1. Introduction
Iron is a trace metal essential for several life-sustaining functions, while excess iron, by virtue of its ability to catalyze the formation of reactive oxygen species (ROS), has the potential to be a causative factor in the age-related mitochondrial deterioration (Kohgo et al. 2008;Duvigneau et al. 2008;Liang et al. 2008). Iron accumulates in senescent cells and most nonhematopoietic tissues with age (Killilea et al. 2003;Killilea et al. 2004;Dunaief 2006;Hofer et al. 2008;Jung et al. 2008). Rapidly emerging evidence suggests that iron accumulation and loss of mitochondrial iron homeostasis may contribute to mitochondrial decay, which subsequently leads to aging (Table 1) (Bitar and Weiner 1983;Atamna et al. 2001;Atamna et al. 2002a;Napoli et al. 2006;Doulias et al. 2008;Seo et al. 2008;Xu et al. 2008;Irazusta et al. 2009;Cantu et al. 2009;Veatch et al. 2009;Chen et al. 2009). Although studies in both yeast and mammalian systems support the conclusion that iron homeostasis may be disrupted with age (Zacharski et al. 2000;Gau et al. 2001), the mechanisms underlying this phenomenon are still unclear. Here, we discuss important features of iron dyshomeostasis with a particular emphasis on its effects on mitochondrial decay and aging.
Table 1.
Summary of studies reporting an association of iron accumulation, mitochondrial dysfunction and aging.
| Stress | Pathway/mechanism | Effects | Prevention | Models | Selected references |
|---|---|---|---|---|---|
| Frataxin deficiency | Mitochondrial ISCs defects, decreased Mn-SOD activity | Increased iron levels, increased protein carbonylation | Copper and manganese treatments | Yeast | Napoli et al. (2006), Irazusta et al. (2009) |
| Paraquat | Iron release from m-aconitase | Increased mitochondrial iron, cell death | Iron chelation | Cell line | Cantu et al. (2009) |
| Aging | Mitochondrial ISCs defects trigger the activation of the iron regulon | Increased iron uptake, genomic instability | N/A | Yeast | Veatch et al. (2009) |
| Aging | N/A | Increased mitochondrial iron, permeability transition | CR | Rat | Seo et al. (2008), Xu et al. (2008) |
| Aging | Inactivation or degradation of IRPs | Free iron overload, increased ferritin, decreased transferritin, decreased lysosomal activity | N/A | Rat and mouse | Chen et al. (2009) |
| Aging | Deficiency in mitochondrial heme synthesis | Increased iron levels, increased ferrochelatase, loss of complex IV | N/A | Cell line and rat | Bitar and Weiner (1983), Atamna et al. (2001, 2002a) |
| Aging | N/A | Increased labile iron | N/A | Human | Doulias et al. (2008) |
Abbreviations: m-aconitase, mitochondrial aconitase; IRPs, iron-regulatory proteins; SOD, superoxide dismutase; CR, calorie restriction; N/A, not available; ISCs, iron-sulfur clusters.
