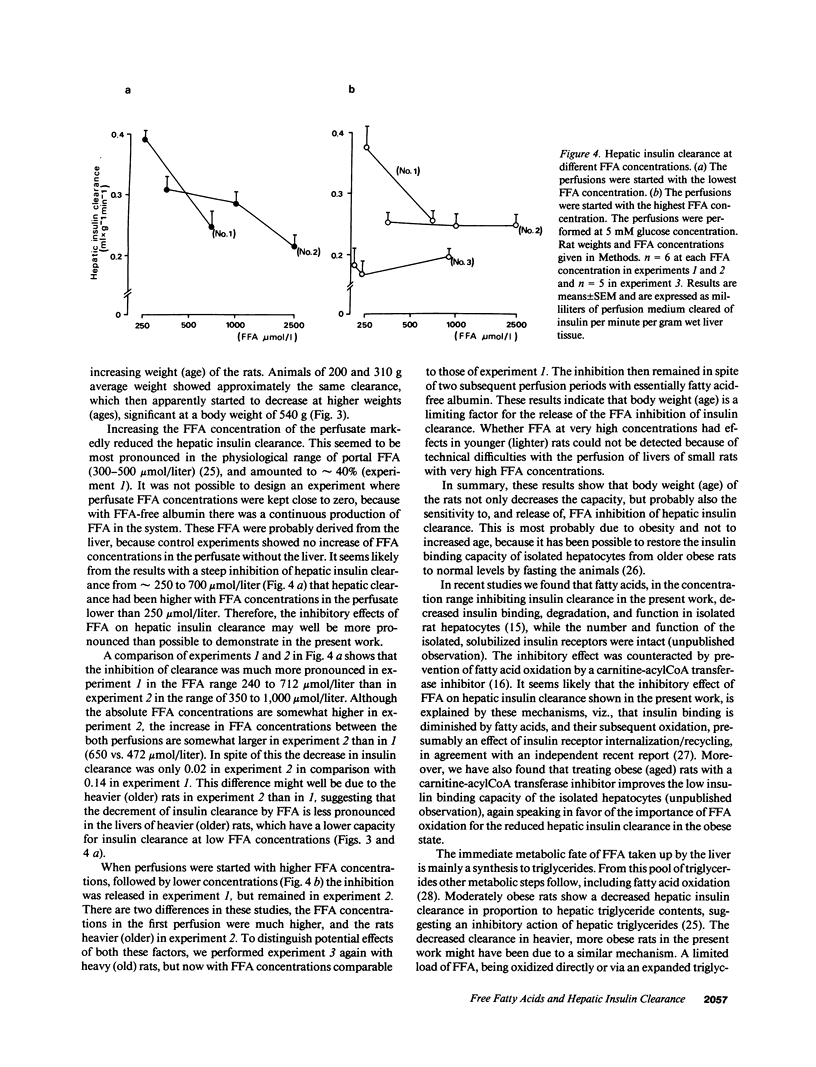
The effects of FFA on hepatic insulin clearance were studied in the in situ perfused rat liver. Clearance decreased with increasing body weight (age) of the rats. When FFA were added to the perfusate a 40% reduction of hepatic removal of insulin was found over the normal, physiological range (less than 1,000 mumol/liter), less pronounced in heavier rats. When perfusion was started with high concentrations of FFA, inhibition was rapidly reversible, a phenomenon again blunted in heavier rats. In contrast to FFA, different glucose concentrations in the perfusate did not affect the hepatic insulin uptake in the presence of FFA within physiological concentrations. Thus, hepatic clearance of insulin is proportional to rat weight (age) and portal FFA concentrations. Other studies have recently shown that fatty acids inhibit insulin binding, degradation, and function in isolated rat hepatocytes, and that hepatic clearance is inversely dependent on hepatic triglyceride concentrations, both inhibitions reversible by prevention of fatty acid oxidation. It is suggested that the diminished hepatic clearance of insulin in heavier (older) rats is at least partly due to their relative obesity and increased hepatic triglyceride contents. This effect as well as that of portal FFA is probably mediated via fatty acid oxidation in the liver. This mechanism may have implications for the regulation of hepatic metabolism, and peripheral insulin concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F., Exton J. H., Jeanrenaud B. Control of gluconeogenesis and glycogenolysis in perfused livers of normal mice. Am J Physiol. 1973 Jul;225(1):25–32. doi: 10.1152/ajplegacy.1973.225.1.25. [DOI] [PubMed] [Google Scholar]
- Björntorp P. "Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990 Jul-Aug;10(4):493–496. [PubMed] [Google Scholar]
- Björntorp P., Bergman H., Varnauskas E., Lindholm B. Lipid mobilization in relation to body composition in man. Metabolism. 1969 Oct;18(10):840–851. doi: 10.1016/0026-0495(69)90059-6. [DOI] [PubMed] [Google Scholar]
- Bonora E., Zavaroni I., Coscelli C., Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism. 1983 May;32(5):438–446. doi: 10.1016/0026-0495(83)90004-5. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Cobelli C. The kinetics of insulin in man. II. Role of the liver. Diabetes Metab Rev. 1987 Apr;3(2):365–397. doi: 10.1002/dmr.5610030202. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Wahren J., Faber O. K., Felig P., Binder C., DeFronzo R. A. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol. 1983 Jun;244(6):E517–E527. doi: 10.1152/ajpendo.1983.244.6.E517. [DOI] [PubMed] [Google Scholar]
- Frank H. J., Davidson M. B. Insulin binding and action in isolated rat hepatocytes: effect of obesity and fasting. Am J Physiol. 1982 Sep;243(3):E240–E245. doi: 10.1152/ajpendo.1982.243.3.E240. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Berry M. N., Williams M. C., Severinghaus E. M. A simple and inexpensive membrane "lung" for small organ perfusion. J Lipid Res. 1974 Mar;15(2):182–186. [PubMed] [Google Scholar]
- Hennes M. M., Shrago E., Kissebah A. H. Receptor and postreceptor effects of free fatty acids (FFA) on hepatocyte insulin dynamics. Int J Obes. 1990 Oct;14(10):831–841. [PubMed] [Google Scholar]
- Jaspan J., Polonsky K. Glucose ingestion in dogs alters the hepatic extraction of insulin. In vivo evidence for a relationship between biologic action and extraction of insulin. J Clin Invest. 1982 Mar;69(3):516–525. doi: 10.1172/JCI110477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakash C., Assimacopoulos-Jeannet F., Jeanrenaud B. An anomaly of insulin removal in perfused livers of obese-hyperglycemic (ob/ob) mice. J Clin Invest. 1976 May;57(5):1117–1124. doi: 10.1172/JCI108378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F., STETTEN D., Jr Metabolism of insulin-I 131; studies in isolated, perfused rat liver and hindlimb preparations. Diabetes. 