Abstract
Background
Previous studies in humans have shown that alcohol consumption decreased the rate of brain glucose utilization. We investigated whether the major metabolite of ethanol, acetate, could account for this observation by providing an alternate to glucose as an energy substrate for brain and the metabolic consequences of that shift.
Methods
Rats were infused with solutions of sodium acetate, ethanol, or saline containing 13C-2-glucose as a tracer elevating the blood ethanol (BEC) and blood acetate (BAcC) concentrations. After an hour, blood was sampled and the brains of animals were removed by freeze blowing. Tissue samples were analyzed for the intermediates of glucose metabolism, Krebs’ cycle, acyl-coenzyme A (CoA) compounds, and amino acids.
Results
Mean peak BEC and BAcC were approximately 25 and 0.8 mM, respectively, in ethanol-infused animals. Peak blood BAcC increased to 12 mM in acetate-infused animals. Both ethanol and acetate infused animals had a lower uptake of 13C-glucose into the brain compared to controls and the concentration of brain 13C-glucose-6-phosphate varied inversely with the BAcC. There were higher concentrations of brain malonyl-CoA and somewhat lower levels of free Mg2+ in ethanol-treated animals compared to saline controls. In acetate-infused animals the concentrations of brain lactate, α-ketoglutarate, and fumarate were higher. Moreover, the free cytosolic [NAD+]/[NADH] was lower, the free mitochondrial [NAD+]/[NADH] and [CoQ]/[CoQH2] were oxidized and the ΔG′ of ATP lowered by acetate infusion from −61.4 kJ to −59.9 kJ/mol.
Conclusions
Animals with elevated levels of blood ethanol or acetate had decreased 13C-glucose uptake into the brain. In acetate-infused animals elevated BAcC were associated with a decrease in 13C-glucose phosphorylation. The co-ordinate decrease in free cytosolic NAD, oxidation of mitochondrial NAD and Q couples and the decrease in ΔG′ of ATP was similar to administration of uncoupling agents indicating that the metabolism of acetate in brain caused the mitochondrial voltage dependent pore to form.
Keywords: Acetate, Brain, Energy Metabolism, 13C-Glucose, ΔG′ ATP
The brain accounts for 20% of the body’s O2 consumption at rest. While under normal resting conditions, the brain’s energy needs are supplied by glucose, during prolonged fasting 60% of the brain’s energy requirements are supplied by ketone bodies (Owen et al., 1967). Volkow and co-workers have shown that ethanol consumption lowered the rate of brain glucose utilization in humans (Volkow et al., 2006; Wang et al., 2003). Since acetate is the major product of liver metabolism of ethanol, we examined whether elevated blood acetate concentrations (BAcC) in laboratory rats could account for a decrease in brain glucose utilization observed in human subjects consuming alcohol.
It has been long recognized that the brain can metabolize acetate specifically in glial cells where it produces glutamine which is then exported to neurons (Berl and Clarke, 1984). Recent studies using 13C MRS have shown that acetate is significantly utilized in brain metabolism (Deelchand et al., 2009). Acetate thus shares with the other monocarboxylates, ketone bodies and lactate, the ability to be transported into brain and to be used in place of glucose (Simpson et al., 2007). Since the usual precursor of elevated blood acetate is ethanol, we studied the effects of both ethanol and acetate on 13C-glucose uptake in brain. Moreover, since differing substrates traversing the Krebs citric acid cycle have different inherent enthalpic values, we examined the effects of ethanol and acetate on brain energy metabolism.
Animals received a bolus of a solution of sodium acetate, ethanol or saline (containing 13C-2-glucose as a tracer) followed by a constant infusion of the same solution for an hour. After which time, blood was sampled, animals were killed, and the brains were analyzed for glycolytic, TCA cycle, and other metabolites. The concentrations of the metabolic intermediates were used to calculate cytosolic and mitochondrial redox potentials and the ΔG of ATP hydrolysis.
MATERIAL AND METHODS
Animal Procedures
All experiments were reviewed and approved by the Animal Care and Use Committee of NIAAA, NIH. Male Wistar rats weighing 280 to 310 g (n = 6 to 8 per group) were obtained from Charles River Laboratories, Wilmington, MA, and divided into 3 groups. Twenty four hours prior to infusion the jugular veins of animals were cannulated using polyethylene tubing (PE-50). Animals recovered from surgery for 24 hours and were maintained on NIH 31 rat diet. Prior to infusions animals were fasted overnight.
Infusions
Each animal received a bolus (0.08 ml/100 g body weight) of 1 of 3 solutions: saline-control (PBS, phosphate buffered saline with 0.73 mg/ml 13C-2 glucose), sodium-acetate (2 M in PBS + 0.73 mg/ml 13C-2 glucose), and ethanol (4 Min PBS+0.73 mg/ml 13C-2 glucose). The bolus dose was followed by infusion of solutions at a rate of 1.4 ml/h/100 g body weight. Following infusions, blood samples were collected and brain removed by freeze blowing (Veech et al., 1973) and stored at −80°C until analyzed.