2. Iron crisis and mitochondrial decay
2.1. Labile iron in mitochondria
Iron taken up by eukaryotic cells must reach mitochondria, the unique site for heme and iron-sulfur cluster (ISC) biosynthesis (Dunn et al. 2007;Levi and Rovida 2009). Since mitochondria are also the major source of intracellular ROS and excess iron has a strong catalytic potential to enhance ROS generation, it is important that mitochondrial iron concentration is maintained within a tightly controlled range. In cells and tissues, iron exists in two pools. Ferritin and iron-containing prosthetic groups in various proteins sequester “non-chelatable” iron that conventional iron chelators like deferoxamine are unable to chelate. The other iron pool is so-called “chelatable or labile” iron that represents both free and loosely bound iron. In hepatocytes, this labile iron is estimated to be about 5 μM (Ma et al. 2006). Most iron transferred from cytoplasm to mitochondria or delivered from late endosomes and lysosomes to mitochondria is sequestered efficiently by the iron storage proteins, frataxin and mitochondrial ferritin (MtF) (Scheme 1) (Kaur and Andersen 2004;Zhang et al. 2005). With aging, a minor amount of mitochondrial iron, either loosely bound to proteins or escaped from storage sites becomes redox-active, and may be harmful, particularly in the presence of high concentration of hydrogen peroxide within the same compartment (Sohal et al. 1999;Kakhlon and Cabantchik 2002;Doulias et al. 2008). Several studies reported iron accumulation with age in mitochondria in rat substantia nigra (Schipper et al. 1998) and skeletal muscle (Seo et al. 2008) as well as human subcortical brain tissue (Schipper and Cisse 1995). Given the fact that labile iron has a strong catalytic potential to generate ROS, iron overload may result in catastrophic cellular damage via increasing oxidative stress accrual.
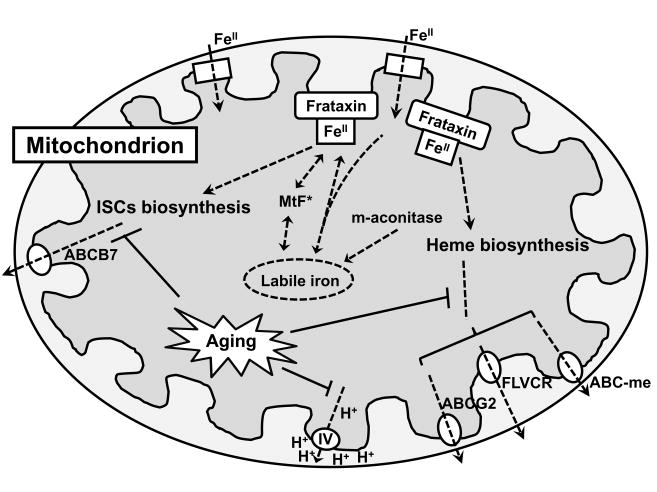
Scheme 1. Schematic illustration of mitochondrial iron dyshomeostasis with aging.
Iron is transported into the mitochondrial matrix by iron importers (e.g. mitoferrin) where it can be directed to different pathways, including storage in frataxin, iron-sulfur cluster (ISC) biosynthesis, heme metabolism, mitochondrial ferritin (MtF) or other currently unknown pathways. The ISCs can be exported to the cytoplasma by ABCB7. Heme is thought to be exported from the mitochondrion by several pathways, including ABCG2, the feline leukemia virus subgroup-C receptor (FLVCR) and ABC-me. Defects in these transporters or defective biosynthesis of heme and ISCs with age impair mitochondrial iron homeostasis and lead to cellular degeneration. Increased labile iron with age, especially in mitochondria, has a strong potential to catalyze the generation of reactive oxygen species (ROS), resulting in cellular damage.
