Abstract
Purpose
To evaluate the effect of vicriviroc (VCV) on peripheral neuropathy (PN), the most prevalent neurological complication of HIV infection in HIV-1–infected treatment-experienced population.
Method
A5211 is a randomized placebo-controlled trial evaluating VCV in treatment-experienced HIV participants failing current therapy. Participants were randomized to VCV (5, 10, or 15 mg) or placebo with optimized ritonavir-containing antiretroviral therapy and followed for 48 weeks. PN was defined as having at least mild loss of vibration bilaterally or ankle reflexes absent or hypoactive bilaterally. We estimated the association between VCV (pooled doses) with PN using a logistic generalized estimating equation. Additional outcomes included symptomatic neuropathy (SPN), painful neuropathy (PPN), and neuropathic signs and symptoms.
Results
118 participants (92% male, 65% white, median age of 46 years, median baseline CD4 139, median HIV-1 RNA 4.58 log) were randomized (90 on VCV and 28 on placebo). VCV therapy did not result in a statistically significant difference relative to placebo in PN (OR = 1.52; P = .39; 95% CI 0.59, 3.90) after controlling for baseline PN status and baseline neurotoxic nucleoside reverse transcriptase inhibitor(s) use.
Conclusion
Treatment with VCV over 48 weeks failed to result in statistically significant effect on PN in treatment-experienced participants with HIV infection relative to placebo, however potentially important effects cannot be ruled out.
Keywords: HIV, peripheral neuropathy, vicriviroc
Peripheral neuropathy (PN) is the most prevalent neurological complication of human immunodeficiency virus (HIV) infection and occurs frequently in treatment-experienced patients. Risk factors include increasing age, advanced HIV disease with low CD4 counts, and neurotoxicity of antiretrovirals (ARV).1–3 Vicriviroc (VCV) is an investigational agent that specifically binds the CCR5 chemokine coreceptor blocking HIV cell entry when this is the coreceptor of the virus.4 Studies have suggested that CCR5-tropic HIV strains may preferentially infect both central and peripheral nervous system cells.5,6 It has been hypothesized that a mechanism of viral neurotoxicity is mediated by CCR5 neuronal receptors7; because CCR5 antagonists may cross the blood-brain barrier, these agents might be neuroprotective.
The AIDS Clinical Trials Group (ACTG) 5211 was a phase II, double-blinded, randomized, placebo-controlled trial designed to evaluate the safety / tolerability and virologic activity of VCV as part of a ritonavir-containing regimen in HIV-1–infected, treatment-experienced participants. In these analyses, we evaluated the effect of VCV on peripheral neuropathy and individual neuropathic signs and symptoms.
METHOD
ACTG 5211
ACTG 5211 was designed to evaluate in treatment-experienced patients with R5 virus the short-term activity, safety, and tolerability of three doses of VCV in comparison to placebo when added to an existing ritonavir-containing antiretroviral regimen over 14 days, as well as the long-term effects to Weeks 24 and 48 following optimization of the antiretroviral regimen at Day 14 based on baseline genotypic and phenotypic resistance testing. Participants were randomized to add one of three doses of VCV (5, 10, or 15 mg once daily) or a matching placebo to their failing antiretroviral regimen; then at Day 14, they were changed to an optimized ritonavir-containing ARV therapy regimen based on antiretroviral drug history and resistance testing and were followed for a total of 48 weeks (Step 1). If virologic failure (a confirmed HIV-1 RNA level of <1 log10 copies/mL below the baseline level at/after 16 weeks) occurred, the participant entered Step 2 and received VCV based on the initial randomized group under Versions 1.0 and 2.0 of the protocol: placebo recipients added VCV 10 mg daily; VCV 5 mg recipients increased VCV to 10 mg; and VCV 10 and 15 mg recipients continued VCV at the same doses. Under Version 3.0 of the protocol, all participants in Step 2 received VCV 15 mg once daily.
