Abstract
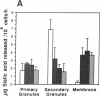
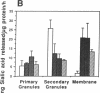
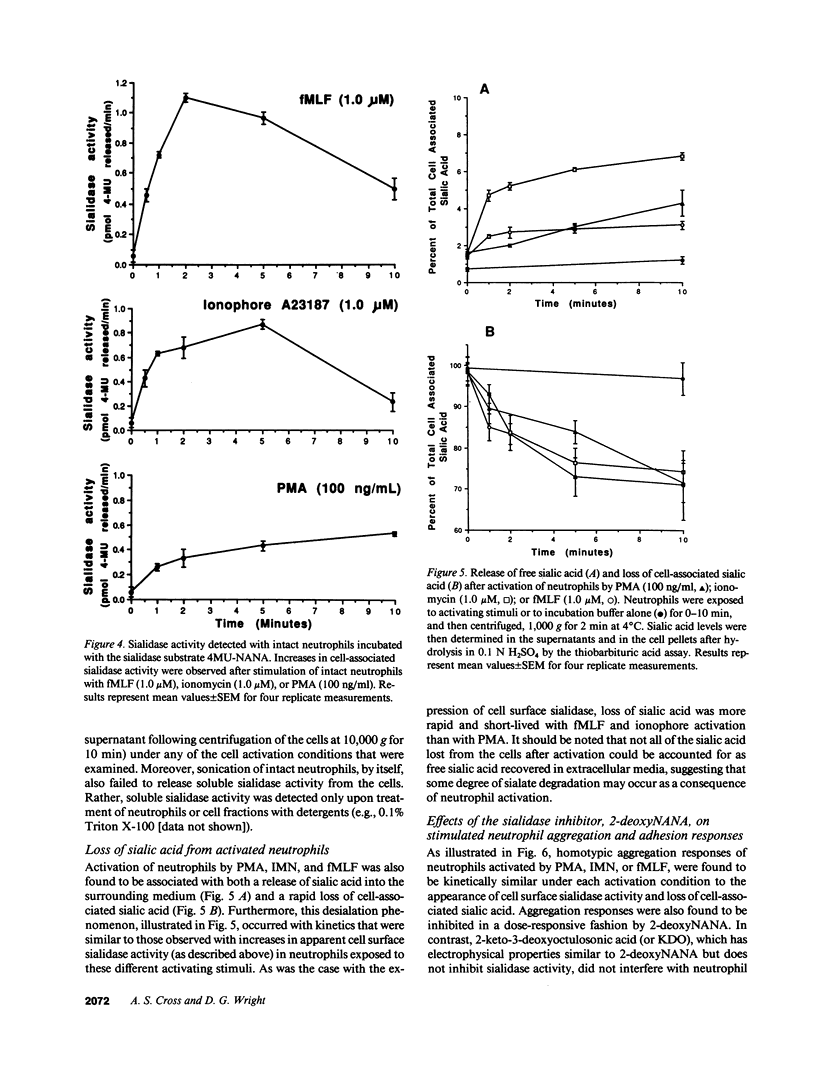
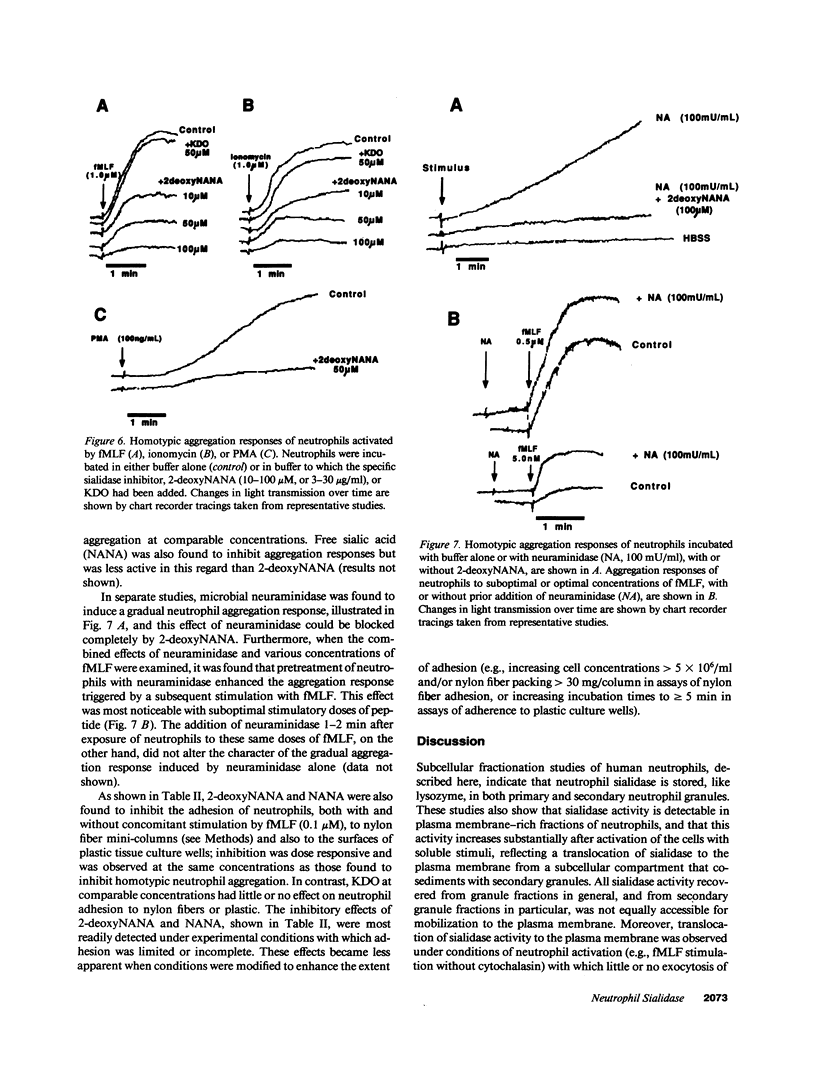
Desialation of cell surfaces has been associated with the initiation or modification of diverse cellular functions. In these studies we have examined the subcellular distribution of sialidase (SE) in human neutrophils as well as the mobilization of this enzyme following neutrophil activation. Separation of subcellular fractions by density gradient centrifugation showed that SE is present not only in neutrophil primary and secondary granule populations, like lysozyme, but also in plasma membrane fractions. Neutrophil activation was associated with a redistribution of SE from secondary granule-enriched fractions to the plasma membrane. Furthermore, SE activity detected on the surface of intact neutrophils with a fluorescent SE substrate increased rapidly after activation with kinetics that matched both the loss of total cell-associated sialic acid and release of free sialic acid from the cells. These activation-dependent events were in each case blocked by incubation of neutrophils with the SE inhibitor, 2-deoxy-N-acetyl-neuraminic acid. Aggregation responses of neutrophils as well as adhesion responses to nylon and plastic surfaces were also inhibited by 2-deoxyNANA. Our findings indicate that the activation-dependent desialation of the neutrophil surface is associated with mobilization of an endogenous SE to the plasma membrane and has a role in stimulated adhesion responses of these cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Christensen L., Bejerrum O. W., Birgens H. S., Clemmensen I. Identification of a highly mobilizable subset of human neutrophil intracellular vesicles that contains tetranectin and latent alkaline phosphatase. J Clin Invest. 1990 Feb;85(2):408–416. doi: 10.1172/JCI114453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon J. P., Slade S. G., Reibman J., Abramson S. B., Philips M. R., Weissmann G., Winchester R. Constitutive and induced phosphorylation of the alpha- and beta-chains of the CD11/CD18 leukocyte integrin family. Relationship to adhesion-dependent functions. J Immunol. 1990 Jan 1;144(1):191–197. [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Borregaard N. Neutrophils autoinactivate secretory products by myeloperoxidase-catalyzed oxidation. Blood. 1985 Feb;65(2):375–381. [PubMed] [Google Scholar]