Since labile iron is transient and exists in dynamic equilibrium with various cellular components, early attempts to identify the labile iron pool were based on cell-disruptive methods, which in turn alter the equilibrium between free and bound iron, as well as the FeII/FeIII redox state (Rothman et al. 1992;Kozlov et al. 1992;Sohal et al. 1999). Nondisruptive techniques that rely on the application of fluorescent metalosensors have been developed to estimate the intracellular chelatable iron (Epsztejn et al. 1997;Kakhlon and Cabantchik 2002). Changes in labile iron in cells and tissues can be visualized by fluorescent probes, including phen green SK and calcein (Petrat et al. 2001;Petrat et al. 2002a) since ferrous iron quenches these fluorescence. However, these fluorophores also bind to other divalent cations like Cu, Ni and Co, which raises an issue of their selectivity (Breuer et al. 1995). The development of iron-sensitive fluorescent probes specifically targeting mitochondria has allowed significant advances in the study of labile iron (Petrat et al. 2002b;Rauen et al. 2007). Probes are comprised of a fluorescent group coupled with a high-affinity iron chelator and must fulfill several requirements. Firstly, probes must be lipophilic and highly membrane-permeable (Petrat et al. 2001). Secondly, fluorescent groups must possess a net positive charge and be electophoretically driven into mitochondria due to the inside negative membrane potential (Dykens and Stout 2001). Thirdly, chelators must possess relatively high affinity for iron and be able to compete with endogenous ligands (i.e., pyruvate, phosphate, and polypeptides) (Rauen et al. 2007). In light of previous studies using the iron indicator rhodamine B 4-[(1,10-phenanthrolin-5-yl)aminocarbonyl]benzyl ester (RPA) to determine chelatable iron concentration in mitochondria of rat hepatocytes and endothelial cells (Petrat et al. 2001;Petrat et al. 2002b), Rauen et al. (2007) have developed an additional selective mitochondrial iron indicator, rhodamine B 4-[(2,2′-bipyridin-4-yl)aminocarbonyl]benzyl ester (RDA), which detected the same iron concentration (16.0 ± 1.9 μM) in rat hepatocyte mitochondria as RPA did (17.0 ± 1.0 μM). A recent study by Cantu et al. (2009) employing an adenoviral construct technique and the iron indicator RPA, has shown that mitochondrial-aconitase (m-aconitase) releases labile iron under oxidative stress in primary ventral mesencephalic cells. This event was followed by mitochondrial dysfunction and cell death. In addition, the observation in the same study that removing labile iron using an iron chelator mitigated mitochondrial damage and cell death strongly suggests its role in oxidative damage and mitochondrial dysfunction during the aging process. A recent study by Ma et al. (2009) also suggests that iron-sulfur containing proteins are targets of ROS in aging, and that m-aconitase is a major target of ROS under conditions of cellular stress. Upon exposure to oxidants, m-aconitase is disassembled and releases labile iron. Moreover, an age-associated decrease of aconitase expression has been observed in brain, heart, and muscle mitochondria in rodents, which contributes to the sensitivity of the enzyme to oxidative stress, as well as the overload of labile iron observed in aging (Dencher et al. 2007;O’Connell et al. 2007;Prokai et al. 2007). In further support of the role of labile iron, H2O2-induced collapse of mitochondrial membrane potential was completely prevented by pre-treatment with the lipophilic iron chelator salicylaldehyde isonicotinoyl hydrazone (SIH) in cultured H9c2 cardiac myoblasts (Simunek et al. 2005;Kurz et al. 2006). This finding indicates that hydrogen peroxide per se is not harmful, but it may become highly toxic if labile iron coexists.
2.2. Loss of iron homeostasis with aging
Heme and ISCs are important for the assembly of electron transfer complexes. Alterations in mitochondrial iron homeostasis cause iron accumulation in this compartment, which may be responsible for the age-related mitochondrial deterioration. Interestingly, multiple defects in mitochondrial heme and ISC biosynthesis have been demonstrated with aging, which may result in bioenergetic crisis and genomic instability (Rouault and Tong 2005;Veatch et al. 2009).
In the early 1980’s, Bitar and Walter (1983) observed that heme biosynthesis declined with aging. This phenomenon was investigated extensively by the Ames group, leading to important findings in this area (Atamna et al. 2001;Atamna et al. 2002a;Atamna et al. 2002b). For instance, heme deficiency in senescent human fibroblasts was found to selectively decrease the expression and activity of cytochrome c oxidase (complex IV) (Atamna et al. 2001). Complex IV, the terminal oxidase in mitochondrial electron transfer chain, is responsible for generating a transmembrane proton gradient across the inner mitochondrial membrane (Saraste 1999;Atamna et al. 2002b). Therefore, age-associated heme deficiency leads to impaired mitochondrial energy production through depressing complex IV. In a follow-up study, a decreased activity of complex IV resulting from defective heme biosynthesis was also observed in human brain cell lines and rat primary hippocampal neurons (Atamna et al. 2002a), further suggesting an association among age-related heme deficiency, mitochondrial decay and the aging process.