Neuropathy Assessment and Neuropathic Outcomes Definitions
A neuropathy assessment was conducted at entry, Week 24, and Week 48 in Step 1, at the entry to Step 2, and at the end of the initial 48 weeks of follow-up or study discontinuation by site coordinators / nurses who were trained and certified to perform the neurological exams via a web-base tutorial maintained by the Neurological AIDS Research Consortium. Two neuropathic signs were defined: loss of vibration was defined as having at least mild loss of vibration sensation in both great toes, and absence of reflexes was defined as having ankle reflexes absent or hypoactive relative to the knees bilaterally. Three neuropathic symptoms were defined: pain was any pain, aching, or burning in feet and/or legs bilaterally; pins and needles was sensation described as feeling pins and needles in feet and/or legs bilaterally; numbness was lack of feeling in feet and/or legs bilaterally. Symptomatic was at least one of the three aforementioned symptoms. Peripheral neuropathy (PN) was defined by having “loss of vibration” or “absence of reflexes.” Symptomatic neuropathy (SPN) was defined as having PN plus being symptomatic. Painful neuropathy (PPN) was defined as having PN plus pain.
Statistical Methods
Descriptive statistics are used to describe the study sample. The primary comparisons are between the combined VCV arms (5 mg, 10 mg, and 15 mg) versus placebo. Within-arm changes in PN and SPN were assessed using transition tables. Posttreatment between-arm comparisons of PN and SPN were performed using a logistic generalized estimating equation (GEE) repeated measures analysis with an unstructured variance-covariance matrix, where treatment arm, baseline PN (or SPN) status, and neurotoxic nucleoside reverse transcriptase inhibitor(s) (RTI; eg, didanosine, stavudine, zalcitabine) use at baseline and at Day 14 were used as binary covariates. Differences between study arms in individual neuropathic signs and symptoms, PN, SPN, and PPN at Weeks 24 and 48 were assessed using exact confidence intervals. For participants who entered Step 2 before Week 24, the results obtained at the entry to Step 2 were used as the Week 24 data. We analyzed the data utilizing three approaches: (1) intent-to-treat (ITT) with missing outcomes imputed as an “event,” (2) using observed data by randomized treatment assignment, and (3) (not shown) using observed data “as-treated.” Given the exploratory nature of this analysis, all significant testing was performed at the .05 level, and all reported P values are two-sided. There was no adjustment for multiple testing.
Summary of Primary A5211 Trial Results
In ACTG 5211, the analyses of the change in plasma HIV-1 RNA levels at Day 14 and Week 24 among treatment-experienced participants showed that VCV was effective at virologic suppression relative to placebo.8 At 14 days and 24 weeks, mean changes in HIV-1 RNA level (log10 copies/mL) were greater in the VCV groups (−0.87 and −1.51 [5 mg], −1.15 and −1.86 [10 mg], and −0.92 and −1.68 [15 mg]) than in the placebo group (+0.06 and −0.29) (P < .01). Grade 3/4 adverse events were similar across the groups.
