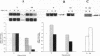Abstract
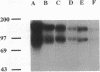
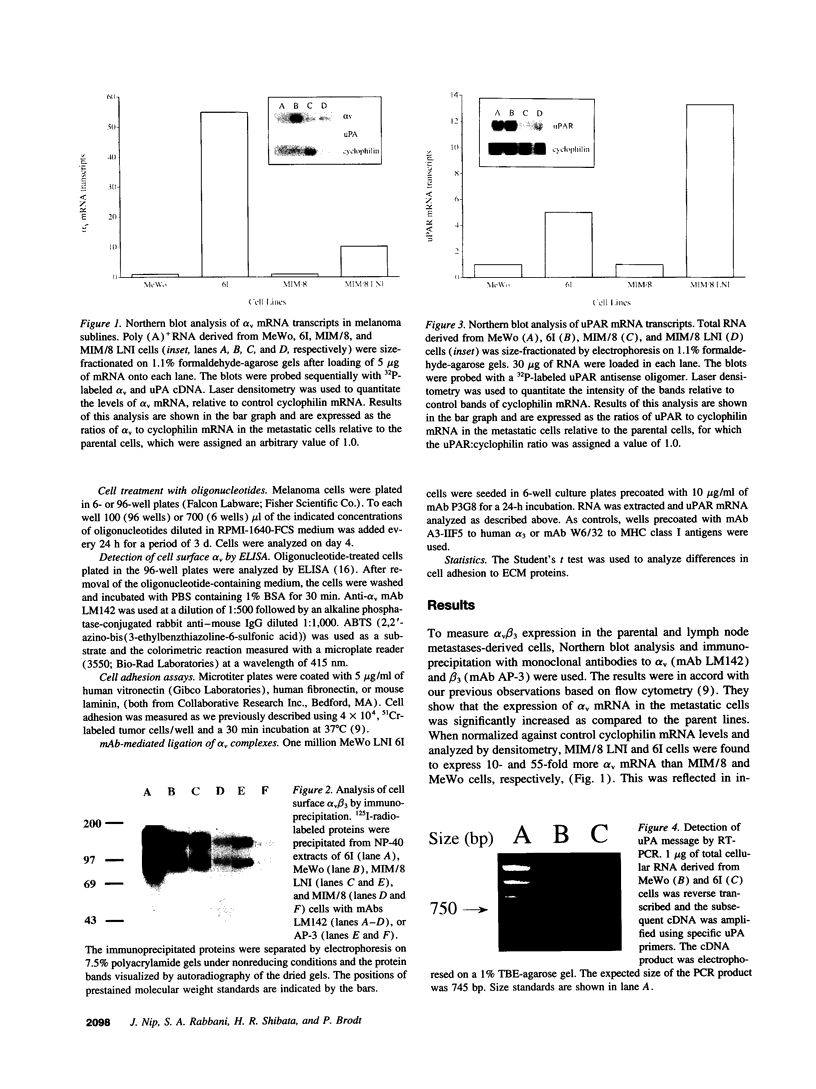
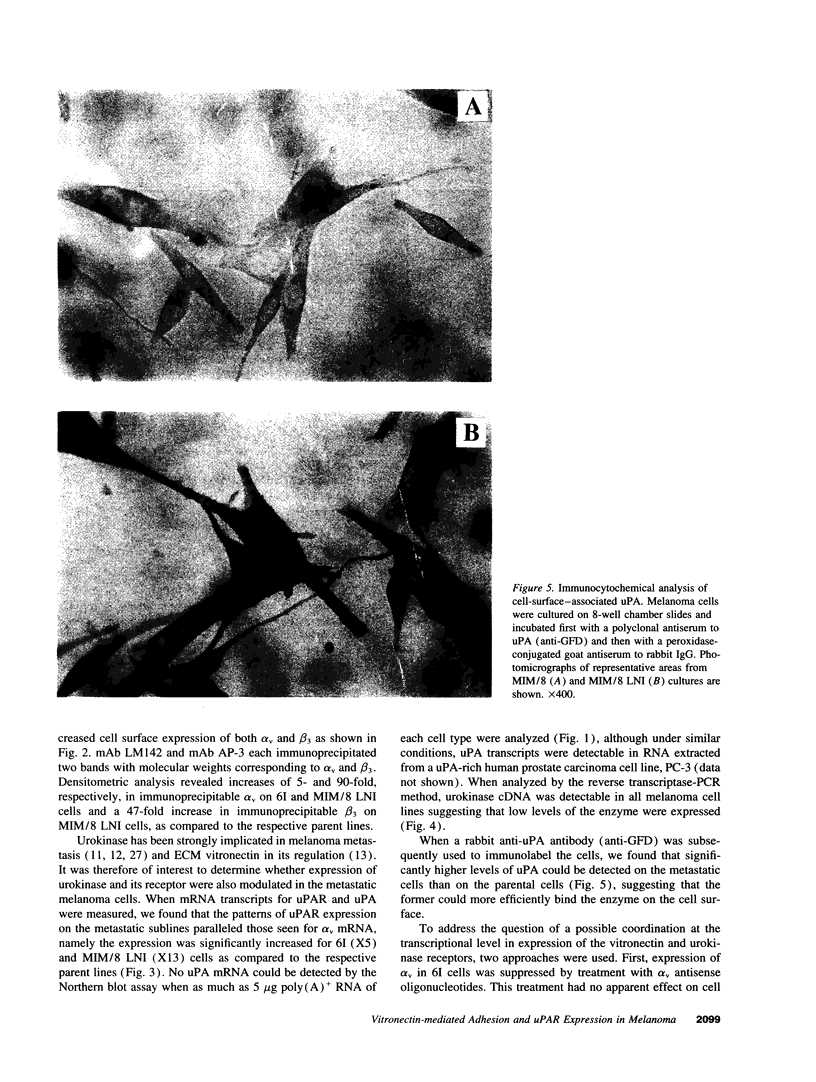
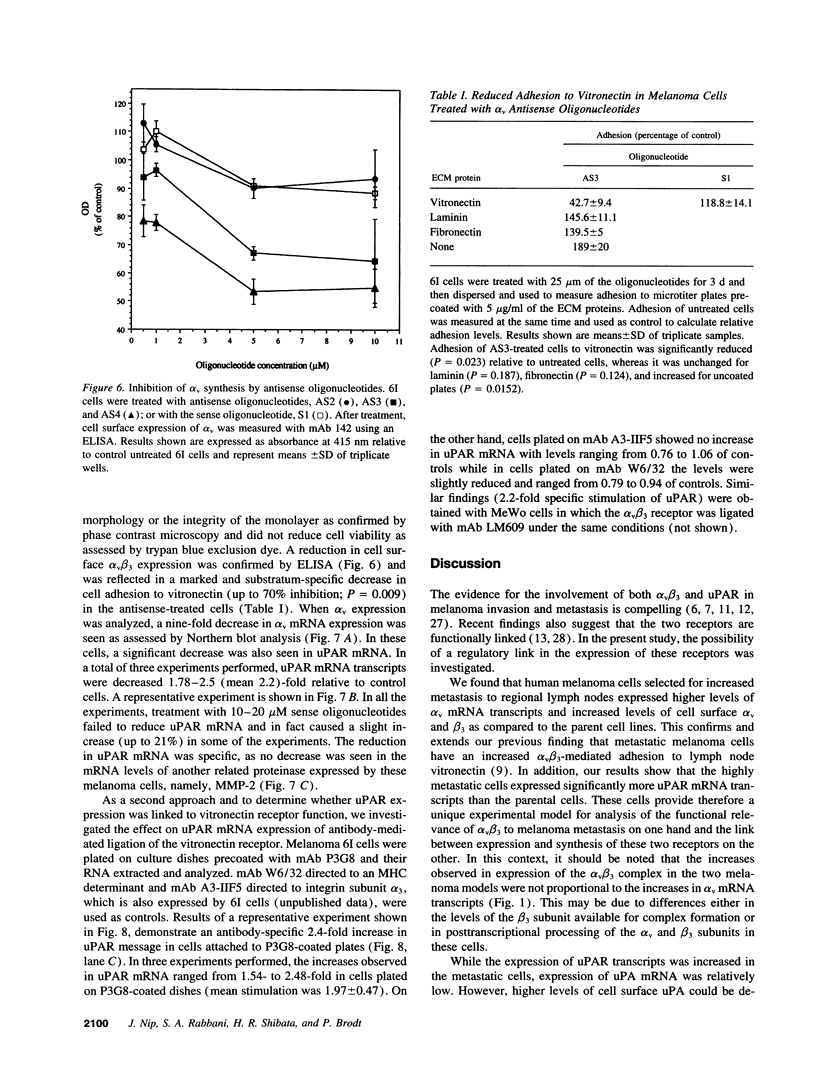
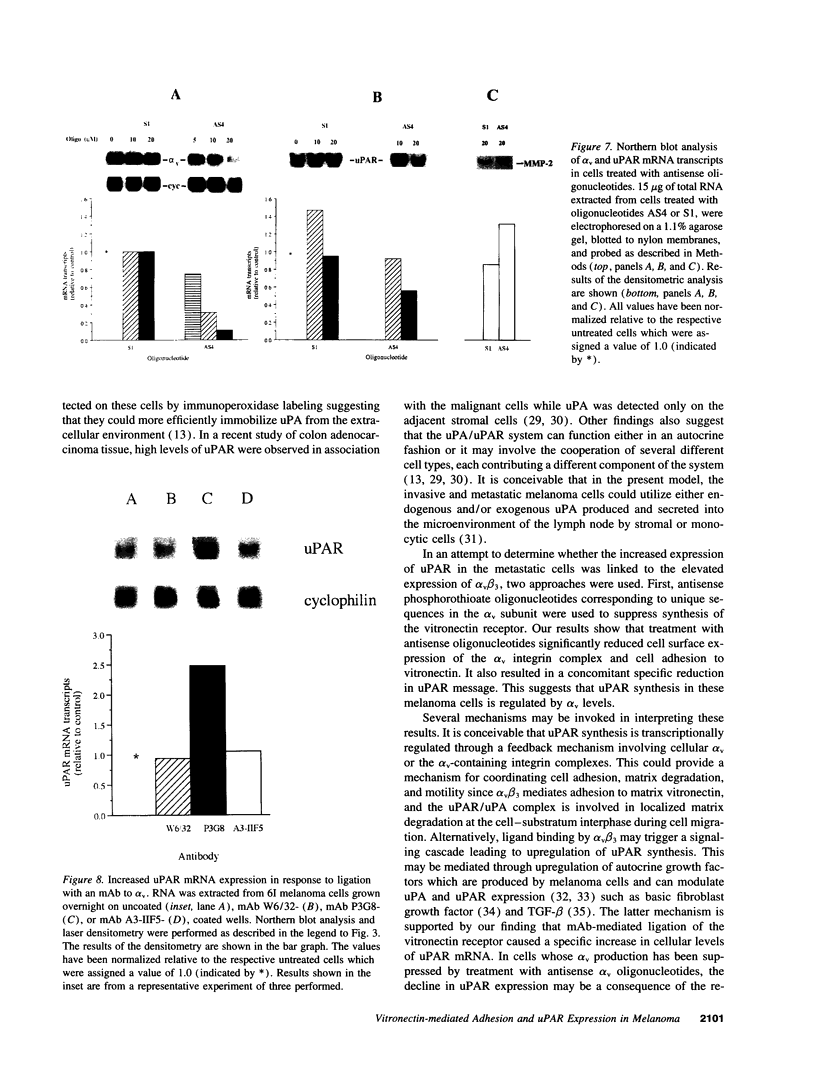
Integrin alpha v beta 3 is a marker of progression in malignant melanoma. Previously we reported that human melanoma cells derived from regional lymph node metastases had increased alpha v beta 3-mediated adhesion to lymph node vitronectin. In the present study, the expression and function of alpha v beta 3 were further investigated with emphasis on the functional relationship between alpha v beta 3 and the urokinase-type plasminogen activator system of proteolysis. We found that metastases-derived melanoma MeWo LNI 6I (6I) and MIM/8 LNI cells had a markedly increased expression of alpha v mRNA transcripts relative to the parent lines which was reflected in significantly elevated levels of the alpha v beta 3 heterodimers on the cell surface. These cells also expressed elevated levels of urokinase plasminogen activator receptor (uPAR) mRNA and had higher levels of surface bound urokinase plasminogen activator as detected by immunolabeling. To determine whether the expression of uPAR and alpha v were linked, alpha v synthesis in the metastatic melanoma cells was suppressed using alpha v antisense phosphorothioate oligonucleotides. This resulted in a marked decrease in detectable alpha v mRNA and protein and a corresponding substratum-specific reduction in cell adhesion to vitronectin. When uPAR expression in these cells was subsequently analyzed, we found a reduction of approximately 50% in uPAR mRNA levels. On the other hand, ligation of the alpha v beta 3 receptor on the melanoma cells by immobilized antibody resulted in a twofold increase in uPAR mRNA. The results suggest that the expression of uPAR in metastatic melanoma cells is linked to the expression and function of the vitronectin receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achbarou A., Kaiser S., Tremblay G., Ste-Marie L. G., Brodt P., Goltzman D., Rabbani S. A. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994 May 1;54(9):2372–2377. [PubMed] [Google Scholar]
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bartfeld N. S., Pasquale E. B., Geltosky J. E., Languino L. R. The alpha v beta 3 integrin associates with a 190-kDa protein that is phosphorylated on tyrosine in response to platelet-derived growth factor. J Biol Chem. 1993 Aug 15;268(23):17270–17276. [PubMed] [Google Scholar]
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993 Feb;15(2):105–111. doi: 10.1002/bies.950150206. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Mueller B. M., Romerdahl C. A., Cheresh D. A. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992 Jun;89(6):2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R., Abe M., Mignatti P., Rifkin D. B. Basic fibroblast growth factor-induced activation of latent transforming growth factor beta in endothelial cells: regulation of plasminogen activator activity. J Cell Biol. 1992 Aug;118(4):901–909. doi: 10.1083/jcb.118.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. G., Hoover R. N. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA. 1989 Oct 20;262(15):2097–2100. [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Ralfkiaer E., Kirkeby L. T., Kristensen P., Lund L. R., Danø K. Localization of urokinase-type plasminogen activator in stromal cells in adenocarcinomas of the colon in humans. Am J Pathol. 1991 Jan;138(1):111–117. [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Law L. W., Corti A., Appella E., Blasi F. Modulation of metastatic potential by cell surface urokinase of murine melanoma cells. Cancer Res. 1988 Mar 1;48(5):1270–1278. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S. Expression of multi-cytokine resistance and multi-growth factor independence in advanced stage metastatic cancer. Malignant melanoma as a paradigm. Am J Pathol. 1992 Sep;141(3):519–524. [PMC free article] [PubMed] [Google Scholar]
- Leavesley D. I., Schwartz M. A., Rosenfeld M., Cheresh D. A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993 Apr;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Lund L. R., Rømer J., Rønne E., Ellis V., Blasi F., Danø K. Urokinase-receptor biosynthesis, mRNA level and gene transcription are increased by transforming growth factor beta 1 in human A549 lung carcinoma cells. EMBO J. 1991 Nov;10(11):3399–3407. doi: 10.1002/j.1460-2075.1991.tb04904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei S., Colombo M. P., Melani C., Silvani A., Parmiani G., Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994 Mar 15;56(6):853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- Meissauer A., Kramer M. D., Schirrmacher V., Brunner G. Generation of cell surface-bound plasmin by cell-associated urokinase-type or secreted tissue-type plasminogen activator: a key event in melanoma cell invasiveness in vitro. Exp Cell Res. 1992 Apr;199(2):179–190. doi: 10.1016/0014-4827(92)90423-6. [DOI] [PubMed] [Google Scholar]
- Nip J., Shibata H., Loskutoff D. J., Cheresh D. A., Brodt P. Human melanoma cells derived from lymphatic metastases use integrin alpha v beta 3 to adhere to lymph node vitronectin. J Clin Invest. 1992 Oct;90(4):1406–1413. doi: 10.1172/JCI116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odekon L. E., Blasi F., Rifkin D. B. Requirement for receptor-bound urokinase in plasmin-dependent cellular conversion of latent TGF-beta to TGF-beta. J Cell Physiol. 1994 Mar;158(3):398–407. doi: 10.1002/jcp.1041580303. [DOI] [PubMed] [Google Scholar]