Analytical Procedures
Krebs cycle intermediates were measured using gas chromatography-mass spectrometry (GC-MS). The coenzyme A’s (CoA) and nitrogenousmetabolites were measured using capillary electrophoresis-mass spectrometry (CE-MS). The number of samples analyzed for each metabolite varied from 5 to 8. All other metabolites were measured enzymatically as described previously (Sato et al., 1995).
Sample Preparation for GC-MS and CE-MS Analyses
Procedures for the determination of citrate, isocitrate, α-ketoglutarate, succinate, fumarate, and malate by GC-MS are described below. The organic acids were analyzed as their tertiary butyl dimethylsilyl ether derivatives (TBDMS) using GC-MS in the electron impact mode and quantified using the 13C-labeled internal standards for each analyte. The N-methyl-N-(tert-butylmethylsilyl) trifluoroacetamide (MTBSTFA) with 1% tert-butyldimethylchlorosilane (TBDMCS) reagent was purchased from Pierce Chemical Co. (Rockford, IL) and the 13C-labeled organic acids, citrate, succinate, fumarate, and malate were procured from Sigma Chemical Co. (St Louis, MO). The 13C-labeled isocitrate and α-ketoglutarate were prepared in the laboratory according to known procedures (Barman, 1969; Ehrlich and Colman, 1987).
Brain samples for GC-MS analysis were prepared by perchloric acid extraction as previously described (Veech et al., 1973). The 13C-labeled internal standards were added in twofold excess of the concentrations of the individual analytes in brain tissue to the neutralized PCA extracts. Extracts (50 μl) were evaporated under a stream of nitrogen to dryness. Samples were immediately reacted with 5 μl of the sylilating reagent in 15 μl of acetonitrile in 1.5 ml screw capped glass vials and heated to 60°C for 5 minutes. Samples were analyzed on an Agilent 5973 quadrupole GC-MS (Agilent, Wilmington, DE). One μl of the sample solutions was injected onto a 250 μm × 30 m capillary DB-1 (Agilent) column in the splitless injection mode using helium as the carrier gas. The injector temperature was set at 270°C and the transfer line at 280°C. The GC oven temperature was programmed from 80 to 325°C at 15°C/min. The mass spectrometer was operated in the electron impact mode (70 eV) and the quadrupole mass analyzer scanned for ions which corresponded to a loss of 15 mass units (-CH3) from the molecular ion and the base peak of each analyte and its corresponding 13C-labeled internal standard using selected ion monitoring. Analytes eluted the column between 10 and 20 minutes following injection and the ratio of peak area counts of the 13C-labeled internal standard to that of the analyte were used to quantify its concentration.
Standards for CE-MS Analysis of CoA, Acetyl CoA, Malonyl CoA, Succinyl CoA, Glutamate, Aspartate
The labeled internal standards, 13C-glutamate, 13C-aspartate, 13C-acetyl CoA, and 13C-malonyl CoA were purchased from Sigma Chemical Co. (St Louis, MO). 13C-succinyl CoA was synthesized in the laboratory.
Sample Extraction for CE-MS Analysis
Brain tissue samples were extracted using a modified chloroform-methanol extraction procedure (Soga et al., 2002). Approximately 50 mg of frozen whole brain tissue was added to a frozen (−80°C) solution of water:chloroform:methanol (300:400:200 μl) containing the labeled internal standards in twofold excess in concentration of the analyte in tissue in 2 ml polypropylene screw capped tubes with 40 to 50 small glass beads. Samples were shaken onMini Bead Beater (Biopsec Products, Bartlesville, OK) for 30 seconds (2×) until samples were homogeneous. Samples were placed on ice until centrifugation at 4°C in a Sorvall benchtop centrifuge at a speed of 10.7 × g for 10 minutes to allow the chloroform and water layers to separate. The water layer was removed and filtered through a protein filter centrifuge tube, MW cut off 10,000 Da at 90 × g. The filtrate (about 300 μl)was used in CE-MS analysis.