RESULTS
Study Participants, Demographics, and Baseline Characteristics
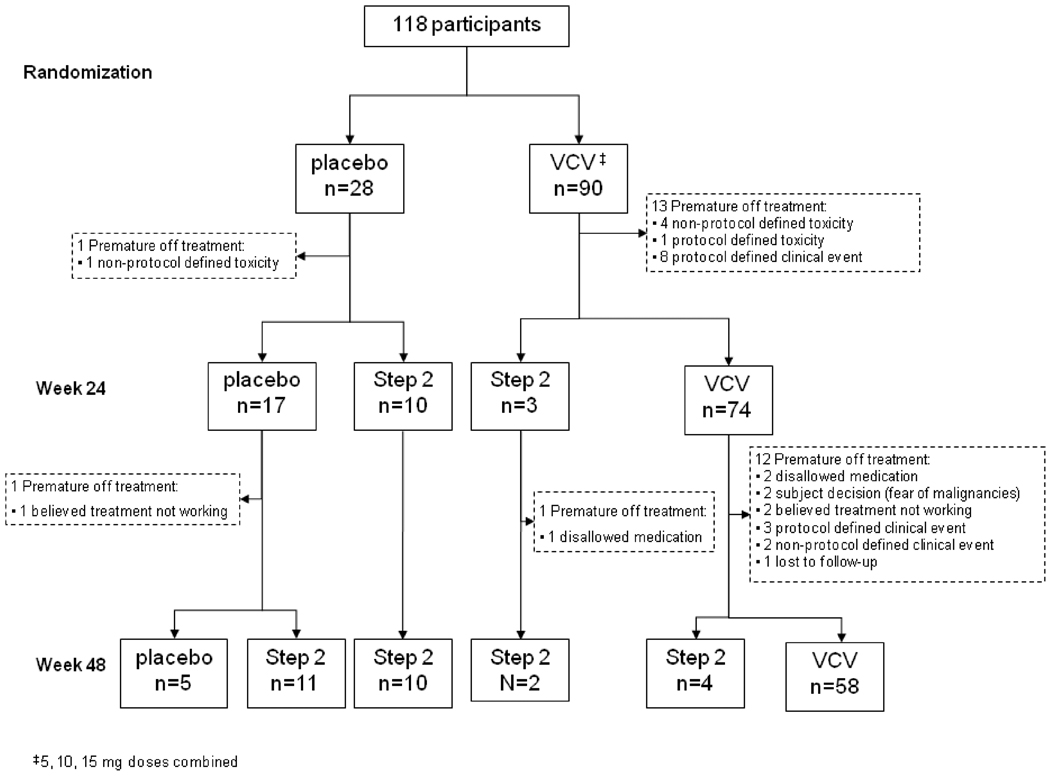
A total of 118 participants were enrolled with 28 and 90 randomized to placebo and VCV (the 5 mg, 10 mg, and 15 mg VCV arms combined), respectively. The VCV 5 mg arm was stopped early by the independent Data and Safety Monitoring Board because of inferior virologic activity, and all participants were offered to increase their VCV to 15 mg. By Week 24, ten participants in the placebo and three in the VCV had experienced virologic failure(s) and entered Step 2 or had their dose increased to 15 mg upon discontinuation of the 5 mg arm. At Week 48, 21 participants in the placebo and 7 in the VCV had entered Step 2 (Figure 1; CONSORT diagram). The study sample was 92% male, 65% white, median (Q1, Q3) age of 46 (41, 53) years, median (Q1, Q3) baseline CD4 count of 139 (63, 265) cells/mm3, and median (Q1, Q3) baseline HIV-1 RNA of 4.58 (4.17, 5.04) log10 copies/mL. Four percent had a history of injection drug use, and 42% were on neurotoxic nucleoside RTI at baseline (Table 1). At baseline, 64%, 39%, and 24% of the participants had PN, SPN, and PPN, respectively.
Figure 1.
CONSORT diagram. VCV = vicriviroc.
Table 1.
