Abstract
We have developed a novel type of duplex enzyme-linked immunosorbent assay (ELISA) for the quanitation of the major plasma proteins, IgG and albumin, in edematous brain tissue. We test this duplex ELISA on our porcine intracerebral hemorrhage (ICH) model and show that it is as accurate and sensitive as independent single ELISAs. This method is useful as a marker of edema in brain tissue and the same design can be applied to other proteins and sample types.
Keywords: ELISA, intracerebral hemorrhage, stroke, edema
1. Introduction
Intracerebral hemorrhage (ICH) is a stroke subtype with high rates of mortality and morbidity (Foulkes et al., 1988; Broderick et al., 1993). ICH can lead to significant interstitial and vasogenic perihematomal edema development. In humans and animals this edema is caused by leakage of plasma proteins into the parenchyma through an open blood-brain-barrier (BBB) and by clot retraction pushing additional proteins into perihematomal tissue (Wagner et al., 1996; Butcher et al., 2004). We have recently shown that these plasma proteins can confound the results of immunoblots by unpredictably inflating the protein measured in a total protein assay (Loftspring et al., 2006). This makes it difficult to load equal amounts of brain parenchyma protein on the electrophoresis gel. Previously, as a solution to this problem we developed two single enzyme-linked immunosorbent assays (ELISAs) to quantify the major plasma proteins, IgG and albumin, which together make up approximately 80% of plasma proteins. We subtracted the concentrations of these proteins from the total protein as a better determination of brain parenchyma protein. However, a straightforward duplex ELISA to measure both proteins simultaneously would be more practicable for this problem as well as for a variety of other applications.
The ability to quantify two or more proteins in the same microwell of a microplate is very useful. Such a method has the advantages of saving time, reducing sample volume needed and providing greater consistency by reducing assay number. Current methods available to do this include multiplex suspension array technology (Fouda, et al., 2006), multiplex bead array assays (Heijmans-Antonissen et al., 2006) and multiplex immunochemiluminescence ELISAs (Franciotta et al., 2006). Although these techniques allow the quantification of many proteins simultaneously, there are no techniques that currently measure IgG and albumin. Additionally, many of these multiplex techniques require specialized equipment to construct and perform. In this brief report we present a novel method to quantify IgG and albumin simultaneously in the same microwell. We tested the method on perihematomal white matter samples from our porcine ICH model and we show that results from this new duplex ELISA are consistent with those obtained by independent single ELISAs for IgG and albumin.
2. Results
We observed prominent perihematomal edema in the ipsilateral hemisphere of ICH animals. In contrast, there was no discernable edema on the contralateral side of ICH animals or sham-operated controls (N = 3 for these groups). In 1 h animals edema is evident as gray-translucent tissue surrounding the hematoma. In later time points edema is marked by the leakage of Evan’s Blue through the open blood-brain-barrier.
The standard curves for both single ELISAs and the duplex ELISA were well described by a quadratic regression equation (r2 was a minimum of 0.990 for all curves). The data are summarized in Table 1. The sample concentrations of IgG and albumin calculated by single ELISAs compared to the duplex ELISA did not statistically differ (p = 0.28, paired t test). These data indicate that there is significant plasma protein contamination of edematous tissue as far as 72 h following experimental ICH (Table 1). The threshold of sensitivity for IgG was 1.2 ng/mL and was 1.1 ng/mL for albumin. In this study we chose different time points than in our initial study (Loftspring et al., 2006), because we wished to study plasma protein contamination of edematous tissue over a wider time span (1 to 72 h).
Table 1.