- Cross A. S., Lowell G. H., Palmblad J., Sadoff J. C., Young L., Berger M. Mechanism of priming of human neutrophils by a soluble lymphoblastoid cell factor. J Immunol. 1985 Sep;135(3):2074–2083. [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R. A modified procedure for the determination of leukocyte alkaline phosphatase. Biochem Med. 1970 Aug;4(1):61–68. doi: 10.1016/0006-2944(70)90103-1. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Lazdins J., Karnovsky M. L. The determination and localization of sialic acid in guinea-pig granulocytes. Biochem J. 1980 Nov 15;192(2):543–550. doi: 10.1042/bj1920543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J., Waller C., Timpl R., Schirrmacher V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature. 1982 Nov 18;300(5889):274–276. doi: 10.1038/300274a0. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Fogel M., Altevogt P., Schirrmacher V. Metastatic potential severely altered by changes in tumor cell adhesiveness and cell-surface sialylation. J Exp Med. 1983 Jan 1;157(1):371–376. doi: 10.1084/jem.157.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Durocher J. R., Kaplan A. P. Interaction of leukocyte chemotactic factors with the cell surface. I. Chemotactic factor-induced changes in human granulocyte surface charge. J Clin Invest. 1975 May;55(5):967–974. doi: 10.1172/JCI108026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görög P., Kovács I. B., Born G. V. Suppression of the intravascular adherence of granulocytes by N-acetyl neuraminic (sialic) acid. Br J Exp Pathol. 1980 Oct;61(5):490–496. [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van Erne-van der Tol M. E., Verhoef J. Partial removal of sialic acid enhances phagocytosis and the generation of superoxide and chemiluminescence by polymorphonuclear leukocytes. J Immunol. 1982 Aug;129(2):745–750. [PubMed] [Google Scholar]
- Hoover R. L., Briggs R. T., Karnovsky M. J. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978 Jun;14(2):423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Kane S. P., Peters T. J. Analytical subcellular fractionation of human granulocytes with reference to the localization of vitamin B12-binding proteins. Clin Sci Mol Med. 1975 Aug;49(2):171–182. doi: 10.1042/cs0490171. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kownatzki E., Uhrich S., Weil B. Adherence and migration of guinea pig granulocytes after enzyme treatment of the cell surface. Immunobiology. 1984 Mar;166(2):111–117. doi: 10.1016/S0171-2985(84)80030-3. [DOI] [PubMed] [Google Scholar]