Demographics and baseline characteristics
| Treatment arm |
|||
|---|---|---|---|
| Total (n = 118) | Placebo (n = 28) | VCV (n = 90) | |
| Gender | |||
| Male | 108 (92%) | 26 (93%) | 82 (91%) |
| Female | 10 (8%) | 2 (7%) | 8 (9%) |
| Race/ethnicity | |||
| White | 77 (65%) | 21 (75%) | 56 (62%) |
| Black | 24 (20%) | 5 (18%) | 19 (21%) |
| Hispanic | 14 (12%) | 1 (4%) | 13 (14%) |
| Other | 3 (3%) | 1 (4%) | 2 (2%) |
| Age, years | |||
| Median | 46 | 48 | 46 |
| 13–29 | 1 (1%) | 0 (0%) | 1 (1%) |
| 30–39 | 15 (13%) | 5 (18%) | 10 (11%) |
| 40–49 | 58 (49%) | 12 (43%) | 46 (51%) |
| 50–59 | 32 (27%) | 10 (36%) | 22 (24%) |
| ≥60 | 12 (10%) | 1 (4%) | 11 (12%) |
| IV drug history | |||
| Never | 113 (96%) | 27 (96%) | 86 (96%) |
| Previously | 5 (4%) | 1 (4%) | 4 (4%) |
| CD4 count, cells/mm3 | |||
| Median (Q1, Q3) | 139 (60, 265) | 163 (66, 228) | 127 (53, 278) |
| Log (HIV-1 RNA), copies/mL | |||
| Median (Q1, Q3) | 4.58 (4.17, 5.04) | 4.38 (4.04, 4.66) | 4.65 (4.19, 5.23) |
| Baseline neurotoxic nucleoside RTIa use | 49 (42%) | 11 (39%) | 38 (42%) |
| Day 14 neurotoxic nucleoside RTIa use | 32 (27%) | 8 (29%) | 24 (27%) |
Note: VCV = vicriviroc; RTI = reverse transcriptase inhibitor.
didanosine, stavudine, zalcitabine
Analyses
Within-arm changes
Using observed data by randomized treatment assignment, at baseline, Week 24, and Week 48, the proportions of PN were 69%, 60%, and 65%; SPN were 35%, 30%, and 30%; and PPN were 18%, 24%, and 13% for the placebo arm. The proportions of PN were 62%, 63%, and 64%; SPN were 40%, 32%, and 30%; and PPN were 26%, 24%, and 24% for the VCV arm (Table 2). Most participants (74% in placebo arm and 76% in VCV arm) kept the same PN status within the fi rst 24 weeks of the study (Table 3).
Table 2.
Specific neuropathic signs and symptoms and neuropathy status [number (% of nonmissing observations)]
| Baseline |
Week 24 |
Week 48 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo n = 28 |
VCV n = 90 |
Placebo n = 28 |
VCV n = 90 |
Difference (95% CI)* |
Difference (95% CI)† |
Placebo n = 28 |
VCV n = 90 |
Difference (95% CI)* |
Difference (95% CI)† |
|
| Loss of vibration | 14 (54%)a | 34 (40%)a | 10 (50%)d | 31 (43%)d | 7% (−17%, 31%) | 10% (−12%, 29%) | 13 (57%)i | 32 (46%)i | 11% (−13%, 33%) | 7% (−15%, 26%) |
| Absence of reflexes | 17 (65%)b | 46 (53%)b | 11 (55%)e | 37 (52%)e | 3% (−22%, 26%) | 6% (−15%, 24%) | 12 (57%)j | 34 (49%)j | 8% (−17%, 31%) | 7% (−15%, 25%) |
| Pain | 7 (25%) | 28 (31%) | 6 (29%)f | 23 (29%)f | –1% (−20%, 23%) | 8% (−13%, 28%) | 3 (13%)k | 21 (28%)k | –15% (−30%, 7%) | –11% (−29%, 10%) |
| Pins & needles | 6 (21%) | 21 (23%) | 5 (24%)f | 18 (23%)f | 1% (−17%, 24%) | 10% (−10%, 30%) | 4 (17%)k | 21 (28%)k | –11% (−27%, 12%) | –8% (−26%, 15%) |
| Numbness | 10 (36%) | 33 (37%) | 7 (33%)f | 27 (35%)f | –1% (−22%, 23%) | 7% (−14%, 27%) | 5 (22%)k | 25 (33%)k | –12% (−29%, 12%) | –9% (−28%, 14%) |
| Symptomatic | 12 (43%) | 42 (47%) | 9 (43%)f | 30 (38%)f | 4% (−18%, 28%) | 10% (−11%, 30%) | 7 (30%)k | 31 (41%)k | –11% (−31%, 13%) | –8% (−29%, 14%) |
| Neuropathy (PN) | 18 (69%)b | 54 (62%)b | 12 (60%)g | 44(63%)g | –3% (−27%, 19%) | 0.3% (−21%, 18%) | 15 (65%)i | 45 (64%)i | 1% (−22%, 22%) | –1% (−22%, 16%) |
| Symptomatic neuropathy (SPN) | 9 (35%)c | 35 (40%)c | 6 (30%)h | 24(32%)h | –2% (−22%, 22%) | 7% (−14%, 27%) | 7 (30%)l | 25 (34%)l | –4% (−23%, 19%) | –4% (−24%, 18%) |
| Painful neuropathy (PPN) | 5 (18%) | 23 (26%) | 5(24%)f | 19 (24%)f | –1% (−18%, 23%) | 8% (−11%, 29%) | 3 (13%)k | 18 (24%)k | –11% (−25%, 10%) | –8% (−26%, 14%) |
Note: VCV = vicriviroc.