CE-MS Analysis
Sample extracts were analyzed on an Agilent capillary electrophoresis-ion trap mass spectrometer (Ultra) using an Agilent 1100 series binary pump to deliver a make-up flow of 50 mM ammonium acetate in methanol:water (50:50) to the electrospray ionization tip (Agilent). Samples were ionized in either the positive or negative ion ionization modes. Anions and cations were analyzed on 50 μm × 100 cm dynamically cationic or anionic coated capillary columns using either positive (+30 kV) or negative (−30 kV) electro-kinetic injections. The capillary column was cooled to 20°C using the thermostatic controlled capillary cassette and the electrospray needle was set orthogonal to the mass spectrometer inlet. The CE electrolyte used for separation was 50 mM ammonium acetate (pH 9.0) and the column was flushed successively between runs with acetic acid (pH 3.4) and ammonium acetate before injection. The applied voltage was set at −30 kV. The binary pump was set to deliver 8 μl of 5 mM ammonium acetate in water:methanol (50:50) as a sheath liquid to the capillary tip. The mass spectrometer was scanned throughout various mass ranges to accommodate the analysis for the amino acids (150 to 300 amu) or the CoA compounds (700 to 1100 amu). Analytes were quantified using the ratio of peak area counts of the 13C-labeled internal standard pseudo molecular ion to that of the analyte.
Calculations
The calculation of free [Mg2+], phosphorylation potential, free cytosolic [ADP], [AMP], [Pi], [oxaloacetate], the ΔG of ATP hydrolysis, cytosolic and mitochondrial redox states were calculated as described previously (Veech et al., 1979, 1994; Veloso et al., 1973).
Statistics
The results are presented as: means ± SEM. A paired t-test with the Bonferroni adjustment for multiple group comparisons were used to determine the level of significance between means of the various experimentally determined intermediates, the derived redox couples and nucleotides between each of the 3 groups. Regression analysis was carried out using StatView statistical software from Adept Scientific (Letchworth Garden City, U.K.) and significance was determined using ANOVA. A p-value<0.05 was considered significant.
RESULTS
Blood Glucose, Acetate, Ethanol Concentrations
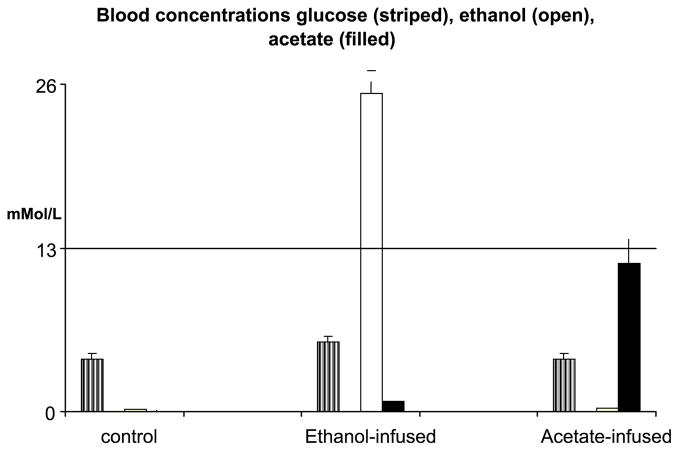
Animals remained alert during infusion procedures, although ethanol-infused animals were less active compared to animals in the other 2 groups. Fasting blood glucose concentrations were within normal limits for all 3 groups (Table 1). Mean blood ethanol concentrations (BEC) increased to 25 mM(range: 8 to 31 mM) an hour after infusion of the ethanol solution. The BAcC increased over baseline values but remained below 1 mM (Fig. 1). As predicted, BAcC in acetate-infused group were elevated to a mean value of 12 mM (range: 2.5 to 20 mM) 1 hour after beginning infusion. Although, mean BEC and BAcC values tended to be proportional to their respective concentrations of the infusate the blood concentration range for both blood ethanol and acetate was variable.
Table 1.
Blood Glucose, Ethanol, and Acetate Concentrations
| Saline (mM/l) | Ethanol (mM/l) | Acetate (mM/l) | |
|---|---|---|---|
| Glucose | 4.15 ± 0.21 | 5.50 ± 0.05 | 4.17 ± 0.44 |
| Ethanol | 0.18 ± 0.03 | 25.3 ± 1.76a,b | 0.23 ± 0.08 |
| Acetate | 0.04 ± 0.01 | 0.77 ± 0.06a | 11.79 ± 1.87a,b |
Animals infused with solutions of phosphate buffered saline (PBS) (n = 5), 4 M ethanol in PBS (n = 8), or 2 M sodium acetate in PBS (n = 6).
p < 0.05 between ethanol- or acetate-infused group and saline.
p < 0.05 between ethanol-infused and acetate-infused, paired t-test with Bonferroni adjustment for multiple group comparisons.
Fig. 1.
Blood glucose, ethanol, and acetate concentrations from rats given bolus doses and infused with solutions of saline (control), sodium acetate (2 M), or ethanol (4 M) in phosphate buffered saline after 1-hour infusion.