A recent study in yeast established a link between defects in ISC biogenesis and age-associated genomic instability (Veatch et al. 2009). Aging yeast cells lose their mitochondrial DNA (mtDNA) over time, leading to decreased inner mitochondrial membrane potential and mitochondrial dysfunction. The reduction of mitochondrial membrane potential, in turn, contributes to the defect in the transport of iron-sulfur proteins into and out of the mitochondria, which is required for mitochondrial ISC assembly and maturation of cytosolic and nuclear iron-sulfur proteins (reviewed in Lill et al., 2008). Defects in the mitochondrial ISC machineries result in an impaired iron homeostasis with increased cellular iron acquisition, iron regulon activation and iron accumulation in mitochondria. The function of iron-sulfur proteins in both the mitochondrial compartment and throughout the cell is either reduced or lost, and eventually cells fail to maintain nuclear genome intergrity.
2.3. Iron manipulation and longevity
The first indication that inhibition of iron absorption could extend life span was provided by Massie et al. (1993), who studied male Drosophila fed an iron-rich diet and tea extracts. The study demonstrated that increased dietary iron reduced longevity and dietary tea prevented the age-related iron accumulation in Drosophila. Brune et al. (1989) and Rossander-Hulthen et al. (1996) reported that the galloyl group in tea polyphenols was responsible for the inhibitory effects, suggesting a unique role of tea polyphenols in iron binding. However, it is possible that polyphenolic compounds in tea extracts exerted a direct antioxidant effect in addition to the inhibition of iron absorption as proposed and that both mechanisms contributed to the reported increase in lifespan. In fact, other studies suggest different mechanisms that may underlie the life extension effects of tea polyphenolics in Drosophila (Li et al. 2007;Berletch et al. 2008;Peng et al. 2009). Additional genetic studies in C. elegans and Drosophila have established a link between frataxin and longevity by demonstrating that frataxin deficiency in C. elegans led to shorter lifespan (Vazquez-Manrique et al. 2006;Zarse et al. 2007) and that frataxin overexpression in Drosophila extended life span (Runko et al. 2008). By stressing Drosophila with paraquat and hydrogen peroxide, Runko et al. (2008) found that transgenic flies were resistant to oxidative stress and that frataxin overexpression extended the median and maximum life span as much as 35% and 28%, respectively. However, the observation that reducing the expression of frataxin by utilizing a bacterial feeding RNAi against the nematode ortholog frh-1 prolongs life span in nematodes (Ventura et al. 2005) provides evidence that there is a threshold effect on life extension in nematodes. Activation of compensatory pathways may allow nematodes with frataxin reduction to exhibit a life-extension phenotype (Rea et al. 2007). These observations also highlight a fundamental difference in frataxin requirements between mammals and nematodes and suggest that the other findings linking reduced mitochondrial function and life extension in invertebrates may not apply to mammals. Aside from the studies in Drosophila (Massie et al. 1993;Runko et al. 2008), little work has been conducted to investigate the effects of frataxin overexpression and iron restriction on longevity in rodents. Such investigations are required to determine whether frataxin overexpression and iron restriction may eventually be employed as a strategy to modulate aging in humans.