Estimates and 95% exact confidence intervals of the differences, based on “observed data” approach, in the proportions between groups (placebo – VCV).
Estimates and 95% exact confidence intervals of the differences, based on “ITT” approach, in the proportions between groups (placebo – VCV).
Missing 2/4 in placebo/VCV.
Missing 2/3 in placebo/VCV.
Missing 2/2 in placebo/VCV.
Missing 8/18 in placebo/VCV.
Missing 8/19 in placebo/VCV.
Missing 7/12 in placebo/VCV.
Missing 8/20 in placebo/VCV.
Missing 8/15 in placebo/VCV.
Missing 5/20 in placebo/VCV.
Missing 7/21 in placebo/VCV.
Missing 5/15 in placebo/VCV.
Missing 5/17 in placebo/VCV.
Table 3.
Transition table of PN and SPN
| Week 24 |
||||||
|---|---|---|---|---|---|---|
| Week 0 | + | − | Missing | |||
| Placebo | PN | + | 18 | 9 (75.0%) | 3 (25.0%) | 6 |
| − | 8 | 2 (28.6%) | 5 (71.4%) | 1 | ||
| Missing | 2 | 1 | 0 | 1 | ||
| Total n | 28 | 12 (57.9%) | 8 (42.1%) | 8 | ||
| SPN | + | 9 | 3 (60.0%) | 2 (40.0%) | 4 | |
| − | 17 | 2 (14.3%) | 12 (85.7%) | 3 | ||
| Missing | 2 | 1 | 0 | 1 | ||
| Total n | 28 | 6 (26.3%) | 14 (73.7%) | 8 | ||
| VCV | PN | + | 54 | 34 (82.9%) | 7 (17.1%) | 13 |
| − | 33 | 10 (34.5%) | 19 (65.5%) | 4 | ||
| Missing | 3 | 0 | 0 | 3 | ||
| Total n | 90 | 44 (62.9%) | 26 (37.1%) | 20 | ||
| SPN | + | 35 | 21 (77.8%) | 6 (22.2%) | 8 | |
| − | 53 | 3 (6.3%) | 45 (93.7%) | 5 | ||
| Missing | 2 | 0 | 0 | 2 | ||
| Total n | 90 | 24 (32.0%) | 51 (68.0%) | 15 | ||
Note: PN = peripheral neuropathy; SPN = symptomatic neuropathy; VCV = vicriviroc.