Brain Glycolytic Intermediates
There were no differences in concentrations of brain phosphorylated intermediates, glucose-6-phosphate, di-hydroxy acetone phosphate, or 3-phosphoglycerate between the ethanol or acetate infused animals and the saline infused controls. However, in the brains of the acetate-infused animals the concentration of lactic acid was greater compared to either the ethanol-treated or saline groups (Table 2). Consequently, the brain free cytosolic [NAD+]/[NADH] (determined by lactate and pyruvate concentrations) were more reduced (mean value: 257) compared to either ethanol-treated (mean: 397) or saline (mean: 392) (Table 3). An increase in the concentration of brain lactate in the presence of a more abundant energy substrate, acetate, suggests a possible decrease in glucose utilization. However, the concentration of acetyl-CoA was no different among any of the groups (Table 4). In ethanol-infused animals, both brain lactate concentrations and cytosolic redox potential (Table 3) were no different from saline-controls, showing that unlike liver where free cytosolic [NAD+]/[NADH] is markedly reduced by ethanol metabolism, the brain which lacks alcohol dehydrogenase (Veloso et al., 1973) may only metabolize ethanol through cytochrome P450 (Anandatheerthavarada et al., 1993) or catalase pathways (Cohen et al., 1980).
Table 2.
Brain Glycolytic Intermediates
| Saline | Ethanol | Acetate | |
|---|---|---|---|
| Glucose-6-phosphate | 0.095 ± 0.007 | 0.086 ± 0.006 | 0.104 ± 0.012 |
| DHAP | 0.027 ± 0.001 | 0.031 ± 0.004 | 0.024 ± 0.003 |
| 3-Phos-glycerate | 0.020 ± 0.002 | 0.023 ± 0.003 | 0.021 ± 0.003 |
| Pyruvate | 0.094 ± 0.012 | 0.091 ± 0.011 | 0.081 ± 0.012 |
| L-Lactate | 1.37 ± 0.04 | 1.29 ± 0.15 | 1.77 ± 0.08a,b |
Animals infused with solutions of phosphate buffered saline (PBS) (n = 5), 4 M ethanol in PBS (n = 8), or 2 M sodium acetate in PBS (n = 6). Values are means in μmole/g wet weight ± SEM.
p < 0.05 between acetate-infused and saline groups.
p < 0.05 between acetate- and ethanol-infused groups, paired t-test with Bonferroni adjustment for multiple group comparisons.
Table 3.
Calculated Free Nucleotide Concentrations and Ratios
| Saline | Ethanol | Acetate | |
|---|---|---|---|
| Free [NAD+]/[NADH] cytosol from lactate/pyruvate | 392.7 ± 57.3 | 397.2 ± 36.9 | 257.3 ± 33.5b |
| Free [NAD+]/[NADH] mitochondria from αKG × NH4+/Glut | 1.03 ± 0.05 | 1.04 ± 0.05 | 1.32 ± 0.08a,b |
| Free [NADP+]/[NADPH] cytosol from αKG × CO2/IsoCit | 0.0130 ± 0.0003 | 0.0133 ± 0.0010 | 0.0165 ± 0.0014 |
| Free mitochondrial [CoQ]/[CoQH2] from measured Fum/Succ | 0.0047 ± 0.0004 | 0.0056 ± 0.0006 | 0.0062 ± 0.0004a |
| Free [Mg2+] mM from [Cit]/[Isocit] | 0.333 ± 0.129 | 0.261 ± 0.068 | 0.750 ± 0.181b |
| Phosphorylation potential from kg + g (Eq. 1)/M | 39,315 ± 5,379 | 43,807 ± 7,528 | 33,082 ± 3,992 |
| ΔG′ ATP kJ/mol | −61.4 ± 0.4 | −61.3 ± 0.5 | −59.9 ± 0.3a,b |
| Free [ADP] cytosol μM/g from PCr/Cr | 0.029 ± 0.006 | 0.043 ± 0.016 | 0.035 ± 0.006 |
| Free [AMP] cytosol μM/g from Kmyokinase | 0.0003 ± 0.00008 | 0.0009 ± 0.0006 | 0.0006 ± 0.0001 |
Animals infused with solutions of phosphate buffered saline (PBS) (n = 5), 4 M ethanol in PBS (n = 8), or 2 M sodium acetate in PBS (n = 6). Values are means in μmole/g wet weight ± SEM. The pH assumed to be 7.2. For methods of calculation, see references Veloso and colleagues (1973) and Veech and colleagues (1979, 1994).
p < 0.05 between acetate-infused and saline.
p < 0.05 between acetate- and ethanol-infused groups, paired t-test with Bonferroni adjustment for multiple group comparisons.
Table 4.