2.4. Diseases related to mitochondrial iron overload
Although the precise molecular components regulating the mitochondrial iron pool are still unknown, frataxin, a 17-KDa mitochondrial protein, is considered central to mitochondrial iron homeostasis (see Scheme 1). Studies in yeast models showed that frataxin was involved in heme biosynthesis (Lange et al. 1999;Seguin et al. 2009), ISC assembly (Muhlenhoff et al. 2002), aconitase repair (Bulteau et al. 2004), and iron storage (Gakh et al. 2006;Gakh et al. 2008;Correia et al. 2010). Ablation of frataxin results in mitochondrial iron overload and Friedreich’s ataxia, a major inheritable neurodegenerative disorder (Pandolfo and Pastore 2009). MtF is another mitochondrial iron storage protein that has been identified so far in testis, neuronal cells and islets of Langherans, and is thought to play an important role in iron storage and regulation in mitochondria (Levi and Arosio 2004;Santambrogio et al. 2007). Levels of MtF are significantly elevated in erythroblasts from patients with sideroblastic anemia, implying that MtF may be induced by iron overloading to sequester excess iron in mitochondria (Drysdale et al. 2002). In agreement with its protective role, studies in frataxin-deficient yeast (Campanella et al. 2004;Campanella et al. 2009) and frataxin-silenced HeLa cells (Zanella et al. 2008) showed that human MtF expression prevented the accumulation of mitochondrial iron, maintained mtDNA integrity, and increased the resistance to oxidative stress by rescuing mitochondrial respiration. Recently, mitochondrial glutaredoxin 5, a thiol-disulfide oxidoreductase, has been shown to be essential for ISC biogenesis and the maintenance of normal mitochondrial and cytosolic iron homeostasis in human RD4 and COS cells (Tong and Rouault 2000). In glutaredoxin 5 deficient HeLa cells, ISC biosynthesis and mitochondrial iron trafficking were impaired, causing mitochondrial iron overload and concomitant cytosolic iron depletion (Ye et al. 2010). In agreement with these observations, glutaredoxin 5 deficiency was associated with sideroblastic anemia in human patients (Camaschella et al. 2007). Besides importer-mediated iron transport to mitochondria, physical interaction between endosomes and mitochondria has also been proposed as a possible mechanism delivering cellular labile iron into the mitochondrion (Zhang et al. 2005;Sheftel et al. 2007). Sec15l1, a mammalian ortholog of the yeast SEC15, has been suggested as a component of the docking machinery between the endosome and the mitochondrion (White et al. 2005). The absence of Sec15l1 causes anemia in mice due to improper iron trafficking in the erythroid transferrin cycle (Lim et al. 2005). Unlike iron import pathways, most iron is exported from mitochondria in the form of heme and ISCs through various transporters (see Scheme 1) (Levi and Rovida 2009). The breast cancer resistance protein (ABCG2), the feline leukemic virus subgroup C receptor (FLVCR), and the ABC-mitochondrial erythroid (ABC-me) are necessary for heme export, whereas ABCB7 is needed for ISCs transport from the mitochondrion to the cytoplasm (Shirihai et al. 2000;Cavadini et al. 2007;Zutz et al. 2009). Defects in these transporters impair mitochondrial iron homeostasis and lead to cellular degeneration and death (Dunn et al. 2007;Rouault and Tong 2008).
Taken together, recent studies on mitochondrial iron overload diseases suggest that redox-active iron accumulation in mitochondria with age may be responsible for increases in cellular and mitochondrial oxidative stress and mitochondrial function decline. These alterations, in turn, may be involved in the pathogenesis of age-related neuro-muscular degeneration as well as in the aging process as a whole.