Between-arm comparison
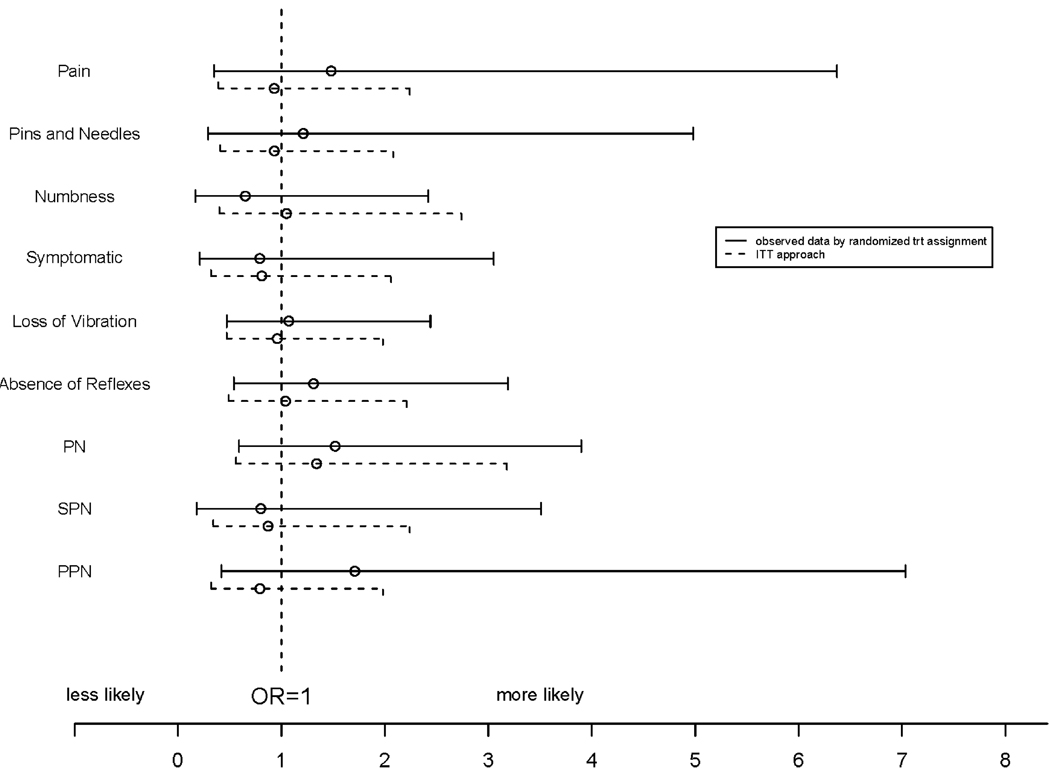
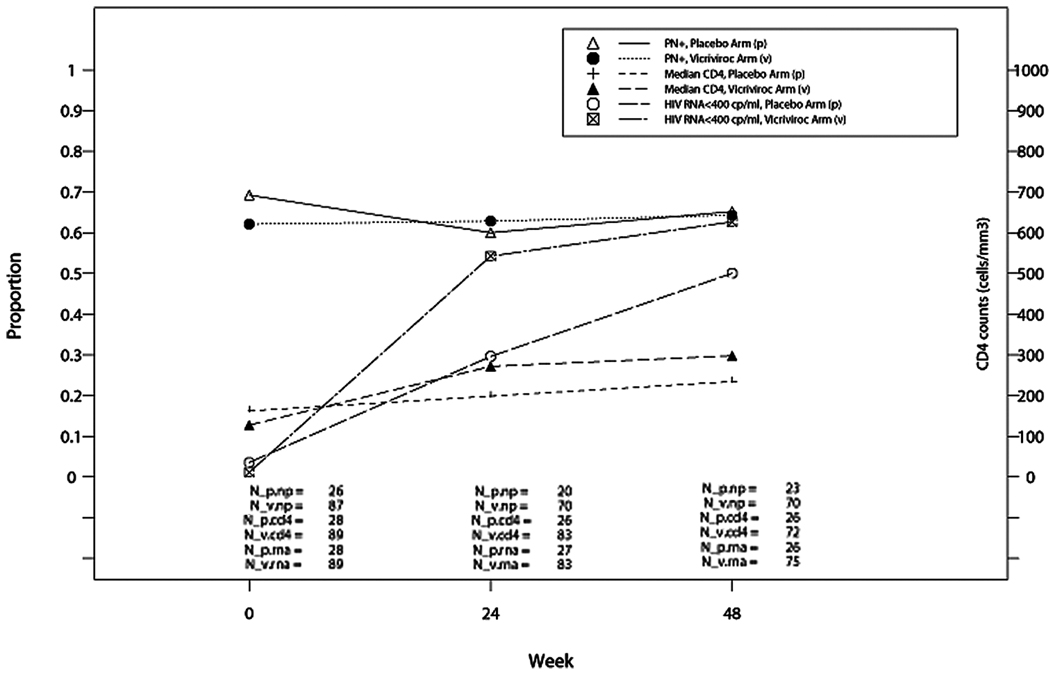
The proportions of participants who had PN, SPN, PPN, and specific neuropathic signs and symptoms were not different between the placebo and VCV groups at any given measurement time during the study (Table 2) based on observed data by treatment assignment and ITT approaches. Treatment with VCV did not result in a significant change compared to placebo in PN (OR = 1.52; P = .39; 95% CI 0.59, 3.90), SPN (OR = 0.80; P = .77; 95% CI 0.18, 3.51), or PPN (OR = 1.71; P = .46; 95% CI 0.42, 7.03) after adjusting for baseline PN, SPN, or PPN status and baseline neurotoxic nucleoside RTI use in the analysis using observed data by randomized treatment assignment (Figure 2). No significant differences were observed when considering individual neurologic signs and symptoms, although odds ratios as large as 6.37 (pain symptoms) or 4.98 (pins and needles symptoms) were still consistent with the data. Participants randomized to VCV, based on observed data by randomized treatment assignment, appeared to have a marginally higher proportion of participants with HIV viral load <400 copies/mL after randomization controlling baseline HIV viral load (OR estimate [95% CI] = 2.07 [0.96, 4.47]; P = .065) and had higher CD4 counts after randomization controlling baseline CD4 counts (effect estimate [95% CI] = 100.8 [55.9, 145.7]; P < .001) (Figure 3).
Figure 2.
Effect of vicriviroc (VCV vs. placebo) on various neuropathic outcomes (odds ratios [OR] and 95% CIs), adjusted for baseline status of neuropathic outcomes and baseline neurotoxic nucleoside reverse transcriptase inhibitor(s) (RTI) use. PN = peripheral neuropathy; PPN = painful neuropathy; SPN = symptomatic neuropathy.
Figure 3.