Brain Krebs Cycle and Coenzyme A (CoA) Intermediates
| Saline | Ethanol | Acetate | |
|---|---|---|---|
| Citrate | 0.286 ± 0.034 | 0.268 ± 0.023b | 0.373 ± 0.047 |
| Isocitrate | 0.022 ± 0.0005 | 0.023 ± 0.001 | 0.022 ± 0.0002 |
| α-Ketoglutarate | 0.170 ± 0.004 | 0.170 ± 0.005 | 0.201 ± 0.007a,b |
| Succinyl CoA (μM) | 3.36 ± 0.47 | 4.15 ± 0.41 | 3.54 ± 0.24 |
| Succinate | 0.160 ± 0.007 | 0.145 ± 0.010 | 0.158 ± 0.008 |
| Fumarate | 0.128 ± 0.010 | 0.133 ± 0.010 | 0.169 ± 0.013a |
| l-Malate | 0.266 ± 0.015 | 0.252 ± 0.024 | 0.275 ± 0.034 |
| Calc oxaloacetate | 0.0068 ± 0.0002 | 0.0063 ± 0.0005 | 0.0089 ± 0.0008a,b |
| Acetyl CoA (μM) | 3.40 ± 1.32 | 3.35 ± 0.31 | 2.78 ± 0.21 |
| Malonyl CoA (μM) | 2.05 ± 0.33 | 3.15 ± 0.31a | 2.32 ± 0.15 |
Animals infused with solutions of phosphate buffered saline (PBS) (n = 5), 4 M ethanol in PBS (n = 8), or 2 M sodium acetate in PBS (n = 6). Values are means in μmole/g wet weight ± SEM. CoA’s are given in nmol/g wet weight.
p < 0.05 between infused and saline group.
p < 0.05 between ethanol-infused and acetate-infused, paired t-test with Bonferroni adjustment for multiple group comparisons.
Uptake and Utilization of 13C-Glucose Into the Brain
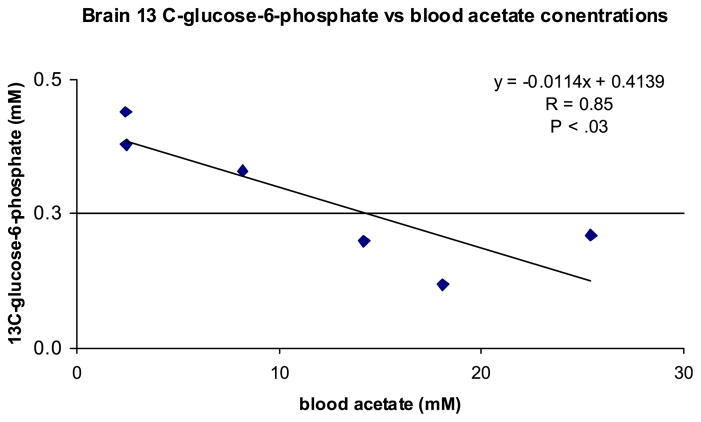
At the end of the infusion period 13C-enrichments of blood 12C-glucose (uncorrected for naturally occurring C-13 abundance) were similar among the 3 groups (Table 5). In the saline controls, 13C-glucose enrichments had equilibrated across the blood-brain interface (blood: 0.442, brain: 0.461). In comparison, in brain extracts from either the ethanol- and acetate-infused animals the 13C-glucose enrichments tended to be lower (ethanol: 0.367, p < 0.06; acetate: 0.295, p < 0.07) than in saline-infused controls. Moreover, 13C-glucose enrichments across the blood-brain interface approached but did not reach equilibrium in either ethanol- or acetate-treated animals (Table 5). Displacement from equilibrium was reflected by a more negative Δ value in these groups and suggested an inhibition in glucose transfer (see Table 5 for the Δ value calculation). Consistent with the observation of a decrease in the uptake and utilization of glucose in acetate-infused animals, there was also an inverse association (correlation coefficient, R = 0.85, p < 0.03) between brain 13C-glucose-6-phosphate concentration and the BAcC (see Fig. 2). However, there was no association between 13C-glucose-6-phosphate and BEC in ethanol-infused animals again demonstrating that the effect on brain glucose 6- phosphate is a result of acetate and not directly from ethanol.
Table 5.
Blood and Brain 13C-Glucose Enrichments and Brain 13C-Glucose-6-Phosphate Concentrations
| Saline | Ethanol | Acetate | |
|---|---|---|---|
| Blood 13C/12C-glucose enrichmentsa | 0.448 ± 0.023 | 0.426 ± 0.008 | 0.360 ± 0.051 |
| Brain 13C/12C-glucose enrichmentsa | 0.461 ± 0.059 | 0.367 ± 0.008 | 0.295 ± 0.048 |
| Δ (percent difference in blood-brain enrichments)b | 2.8 ± 1.1 | −16.4 ± 3.5a | −27.3 ± 7.7d |
| Brain 13C-glucose-6-phosphatec | 0.091 ± 0.013 | 0.070 ± 0.016 | 0.066 ± 0.007e |
Animals infused with solutions of phosphate buffered saline (PBS) (n = 5), 4 M ethanol in PBS (n = 8), or 2 M sodium acetate in PBS (n = 6). Infusates contained 1.8 mg/ml of 13C-2-glucose as tracer.