- Pasqualini R., Bodorova J., Ye S., Hemler M. E. A study of the structure, function and distribution of beta 5 integrins using novel anti-beta 5 monoclonal antibodies. J Cell Sci. 1993 May;105(Pt 1):101–111. doi: 10.1242/jcs.105.1.101. [DOI] [PubMed] [Google Scholar]
- Pelletier A. J., Bodary S. C., Levinson A. D. Signal transduction by the platelet integrin alpha IIb beta 3: induction of calcium oscillations required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transfected cells. Mol Biol Cell. 1992 Sep;3(9):989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Sappino A. P., Stöcklin R., Montesano R., Orci L., Vassalli J. D. Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol. 1993 Aug;122(3):673–684. doi: 10.1083/jcb.122.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Grøndahl-Hansen J., Eriksen J., Blasi F., Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [PMC free article] [PubMed] [Google Scholar]
- Rabbani S. A., Mazar A. P., Bernier S. M., Haq M., Bolivar I., Henkin J., Goltzman D. Structural requirements for the growth factor activity of the amino-terminal domain of urokinase. J Biol Chem. 1992 Jul 15;267(20):14151–14156. [PubMed] [Google Scholar]
- Rigel D. S. Epidemiology and prognostic factors in malignant melanoma. Ann Plast Surg. 1992 Jan;28(1):7–8. doi: 10.1097/00000637-199201000-00003. [DOI] [PubMed] [Google Scholar]
- Rodeck U., Bossler A., Graeven U., Fox F. E., Nowell P. C., Knabbe C., Kari C. Transforming growth factor beta production and responsiveness in normal human melanocytes and melanoma cells. Cancer Res. 1994 Jan 15;54(2):575–581. [PubMed] [Google Scholar]