Neuropathy (PN) prevalence (using observed data by randomized treatment assignment), median CD4, and HIV RNA over time.
Sensitivity analyses were also conducted based on as-treated approach, and the results were not qualitatively different.
DISCUSSION
These analyses failed to identify an association between VCV and neuropathic outcomes in the treatment-experienced participants. Most participants entering the study with neuropathy continued to have neuropathy in both placebo and VCV arms. The analyses were limited by the small sample size with consequently low power to demonstrate an effect of VCV on neuropathic outcomes. In addition, a substantial number of the participants who were randomized to the placebo group subsequently entered Step 2 and received VCV treatment (ie, making the two randomization groups more alike). The analysis approaches (ie, ITT and observed data by randomized treatment assignment) are conservative. However, as-treated analysis (ie, using observed data based upon the treatments the participants received) also failed to detect an effect of VCV.
The key finding we were interested in was whether VCV might be neuroprotective over the course of 48 weeks. Comparison with the placebo group, with only 5/28 patients remaining on placebo through this period due to suboptimal virologic responses, make this comparison virtually impossible. However, had neuroprotection occurred we might have seen less neuropathy than at the beginning of the study, assuming healing is possible with PN. Instead, the percentage with neuropathy remained stable throughout the study. Recovery did not occur. We conclude that there is insufficient evidence of neuroprotection from VCV, although long-standing fixed peripheral nerve deficits in advanced HIV patients such as these may have limited capacity for healing, particularly without additional growth factors to stimulate new nerve growth.