Values are glucose 13C/12C enrichments ± SEM.
Values are means in μmole/g wet weight ± SEM.
p < 0.01 between ethanol- or acetate-infused animals and saline, paired t-test with Bonferroni adjustment for multiple group comparisons.
p < 0.03 between acetate-infused animals and saline controls, ANOVA.
Fig. 2.
Linear regression of blood acetate concentration versus brain 13C-glucose-6-phosphate in rats infused with sodium acetate (2 M) after 1-hour infusion. Correlation coefficient, R = 0.85 and p < 0.03 (ANOVA).
Krebs Cycle Intermediates
Ethanol-infused animals had a small but statistically significant lower concentrations of brain citric acid compared to the saline-infused controls and significantly lower concentrations with respect to the acetate-infused group (Table 4). As reported previously the citrate/isocitrate ratio may be used as a determinant of the concentration of freeMg2+ in the system (Veloso et al., 1972). Acetate-infused animals had higher free Mg2+ concentrations compared to the saline- or ethanolinfused groups. Ethanol-treated animals had significantly higher concentrations of malonyl CoA than saline-infused animals. In acetate-infused animals there were higher concentrations of fumarate compared to the saline controls and higher amounts of α-ketoglutarate than either the saline- or ethanol-infused animals. However, there were no differences in isocitrate, succinate, succinyl CoA, and malate concentrations in animals from either acetate- or ethanol-infused groups compared to the saline group (Table 4).
Nucleotide and Amino Acid Concentrations
There were several differences in the values of the reduced and oxidized nucleotides (calculated from the concentrations of various metabolic intermediates) in acetate-infused animals compared to either the saline- or ethanol-infused groups (Table 3). Acetate-infused animals had a lower cytosolic free [NAD+]/[NADH] ratio compared to ethanol-infused animals and a lower but nonsignificant (p < 0.057) redox potential compared to the saline controls. Acetate infused animals had an increased free mitochondrial [NAD+]/[NADH] ratio and an increased free [CoQ]/[CoQH2] ratio (Table 3). This reduction in the ratio of free cytosolic [NAD+]/[NADH] in the presence of a more oxidized free mitochondrial redox potential [NAD+]/[NADH] is an unusual pattern, found only rarely in situations such as the administration of uncoupling agents such as carbonyl cyanide p-trifluoromethoxyphenyl hydrazone (FCCP) (Spry, 1971). Compatible with this inference was also a significant decrease in the energy of ATP hydrolysis in acetate- infused animals compared to either the ethanol-infused or saline-infused group. There were no differences in the concentrations neither of brain glutamate or aspartate nor of their 13C-labeled analogues (Table 6) nor high energy intermediates between any of the groups (Table 7).
Table 6.
Brain Amino Acids
| Saline | Ethanol | Acetate | |
|---|---|---|---|
| L-Glutamate | 10.59 ± 0.32 | 10.48 ± 0.29 | 9.78 ± 0.50 |
| L-Aspartate | 2.79 ± 0.11 | 2.55 ± 0.17 | 2.85 ± 0.16 |
Animals infused with solutions of phosphate buffered saline (PBS) (n = 5), 4 M ethanol in PBS (n = 8), or 2 M sodium acetate in PBS (n = 6). Values are means in μmole/g wet weight ± SEM.
Table 7.
Brain High Energy Intermediates
| Saline | Ethanol | Acetate | |
|---|---|---|---|
| ATP | 2.49 ± 0.173 | 2.64 ± 0.19 | 2.39 ± 0.19 |
| P-Creatine | 3.30 ± 0.12 | 3.37 ± 0.11 | 3.54 ± 0.10 |
Animals infused with solutions of phosphate buffered saline (PBS) (n = 5), 4 M ethanol in PBS (n = 8), or 2 M sodium acetate in PBS (n = 6). Values are means in μmole/g wet weight ± SEM.
DISCUSSION
This is the first study to our knowledge investigating the effects of elevated blood acetate and ethanol concentrations on alterations of brain energetic profiles. Previous studies have investigated the effects of labeled-acetate in supplanting glucose utilization on brain metabolites (Deelchand et al., 2009; Serres et al., 2008; Tyson et al., 2003). Aside from blood acetate derived from the metabolism of ethanol, patients with renal failure undergoing dialysis treatment may also have highly elevated BAcC (Mansell et al., 1979).