2.5. Iron accumulation and sarcopenia
The age-related loss of muscle mass and strength, referred to as sarcopenia of aging, is a highly prevalent condition among older adults and severely impacts functional status, quality of life and mortality (Marzetti and Leeuwenburgh 2006). Older individuals are also especially vulnerable to disuse-induced muscle atrophy (Edgerton et al. 2002), the recovery from which is impaired at old age (Zarzhevsky et al. 2001). The socio-economic burden associated with sarcopenia and disuse muscle atrophy has instigated an intensive research on the etiopathogenesis of these conditions, leading to the discovery of several potential contributing factors (Marzetti et al. 2009b). In particular, recent experimental evidence indicates that abnormal iron homeostasis may be involved in the pathogenesis of both sarcopenia (Altun et al. 2007;Hofer et al. 2008;Seo et al. 2008;Jung et al. 2008;Xu et al. 2008) and acute muscle atrophy in old animals (Kondo et al. 1992;Hofer et al. 2008). Hofer et al. (2008) recently demonstrated that non-heme iron levels were over 2-fold higher in the gastrocnemius muscle of old rats compared to younger controls. Acute atrophy induced by hind limb suspension resulted in a further elevation in muscle non-heme iron content in old, but not young rats. Notably, histochemical analysis revealed that iron accumulated in atrophied rather than normal fibers, suggesting a mechanistic link between iron overload and the loss of muscle mass. In addition, Kondo et al. (1992) demonstrated that the accrual of oxidative damage and the severity of muscle atrophy in hind limb immobilized rats were significantly attenuated by the administration of the iron chelator deferoxamine. More recently, Xu et al. (2008) found that total non-heme iron levels increased progressively over the course of aging in the rat gastrocnemius muscle and correlated with decreased muscle mass and grip strength. Importantly, lifelong 40% calorie restriction, an intervention known to mitigate the severity of sarcopenia in rodents, preserved muscle iron homeostasis into very old age (Marzetti et al. 2009b). This adaptation was paralleled by a reduced decline in muscle mass and strength, further supporting a role for muscle iron overload in the pathogenesis of sarcopenia.
Since the mitochondrion is one of the most important sites for cellular iron utilization (Levi and Rovida 2009), Seo et al. (2008) investigated whether mitochondrial iron homeostasis was altered in the aged muscle. Advanced age was associated with increased mitochondrial non-heme iron levels in the rat quadriceps muscle, which correlated with levels of mitochondrial RNA (mtRNA) oxidation and susceptibility to mPTP opening. This finding is of particular relevance, since mPTP opening is considered a central event in the induction of apoptosis, which in turn is thought to be involved in the pathogenesis of sarcopenia and acute muscle atrophy (Marzetti et al. 2009a). Further research is warranted to unveil the mechanisms underlying muscle iron dyshomeostasis during aging as well as in other atrophying conditions. This knowledge will likely allow the development of new therapeutic tools for the prevention and treatment of sarcopenia and disuse muscle atrophy.
2.6. Iron accumulation and brain aging
During aging a progressive structural derangement of biomolecules and cellular compartments takes places in the brain, causing a decline in motor plasticity and cognitive performance (Moos and Morgan 2004;Droge and Schipper 2007;Stankiewicz and Brass 2009). The mechanisms responsible for brain aging are complex and not yet completely understood; however, growing evidence indicates that the aging process partly stems from the accumulation of damage to macromolecules and cell components caused by distorted cellular redox balance and aberrant metal homeostasis (Levenson 2005;Lee and Andersen 2010). In fact, oxidant-mediated damages of macromolecules disturb redox-sensitive signaling (Altamura and Muckenthaler 2009) and cause mitochondrial dysfunction (Lin and Beal 2006) as well as brain cell death (Mattson 2006). Additionally, accumulation of metal ions, especially iron, has been observed in the aged central nervous system in humans (Aquino et al. 2009) and animal models (Hahn et al. 2009) as well as in disorders such as Alzheimer’s disease (Honda et al. 2005;Altamura and Muckenthaler 2009), Huntington’s disease (Simmons et al. 2007;Bartzokis et al. 2007), Parkinson’s disease (Lee and Andersen 2010) and Friedreich’s ataxia (Pandolfo and Pastore 2009).