- Kumar V., Kessler J., Scott M. E., Patwardhan B. H., Tanenbaum S. W., Flashner M. 2,3-Dehydro-4-epi-N-acetylneuraminic acid; a neuraminidase inhibitor. Carbohydr Res. 1981 Aug 1;94(2):123–130. doi: 10.1016/s0008-6215(00)80711-9. [DOI] [PubMed] [Google Scholar]
- Larsen E., Celi A., Gilbert G. E., Furie B. C., Erban J. K., Bonfanti R., Wagner D. D., Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989 Oct 20;59(2):305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Electrophoretic mobility and N-acetyl neuraminic acid content of human normal and leukemic lymphocytes and granulocytes. Blood. 1970 Jan;35(1):12–22. [PubMed] [Google Scholar]
- Lloyd C. W. Sialic acid and the social behaviour of cells. Biol Rev Camb Philos Soc. 1975 Aug;50(3):325–350. doi: 10.1111/j.1469-185x.1975.tb00832.x. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989 Jul;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendla K., Cantz M. Specificity studies on the oligosaccharide neuraminidase of human fibroblasts. Biochem J. 1984 Mar 1;218(2):625–628. doi: 10.1042/bj2180625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski J. C., Corfield A. P., Schauer R. Properties of human liver lysosomal sialidase. Biol Chem Hoppe Seyler. 1986 Aug;367(8):715–722. doi: 10.1515/bchm3.1986.367.2.715. [DOI] [PubMed] [Google Scholar]
- Michalski J. C., Corfield A. P., Schauer R. Solubilization and affinity chromatography of a sialidase from human liver. Hoppe Seylers Z Physiol Chem. 1982 Sep;363(9):1097–1102. doi: 10.1515/bchm2.1982.363.2.1097. [DOI] [PubMed] [Google Scholar]
- Miyagi T., Tsuiki S. Purification and characterization of cytosolic sialidase from rat liver. J Biol Chem. 1985 Jun 10;260(11):6710–6716. [PubMed] [Google Scholar]
- Nojiri N., Takaku F., Tetsuka T., Saito M. Stimulation of sialidase activity during cell differentiation of human promyelocytic leukemia cell line HL-60. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1239–1246. doi: 10.1016/0006-291x(82)91383-3. [DOI] [PubMed] [Google Scholar]
- Noseworthy J., Jr, Korchak H., Karnovsky M. L. Phagocytosis and the sialic acid of the surface of polymorphonuclear leukocytes. J Cell Physiol. 1972 Feb;79(1):91–96. doi: 10.1002/jcp.1040790110. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Salk P. L., Yogeeswaran G., Karpatkin S. Correlation between spontaneous metastatic potential, platelet-aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic-variant derivatives of a rat renal sarcoma cell line. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4336–4339. doi: 10.1073/pnas.77.7.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A rapid and sensitive assay for neuraminidase using peanut lectin hemagglutination: application to Vibrio cholera and Trypanosoma cruzi. J Immunol Methods. 1983 Sep 30;63(1):25–34. doi: 10.1016/0022-1759(83)90206-5. [DOI] [PubMed] [Google Scholar]