Summary of plasma protein measurements by the duplex ELISA.
| Sample | Avg PP (% of total protein) | SD | N measured | N below detection limit |
|---|---|---|---|---|
| 1 h ipsi | 11.8 | 5.4 | 4 | 0 |
| 1 h contra | 0.68 | n/a | 3 | 2 |
| 24 h ipsi | 19.3 | 10.8 | 4 | 0 |
| 24 h contra | n/a | n/a | 3 | 3 |
| 48 h ipsi | 21.2 | 0.39 | 2 | 0 |
| 48 h contra | 1.07 | 0.72 | 4 | 2 |
| 72 h ipsi | 10.9 | 5.7 | 3 | 0 |
| 72 h contra | 12.6 | n/a | 3 | 2 |
| 2 h sham ipsi | n/a | n/a | 3 | 3 |
| 2 h sham contra | n/a | n/a | 3 | 3 |
| Normal | n/a | n/a | 2 | 2 |
Ipsi, ipsilateral; contra, contralateral; sham, identical to ICH but no blood infused; normal, euthanized without any surgery; PP, plasma proteins (IgG + Albumin).
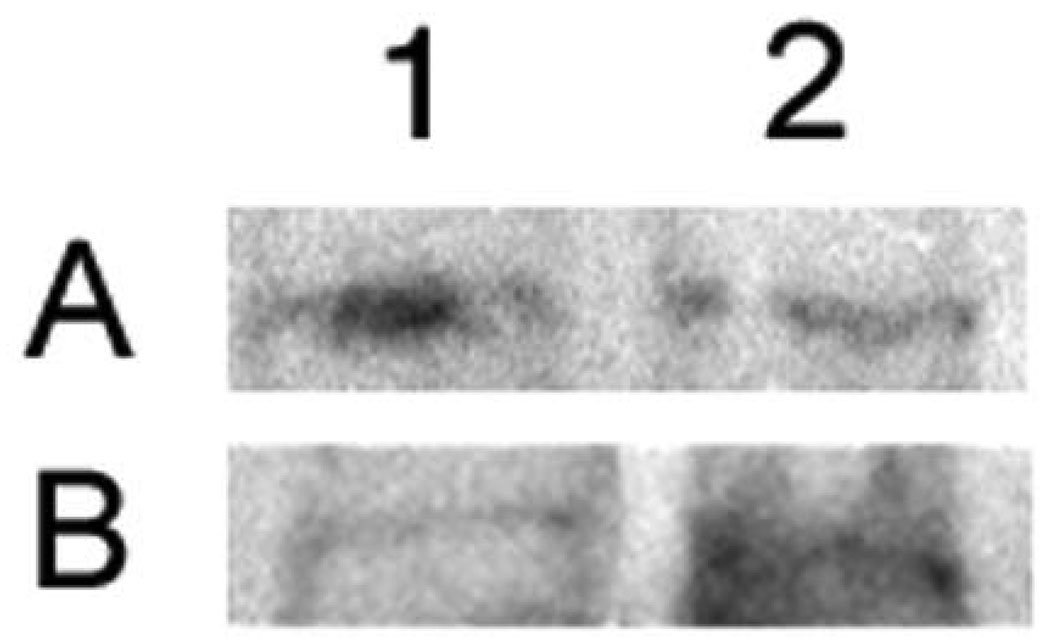
In Figure 1 we present an example of the potential pitfalls that may occur if plasma proteins are not accounted for during the immunoblotting process. These are previously unpublished data examining the activation of the p65 subunit of NFκB in our porcine ICH model. If plasma proteins are not accounted for, a slight decrease in NFκB is observed from 1 h to 16 h (mean gray area values are 57.4 and 55.4, respectively). However, when we measured plasma proteins with this method the same samples show a marked increase from 1 h to 16 h (mean gray area values are 55.3 and 67.4, respectively).
Figure 1.
This figure demonstrates the potential pitfalls involved in not correcting for the presence of plasma proteins. We studied activation of the p65 subunit of NFκB by immunoblotting. Lane 1, 1 h post-ICH; lane 2, 16 h post-ICH. Panel A is without correction and panel B is the same samples as in A but with correction for plasma proteins. As determined from densitometric analysis, panel A shows a decrease form 1 h to 16 h, but panel B shows an increase.