- Roldan A. L., Cubellis M. V., Masucci M. T., Behrendt N., Lund L. R., Danø K., Appella E., Blasi F. Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J. 1990 Feb;9(2):467–474. doi: 10.1002/j.1460-2075.1990.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saksela O., Hovi T., Vaheri A. Urokinase-type plasminogen activator and its inhibitor secreted by cultured human monocyte-macrophages. J Cell Physiol. 1985 Jan;122(1):125–132. doi: 10.1002/jcp.1041220119. [DOI] [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Gehlsen K. R., Stetler-Stevenson W. G., Brown P. D., Ruoslahti E., Hendrix M. J. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A., Mueller B. M. Binding of urokinase to its receptor promotes migration and invasion of human melanoma cells in vitro. Cancer Res. 1994 Jun 1;54(11):3066–3071. [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Pytela R., Arai H., Krusius T., Pierschbacher M. D., Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Mateos P., Arroyo A. G., Balboa M. A., Sánchez-Madrid F. Post-receptor occupancy events in leukocytes during beta 1 integrin-ligand interactions. Eur J Immunol. 1993 Oct;23(10):2642–2648. doi: 10.1002/eji.1830231038. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz D. A., Chapman H. A. Reversible cellular adhesion to vitronectin linked to urokinase receptor occupancy. J Biol Chem. 1994 May 20;269(20):14746–14750. [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman J. B., Pasqualini R., Takada Y., Hemler M. E. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993 Apr 25;268(12):8651–8657. [PubMed] [Google Scholar]
- Yurochko A. D., Liu D. Y., Eierman D., Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries T. J., Quax P. H., Denijn M., Verrijp K. N., Verheijen J. H., Verspaget H. W., Weidle U. H., Ruiter D. J., van Muijen G. N. Plasminogen activators, their inhibitors, and urokinase receptor emerge in late stages of melanocytic tumor progression. Am J Pathol. 1994 Jan;144(1):70–81. [PMC free article] [PubMed] [Google Scholar]