- Perez H. D., Elfman F., Lobo E. Removal of human polymorphonuclear leukocyte surface sialic acid inhibits reexpression (or recycling) of formyl peptide receptors. A possible explanation for its effect on formyl peptide-induced polymorphonuclear leukocyte chemotaxis. J Immunol. 1987 Sep 15;139(6):1978–1984. [PubMed] [Google Scholar]
- Perez H. D., Ong R. R., Elfman F. Removal or oxidation of surface membrane sialic acid inhibits formyl-peptide-induced polymorphonuclear leukocyte chemotaxis. J Immunol. 1985 Mar;134(3):1902–1908. [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Samollow P. B., VandeBerg J. L., Kunz H. W., Gill T. J., 3rd Analysis of neuraminidase isozyme phenotypes in mammalian tissues: an electrophoretic approach. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1182–1188. doi: 10.1016/0006-291x(85)90310-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaltro J., Alhadeff J. A. Solubilization, stabilization and isoelectric focusing of human liver neuraminidase activity. Biochim Biophys Acta. 1984 Jul 30;800(2):159–165. doi: 10.1016/0304-4165(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kurita T., Kakinuma K. Effects of neuraminidase on O2 consumption and release of O2 and H2O2 from phagocytosing human polymorphonuclear leukocytes. Blood. 1982 Aug;60(2):446–453. [PubMed] [Google Scholar]
- Takeda A. Sialylation patterns of lymphocyte function-associated antigen 1 (LFA-1) differ between T and B lymphocytes. Eur J Immunol. 1987 Feb;17(2):281–286. doi: 10.1002/eji.1830170220. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen F. W., Janse H. C., van Diggelen O. P., Bakker H. D., Loonen M. C., Durand P., Galjaard H. Two genetically different MU-NANA neuraminidases in human leucocytes. Biochem Biophys Res Commun. 1983 Dec 16;117(2):470–478. doi: 10.1016/0006-291x(83)91224-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Bralove D. A., Gallin J. I. The differential mobilization of human neutrophil granules. Effects of phorbol myristate acetate and ionophore A23187. Am J Pathol. 1977 May;87(2):273–284. [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Wright D. G. Human neutrophil degranulation. Methods Enzymol. 1988;162:538–551. doi: 10.1016/0076-6879(88)62102-1. [DOI] [PubMed] [Google Scholar]