3. Discussion
Our results indicate that we have developed an accurate and sensitive duplex ELISA method for the quantitation of plasma proteins in edematous brain tissue following ICH. The value of 12.6% in the 72 h contralateral brain is higher than expected. Occasionally a subarachnoid hemorrhage occurs in our ICH model. We believe this value is due to blood or plasma that was in sulci in the frontal lobe and that it was sampled with the tissue.
Because plasma proteins have the potential to interfere with the semi-quantitation of immunoblots by unpredictably inflating the total protein amounts (Loftspring et al., 2006; Figure 1), it is important to account for them during the immunoblotting process. The method we have chosen is to quantify the main plasma proteins, IgG and albumin, by ELISA and subtract their concentration from the total protein concentration. This allows loading of equal cellular protein on the PAGE gel. The duplex ELISA method we describe here allows for the easy and accurate quantitation of these plasma proteins.
Although we have tested this method on tissue samples from our ICH model, there are other brain pathologies that produce significant amounts of edema. Traumatic brain injury, subarachnoid hemorrhage, cerebral ischemia, hydrocephalus and multiple sclerosis have been shown to lead to brain edema (Liu and Sturner, 1988; Ostrowski et al., 2006; Simard et al., 2007; Del Bigio, 1993; Gay and Esiri, 1991; Lucchinetti, 2005;). However, it is not known whether plasma protein contamination in models of these conditions is significant enough to confound the results of immunoblots as in our porcine ICH model. In summary, our duplex ELISA method is very straightforward and the same basic design can potentially be applied to a variety of proteins and sample types.
4. Experimental Procedure
Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC). All surgical methods to experimentally induce ICH have been previously described (Wagner et al., 1996, 1998, 1999). Briefly, 3 mL of autologous blood was injected into frontal hemispheric white matter of pentobarbital-anaesthetized pigs. Brains were frozen in situ with liquid nitrogen at various time points up to 72 h. Perihematomal, edematous tissue (approximately 20 mg) was sampled and homogenized in 200 µL of homogenization buffer, as previously described (Loftspring et al., 2006). The BCA assay (Pierce, Rockford, IL) was used for total protein determination with bovine serum albumin as the standard.
Methods for immunoblots and quantification have been previously described (Loftspring et al., 2006). Briefly, 20 µg of protein was loaded onto 8% polyacrylamide gels and eletrcophoresed at 200 V for 1 h. Proteins were transferred to polyvinylidene fluoride (PVDF) membranes at 115 V for 1.25 h. Membranes were blocked with 4.5% nonfat dried milk. The primary antibody (Chemicon, Cemucula, CA) was directed against the activated form of the NFκB p65 subunit and was diluted 1:1250. The secondary antibody (Kirkegaard and Perry, Gaithersburg, MD) was diluted 1:5000. Bands were visualized with the ECL-plus kit (Amersham, Piscataway, NJ) and quantified with ImageJ software (NIH).
Individual ELISAs for IgG and albumin were carried out as described in our previous report (Loftspring et al., 2006) with the following exceptions: round bottom ELISA plates were used and an alkaline phosphatase-conjugated secondary antibody was used for the IgG ELISA.