In the 1950s, Hallgren and Sourander (1958) reported non-heme iron accumulation in several regions of the aged human brain such as putamen, motor cortex, prefrontal cortex, sensory cortex and thalamus (Hallgren and Sourander 1958). In addition to these observations, studies in post-mortem brains showed an age-dependent accumulation of ferritin, indicative of increased storage iron, in different brain regions (Thomas et al. 1993;Connor et al. 1995;Zecca et al. 2001). Recent studies using high-field magnetic resonance imaging revealed an increase in iron levels in various regions of the aged brain (Pfefferbaum et al. 2009;Aquino et al. 2009;Peran et al. 2009;Cherubini et al. 2009). Excessive iron content in the aged brain may generate cellular toxic stress, which partly explains the age-related decline in cognitive performance and other neurodegenerative disorders (Polla et al. 2003;Zecca et al. 2004;Sen et al. 2007;Hahn et al. 2009).
Neurodegenerative diseases associated with iron accumulation in the brain, especially in the basal ganglia, are caused by specific gene mutations (Gregory et al. 2009). For instance, a defect in the PANK2 gene encoding pantothenate kinase 2 causes pantothenate kinase-associated neurodegeneration, in which pathologic accumulation of iron in the brain is observed (Zhou et al. 2001;Gregory and Hayflick 2005). Patients lacking circulating serum ceruloplasmin and ferritin light polypeptide bear mutations in the CP and FTL genes, respectively (Curtis et al. 2001;Texel et al. 2008). About half of cases of infantile neuroaxonal dystrophy and atypical neuroaxonal dystrophy exhibit iron deposition in the brain, which is associated with mutations in the gene PLA2G6. Much research effort is directed at exploration of noninvasive therapeutics to combat these neurodegenerative diseases associated with brain iron accumulation (Miyajima et al. 1997;Gregory et al. 2009).
3. Future research
If indeed iron accumulation and alterations in labile iron are significant factors in the aging process, iron scavenging and removal might therefore prevent cellular and mitochondrial oxidative damage and attenuate age-related mitochondrial decay. This goal could be achieved through phlebotomy, iron chelation, overexpression of iron storage proteins or limitation of dietary iron (Polla et al. 2003;Saito et al. 2003;Sullivan 2009). Several studies have shown that iron chelation may be beneficial in the treatment of iron overload diseases, such as Alzheimer’s disease (Weinberg and Miklossy 2008;Liu et al. 2010), Parkinson’s disease (Kaur and Andersen 2004;Ghosh et al. 2010), Friedreich’s ataxia (Whitnall et al. 2008;Goncalves et al. 2008) and retinal disease (Dunaief 2006;Lukinova et al. 2009). An important issue associated with iron chelation therapy is that compounds available to date do not possess enough selectivity for organs or macromolecular structures, and once penetrated in tissues chelate iron indiscriminately. This may dramatically limit the use of iron chelators in elderly people, in whom iron-deficient anemia is highly prevalent (Darnton-Hill et al. 2005). Therefore, we need safer therapeutic interventions with a high selectivity to reduce labile iron levels while maintaining the bioavailable iron pool.
Calorie restriction, the only non-genetic intervention extending life and health span in all organisms studied to date, has been shown to be effective in alleviating the age-associated iron accumulation in rat muscle, liver, brain and kidney (Cook and Yu 1998;Xu et al. 2008). However, one major issue associated with calorie restriction involves feasibility and tolerability, especially for the old frail elderly. Tea polyphenolics, on the other hand, show promise in terms of feasibility and may provide a valid alternative to calorie restriction to reduce iron absorption, but this intervention has not been fully tested. Thus, a major research challenge will be the development of novel, safe and feasible interventions to preserve iron homeostasis into old age.