Although we were only able to rule out very large effects, modest effects are still possible. Given that the resolution of existing PN may be unlikely, it may be worth evaluating any neuroprotective effects of CCR5 inhibitors, such as VCV, with respect to the incidence of PN in future studies involving participants with earlier stage of HIV infection. In addition, given that the VCV doses used in this study are lower than those used in subsequent phase III VCV studies, future studies assessing the effect of higher doses of VCV on neuropathy should be considered.
ACKNOWLEDGMENTS
The authors thank the ACTG 5211 study team, study participants, and ACTG sites that participated in this project. We also wish to acknowledge the support of the AIDS Clinical Trials Group, National Institute of Allergy and Infectious Diseases, with the following grant numbers: AI-68636 (ACTG), AI-68634 (Statistical and Data Analysis Center, Harvard School of Public Health), AI-25859 (University of Indiana Clinical Trials Unit [CTU]), AI-27661 (University of Minnesota CTU), AI-32782 ( University of Texas, Galveston CTU), AI-32783 ( University of Pennsylvania CTU), AI-34853 ( University of Hawaii CTU), AI-46370 (Beth Israel Medical Center CTU, New York ), AI-46381 (Brown University ACTU), AI-69411 ( University of Rochester CTU), AI-69418 (Emory University CTU), AI-69419 (Cornell University CTU), AI-69423 (University of North Carolina CTU), AI-69424 (University of California, Los Angeles CTU), AI-69432 (University of California, San Diego CTU), AI-69434 ( University of Washington CTU), AI-69439 (Vanderbilt University CTU), AI-69450 (University of Colorado CTU), AI-69465 (Johns Hopkins University CTU), AI-69471 (North-western University CTU), AI-69472 (Harvard University/Boston Medical Center CTU), AI-69474 (Ohio State University CTU), AI-69494 ( University of Pittsburgh/Georgetown University CTU), AI-69495 (Washington University CTU), AI-69501 (Case Western Reserve University CTU), AI-69502 (University of California, San Francisco CTU), AI-69532 (New York University CTU), AI-69556 (Stanford University CTU); K24-AI-51966 (R.M.G.); the Neurological AIDS Research Consortium NS32228; and Schering-Plough Research Institute for providing the study drug. Also supported in part by the General Clinical Research Centers (GCRC) Units of the National Center for Research Resources: grants M01-RR00044 (University of Rochester GCRC), M01-RR00046 (University of North Carolina GCRC), M01-RR00047 (Cornell University GCRC), M01-RR00051 (University of Colorado GCRC), M01-RR00052 (Johns Hopkins University GCRC), M01-RR00096 (New York University GCRC), P30-AI-45008 (University of Pennsylvania Center for AIDS Research [CFAR]), and P30-AI-50410 (University of North Carolina CFAR).
REFERENCES
- 1.Verma S, Simpson DM. Peripheral neuropathy in HIV infection. In: Portegies P, Berger J, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier; 2007. pp. 129–137. [DOI] [PubMed] [Google Scholar]
- 2.Simpson D, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr. 1995;9:153–161. [PubMed] [Google Scholar]
- 3.Evans SR, Clifford DB, Chen H, et al. HIV-Associated Peripheral Neuropathy in the HAART Era: Results from AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) Protocol A5001. Presented at: 16th CROI Conference; February 8–11, 2009; Montreal, QC, Canada. [Google Scholar]
- 4.Strizki JM, Tremblay C, Xu S, et al. Discovery and chracterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2005;49:4911–4919. doi: 10.1128/AAC.49.12.4911-4919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson KA, Churchill MJ, Gorry PR, et al. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56(6):873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- 6.Jones G, Zhu Y, Silva C, et al. Peripheral nerve-derived HIV-1 is predominantly CCR5-dependent and causes neuronal degeneration and neuroinflammation. Virology. 2005;334(2):178–193. doi: 10.1016/j.virol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Keswani SC, Polley M, Pardo CA, et al. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54(3):287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 8.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a ccr5 inhibitor, in hiv-1–infected, treatment-experienced patients: AIDS Clinical Trials Group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]