The duplex ELISA was constructed and carried out in the following way: polyclonal anti-pig albumin antibody (Bethyl, Montgomery, TX) and polyclonal anti-swine IgG antibody (Kirkegaard and Perry) were diluted in 100 mM NaHCO3, pH 8.4, and coated onto each well of a 96-well round bottom microplate (Fisher, Waltham, MA) for 1 h at 37°C; 0.8 µg of each antibody was coated onto each microwell. All incubations with the exception of those with enzyme substrates were for 1 h at 37°C. Nonspecific binding was blocked by 200 µL of nonfat dried milk (5%) in phosphate-buffered saline (PBS [137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4]). We purchased porcine IgG and albumin (Sigma, St. Louis) and prepared five standards by serial dilution in PBS. Concentrations of IgG and albumin in each standard were, respectively, 100/300, 50/150, 25/75, 12.5/37.5 and 6.25/18.75 ng/mL. Samples were diluted 1:10,000 in PBS. Standards and samples were plated 50 µL per well in duplicate. A horseradish peroxidase (HRP)-conjugated anti-pig albumin antibody and an alkaline phosphatase (AP)-conjugated anti-pig IgG antibody were purchased from Bethyl. The HRP and AP antibodies were diluted 1:20,000 and 1:1000, respectively, together in PBS and added at 50 µL per well. Plates were washed with both distilled water and PBS containing 0.05% Tween-20 and aspirated in between all incubations. Microwells were incubated with 50 µL of blue AP microwell solution (Sigma) at room temperature and then read at 610 nm with a plate reader (Bio-Tek Instruments, Winooski, VT). Plates were then washed and incubated with 50 µL of tetramethyl benzidine (Sigma). The reaction was stopped with 50 µL of 1M H2SO4 and plates were read at 450 nm.
Concentrations of IgG and albumin were interpolated from separate standard curves fitted with quadratic regression curves. The plasma protein concentrations determined from the duplex ELISA were compared to those determined from single ELISAs using a paired t-test. All data are presented as mean ± SD. The level of significance was 0.05
ACKNOWEDGEMENTS
This work was supported by NIH grants 5R01NS050569-02 (JFC) and 5R01NS030652-11 (KRW).
Non-standard abbreviations
- HRP
horseradish peroxidase
- AP
alkaline phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 2.Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke. 2004;35:1879–1885. doi: 10.1161/01.STR.0000131807.54742.1a. [DOI] [PubMed] [Google Scholar]
- 3.Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol (Berl) 1993;85:573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- 4.Fouda GG, Leke RF, Long C, Druilhe P, Zhou A, Taylor DW, Johnson AH. Multiplex assay for simultaneous measurement of antibodies to multiple Plasmodium falciparum antigens. Clin Vaccine Immunol. 2006;13:1307–1313. doi: 10.1128/CVI.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 6.Franciotta D, Zardini E, Ravaglia S, Piccolo G, Andreoni L, Bergamaschi R, Romani A, Tavazzi E, Naldi P, Ceroni M, Marchioni E. Cytokines and chemokines in cerebrospinal fluid and serum of adult patients with acute disseminated encephalomyelitis. J Neurol Sci. 2006;247:202–207. doi: 10.1016/j.jns.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 7.Gay D, Esiri M. Blood-brain barrier damage in acute multiple sclerosis plaques. An immunocytological study. Brain. 1991;114(Pt 1B):557–572. doi: 10.1093/brain/114.1.557. [DOI] [PubMed] [Google Scholar]
- 8.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, Hooijkaas H, Zijlstra FJ. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators Inflamm. 2006;2006:28398. doi: 10.1155/MI/2006/28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu HM, Sturner WQ. Extravasation of plasma proteins in brain trauma. Forensic Sci Int. 1988;38:285–295. doi: 10.1016/0379-0738(88)90174-0. [DOI] [PubMed] [Google Scholar]
- 10.Loftspring MC, Beiler S, Beiler C, Wagner KR. Plasma proteins in edematous white matter after intracerebral hemorrhage confound immunoblots: an ELISA to quantify contamination. J Neurotrauma. 2006;23:1904–1911. doi: 10.1089/neu.2006.23.1904. [DOI] [PubMed] [Google Scholar]
- 11.Lucchinetti CF. Update on the international project on pathologic correlates in MS. Mult Scler. 2005;11:99–100. doi: 10.1177/135245850501100120. [DOI] [PubMed] [Google Scholar]
- 12.Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 13.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, Broderick JP, Brott TG. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke. 1996;27:490–497. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- 15.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88:1058–1065. doi: 10.3171/jns.1998.88.6.1058. [DOI] [PubMed] [Google Scholar]
- 16.Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, Brott TG. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–498. doi: 10.3171/jns.1999.90.3.0491. [DOI] [PubMed] [Google Scholar]