Previously, Volkow and coworkers using PET imaging demonstrated that human subjects consuming even low amounts of alcohol had decreased brain glucose uptake (Volkow et al., 2006). Yet, it remains unclear whether this effect is primarily due to blood ethanol concentrations or to a combination of increases in blood ethanol and its metabolite, acetate (Lundquist, 1962).
We found that when rats were infused with solutions of ethanol or sodium acetate, both substances decreased 13C-glucose enrichments in the brains. However, in the present study highly elevated BEC together with increases in BAcC (<1 mM) did not cause a significant decrease in the phosphorylation of 13C-glucose whereas, in acetate-infused animals the decrease in brain 13C-glucose-6-phosphate was proportional to the blood acetate concentration suggesting a probable cause and effect relationship.
Elevations in blood acetate also had widespread effects on brain cytosolic and mitochondrial redox potentials and on the phosphorylation potential. In acetate-infused animals there was a decrease in the free cytosolic [NAD+]/[NADH] ratio, an increase of the free mitochondrial [NAD+]/[NADH] ratio, and the free mitochondrial [CoQ]/[CoQH2], and a decrease in the ΔG′ of ATP hydrolysis. This pattern of changes was also seen with the administration of uncoupling agents (Spry, 1971). However, a more plausible explanation for these observations is that high concentrations of acetate elevated the inorganic pyrophosphate (PPi) levels in the brain. Highly elevated PPi concentrations were previously reported in the liver of rats given intraperitoneal (IP) injections of sodium acetate or other short chain fatty acids (Veech et al., 1986). Elevated acetate concentrations were shown to cause an influx of divalent cations, Ca2+ and Mg2+ into the liver mitochondria forming precipitates in the presence of 2 mM CaMgPPi. This process of rapid formation of complexes of CaMgPPi within the mitochondria is only compatible with the formation of the mitochondrial permeability transition pore provided that an opening of a calcium ion channel and activation of protein kinase A also occurs (Yamada et al., 1988). Beavis and Garlid demonstrated that depletion of Mg2+ from the mitochondria matrix causes the formation of the unregulated mitochondrial permeability transition pore (Beavis and Garlid, 1987). It is likely that the activation of acetate in the brain via,
| (1) |
by acetate thiokinase (EC 6.2.1.1) leads to formation of PPi in the matrix depleting free mitochondrial Mg2+ by forming CaMgPPi and forming the mitochondrial permeability transition pore in the brain as it does in liver. In the current study the threefold influx of Mg2+ into the cytosol of brains of acetate- treated animals is compatible with processes that cause a depletion of free mitochondrial Mg2+ through the formation of CaMgPPi complexes. Excitotoxic neuronal death resulting from an overstimulation of glutamate receptors, also is known to cause large influxes of divalent cations into the mitochondria effecting formation of mitochondrial permeability transition pore (Schinder et al., 1996).
Formation of the mitochondrial permeability transition pore, with a necessary destruction of the mitochondrial H+ gradient would account for the reduction of the cytosolic [NAD+]/[NADH], the oxidation of the free mitochondrial NAD and CoQ couples and the decrease in the phosphorylation potential observed in the acetate treated group.
This study confirms that high elevations in blood acetate (above 2 mM) alter several metabolic parameters related to energy production in the brain in rats. The metabolism of acetate in the brain decreased the ΔG of ATP hydrolysis whereas high concentrations of blood ethanol with lower concentrations of blood acetate did not alter similar metabolic processes. In man, blood acetate can reach as high a level as 1.5 mM when consuming variable quantities of alcohol (Chen et al., 2009; Lundquist, 1962). It is therefore possible under certain hormonal conditions (Yamada et al., 1988), pore opening could occur during ethanol consumption and its metabolism in man. It is therefore possible that the ability of acetate to form the mitochondrial permeability transition pore, may under certain conditions, account for some ethanol related cerebral pathologies.