1959 Jul-Aug;8(4):307–314. doi: 10.2337/diab.8.4.307. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Batchelder T., Colome S., Reaven G. M. Effect of intravenous glucose infusion on plasma insulin removal rate. Metabolism. 1974 Jun;23(6):543–548. doi: 10.1016/0026-0495(74)90082-1. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Peiris A. N., Mueller R. A., Smith G. A., Struve M. F., Kissebah A. H. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest. 1986 Dec;78(6):1648–1657. doi: 10.1172/JCI112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris A. N., Mueller R. A., Smith G. A., Struve M. F., Kissebah A. H. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest. 1986 Dec;78(6):1648–1657. doi: 10.1172/JCI112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky K. S., Given B. D., Hirsch L., Shapiro E. T., Tillil H., Beebe C., Galloway J. A., Frank B. H., Karrison T., Van Cauter E. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988 Feb;81(2):435–441. doi: 10.1172/JCI113338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuffé-Scrive M., Anderson B., Olbe L., Björntorp P. Metabolism of adipose tissue in intraabdominal depots in severely obese men and women. Metabolism. 1990 Oct;39(10):1021–1025. doi: 10.1016/0026-0495(90)90160-e. [DOI] [PubMed] [Google Scholar]
- Rebuffé-Scrive M., Andersson B., Olbe L., Björntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989 May;38(5):453–458. doi: 10.1016/0026-0495(89)90198-4. [DOI] [PubMed] [Google Scholar]
- Rossell R., Gomis R., Casamitjana R., Segura R., Vilardell E., Rivera F. Reduced hepatic insulin extraction in obesity: relationship with plasma insulin levels. J Clin Endocrinol Metab. 1983 Mar;56(3):608–611. doi: 10.1210/jcem-56-3-608. [DOI] [PubMed] [Google Scholar]
- Röjdmark S., Bloom G., Chou M. C., Jaspan J. B., Field J. B. Hepatic insulin and glucagon extraction after their augmented secretion in dogs. Am J Physiol. 1978 Jul;235(1):E88–E96. doi: 10.1152/ajpendo.1978.235.1.E88. [DOI] [PubMed] [Google Scholar]
- Shapiro E. T., Tillil H., Rubenstein A. H., Polonsky K. S. Peripheral insulin parallels changes in insulin secretion more closely than C-peptide after bolus intravenous glucose administration. J Clin Endocrinol Metab. 1988 Nov;67(5):1094–1099. doi: 10.1210/jcem-67-5-1094. [DOI] [PubMed] [Google Scholar]
- Sims E. A., Danforth E., Jr, Horton E. S., Bray G. A., Glennon J. A., Salans L. B. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- Smith U. Adrenergic control of human adipose tissue lipolysis. Eur J Clin Invest. 1980 Oct;10(5):343–344. doi: 10.1111/j.1365-2362.1980.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Strömblad G., Björntorp P. Reduced hepatic insulin clearance in rats with dietary-induced obesity. Metabolism. 1986 Apr;35(4):323–327. doi: 10.1016/0026-0495(86)90148-4. [DOI] [PubMed] [Google Scholar]
- Svedberg J., Björntorp P., Lönnroth P., Smith U. Prevention of inhibitory effect of free fatty acids on insulin binding and action in isolated rat hepatocytes by etomoxir. Diabetes. 1991 Jun;40(6):783–786. doi: 10.2337/diab.40.6.783. [DOI] [PubMed] [Google Scholar]
- Svedberg J., Björntorp P., Smith U., Lönnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes. 1990 May;39(5):570–574. doi: 10.2337/diab.39.5.570. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. R., Sussman K. E. Plasma insulin after diversion of portal and pancreatic venous blood to vena cava. J Appl Physiol. 1967 Apr;22(4):808–812. doi: 10.1152/jappl.1967.22.4.808. [DOI] [PubMed] [Google Scholar]
- Waldhäusl W., Bratusch-Marrain P., Gasic S., Korn A., Nowotny P. Insulin production rate following glucose ingestion estimated by splanchnic C-peptide output in normal man. Diabetologia. 1979 Oct;17(4):221–227. doi: 10.1007/BF01235858. [DOI] [PubMed] [Google Scholar]
- Wirth A., Holm G., Björntorp P. Effect of physical training on insulin uptake by the perfused rat liver. Metabolism. 1982 May;31(5):457–462. doi: 10.1016/0026-0495(82)90234-7. [DOI] [PubMed] [Google Scholar]