Footnotes
No claim to original U.S. government works
References
- Anandatheerthavarada HK, Shankar SK, Bhamre S, Boyd MR, Song BJ, Ravindranath V. Induction of brain cytochrome P-450IIE1 by chronic ethanol treatment. Brain Res. 1993;601:29–85. doi: 10.1016/0006-8993(93)91721-4. [DOI] [PubMed] [Google Scholar]
- Barman TE. Enzyme Handbook. Springer-Verlag; Berlin: 1969. [Google Scholar]
- Beavis AD, Garlid KD. The mitochondrial inner membrane anion channel. J Biol Chem. 1987;262(31):15085–15093. [PubMed] [Google Scholar]
- Berl S, Clarke DD. Cerebral glutamine/glutamate interrelationships and metabolic compartmentation. In: Haussinger D, Sies H, editors. Glutamine Metabolism in Mammalian Tissues. Springer-Verlag; New York: 1984. pp. 150–222. [Google Scholar]
- Chen YC, Peng GS, Tsao TP, Wang MF, Lu RB, Yin SJ. Pharmacokinetic and pharmacodynamic basis for overcoming acetaldehyde-induced adverse reaction in Asian alcoholics, heterozygous for the variant ALDH2*2 gene allele. Pharmacogenet Genomics. 2009;19(8):588–599. doi: 10.1097/FPC.0b013e32832ecf2e. [DOI] [PubMed] [Google Scholar]
- Cohen G, Sinet PM, Heikkila R. Ethanol oxidation by rat brain in vivo. Alcohol Clin Exp Res. 1980;4(4):366–370. doi: 10.1111/j.1530-0277.1980.tb04833.x. [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Shestov AA, Koski DM, Ug¢urbil K, Henry PG. Acetate transport and utilization in the rat brain. J Neurochem. 2009;109(Suppl):46–54. doi: 10.1111/j.1471-4159.2009.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich RS, Colman RF. Ionization of isocitrate bound to pig heart NADP+-dependent isocitrate dehydrogenase: 13C NMR study of substrate binding. Biochemistry. 1987;26:3461–3466. doi: 10.1021/bi00386a032. [DOI] [PubMed] [Google Scholar]
- Lundquist R. Production and utilization of free acetate in man. Nature. 1962;193:579–580. doi: 10.1038/193579b0. [DOI] [PubMed] [Google Scholar]
- Mansell MA, Nunan TO, Laker MF, Boon NA, Wing AJ. Incidence and significance of rising blood acetate levels during hemodialysis. Clin Nephrol. 1979;12(1):22–25. [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr Brain metabolism during fasting. J Clin Invest. 1967;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16(19):6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres S, Raffard G, Franconi J-M, Merle M. Close coupling between astrocytic and neuronal metabolisms to fulfill anaplerotic and energy needs in the rat brain. J Cereb Blood Flow Metab. 2008;28:712–724. doi: 10.1038/sj.jcbfm.9600568. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood FlowMetab. 2007;27(11):1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga T, Ueno Y, Naraoka H, Ohashi Y, Tomita M, Nishiokat T. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem. 2002;74:2233–2239. doi: 10.1021/ac020064n. [DOI] [PubMed] [Google Scholar]
- Spry MS. BSc Thesis: Relationships between the redox state of the pyridine nucleotides, the phosphorylation state of the adenine nucleotides and substrate utilization in animal tissues. St. Anne’s College, Oxford University; Oxford, England: 1971. [Google Scholar]
- Tyson RL, Gallagher C, Sutherland GR. 13C-labeled substrates and the cerebral metabolic compartmentalization of acetate and lactate. Brain Res. 2003;992:43–52. doi: 10.1016/j.brainres.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Veech RL, Gates DN, Crutchfield CW, Gitomer WL, Kashiwaya Y, King MT, Wondergem R. Metabolic hyperpolarization of liver by ethanol: the importance of Mg2+ and H+ in determining impermeant intracellular anionic charge and energy of metabolic reactions. Alcohol Clin Exp Res. 1994;18:1040–1056. doi: 10.1111/j.1530-0277.1994.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Veech RL, Gitomer WL, King MT, Balaban RS, Costa JL, Eanes ED. The effect of short chain fatty acid administration on hepatic glucose, phosphate, magnesium and calcium metabolism. Myocardial and Skeletal Muscle. In: Nachman B, editor. Bioenergetics. Plenum Publishing Corp; New York: 1986. pp. 617–646. [DOI] [PubMed] [Google Scholar]
- Veech RL, Harris RL, Veloso D, Veech EH. Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem. 1973;20:183–188. doi: 10.1111/j.1471-4159.1973.tb12115.x. [DOI] [PubMed] [Google Scholar]
- Veech RL, Lawson JWR, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. J Biol Chem. 1979;254:6538–6547. [PubMed] [Google Scholar]
- Veloso D, Guynn RW, Oskarsson M, Veech RL. The concentrations of free and bound magnesium in rat tissues. J Biol Chem. 1973;248(13):4811–4819. [PubMed] [Google Scholar]
- Veloso D, Passonneau JV, Veech RL. The effects of intoxicating doses of ethanol upon intermediary metabolism in rat brain. J Neurochem. 1972;19(11):2679–2686. doi: 10.1111/j.1471-4159.1972.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, Kai Li T. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. 2006;29(1):295–301. doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, Netusil N, Zhu W, Felder C, Ma Y. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcohol Clin Exp Res. 2003;27(6):909–917. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- Yamada T, Inoue T, Nishida T, Furuya E, Tagawa K. Hepatic Accumulation of pyrophosphate during acetate metabolism. J Biochem. 1988;104:847–850. doi: 10.1093/oxfordjournals.jbchem.a122561. [DOI] [PubMed] [Google Scholar]