Acknowledgments
This research was supported by grants to CL (NIA R01 AG17994) and J-SK (NIDDK R01 DK079879), the University of Florida Institute on Aging and the Claude D. Pepper Older Americans Independence Center (1 P30 AG028740), and fellowship award to JX (AHA 09POST2060112).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Altamura S, Muckenthaler MU. Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J Alzheimers Dis. 2009;16:879–895. doi: 10.3233/JAD-2009-1010. [DOI] [PubMed] [Google Scholar]
- Altun M, Edstrom E, Spooner E, Flores-Moralez A, Bergman E, Tollet-Egnell P, Norstedt G, Kessler BM, Ulfhake B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve. 2007;36:223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252:165–172. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- Atamna H, Killilea DW, Killilea AN, Ames BN. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc Natl Acad Sci USA. 2002a;99:14807–14812. doi: 10.1073/pnas.192585799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H, Liu J, Ames BN. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: revelance to aging. JBiol Chem. 2001;276:48410–48416. doi: 10.1074/jbc.M108362200. [DOI] [PubMed] [Google Scholar]
- Atamna H, Walter PB, Ames BN. The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch Biochem Biophys. 2002b;397:345–353. doi: 10.1006/abbi.2001.2671. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S. Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res. 2007;32:1655–1664. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. JCell Biochem. 2008;103:509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar M, Weiner M. Modification of age-induced changes in heme and hemoproteins by testosterone in male rats. Mech Ageing Dev. 1983;23:285–296. doi: 10.1016/0047-6374(83)90029-5. [DOI] [PubMed] [Google Scholar]
- Breuer W, Epsztejn S, Millgram P, Cabantchik IZ. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am JPhysiol. 1995;268:C1354–C1361. doi: 10.1152/ajpcell.1995.268.6.C1354. [DOI] [PubMed] [Google Scholar]
- Brune M, Rossander L, Hallberg L. Iron absorption and phenolic compounds: importance of different phenolic structures. Eur JClin Nutr. 1989;43:547–557. [PubMed] [Google Scholar]
- Bulteau AL, O’Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305:242–245. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- Camaschella C, Campanella A, De FL, Boschetto L, Merlini R, Silvestri L, Levi S, Iolascon A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110:1353–1358. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- Campanella A, Isaya G, O’Neill HA, Santambrogio P, Cozzi A, Arosio P, Levi S. The expression of human mitochondrial ferritin rescues respiratory function in frataxin-deficient yeast. Hum Mol Genet. 2004;13:2279–2288. doi: 10.1093/hmg/ddh232. [DOI] [PubMed] [Google Scholar]
- Campanella A, Rovelli E, Santambrogio P, Cozzi A, Taroni F, Levi S. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: hypothesis for a protective role in Friedreich ataxia. Hum Mol Genet. 2009;18:1–11. doi: 10.1093/hmg/ddn308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D, Schaack J, Patel M. Oxidative inactivation of mitochondrial aconitase results in iron and H2O2-mediated neurotoxicity in rat primary mesencephalic cultures. PLoSONE. 2009;4:e7095. doi: 10.1371/journal.pone.0007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavadini P, Biasiotto G, Poli M, Levi S, Verardi R, Zanella I, Derosas M, Ingrassia R, Corrado M, Arosio P. RNA silencing of the mitochondrial ABCB7 transporter in HeLa cells causes an iron-deficient phenotype with mitochondrial iron overload. Blood. 2007;109:3552–3559. doi: 10.1182/blood-2006-08-041632. [DOI] [PubMed] [Google Scholar]
- Chen H, Lukas TJ, Du N, Suyeoka G, Neufeld AH. Dysfunction of the retinal pigment epithelium with age: increased iron decreases phagocytosis and lysosomal activity. Invest Ophthalmol Vis Sci. 2009;50:1895–1902. doi: 10.1167/iovs.08-2850. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Peran P, Caltagirone C, Sabatini U, Spalletta G. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage. 2009;48:29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Connor JR, Snyder BS, Arosio P, Loeffler DA, LeWitt P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer’s diseased brains. JNeurochem. 1995;65:717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech Ageing Dev. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Correia AR, Wang T, Craig EA, Gomes CM. Iron-binding activity in yeast frataxin entails a trade off with stability in the alpha1/beta1 acidic ridge region. Biochem J. 2010;426:197–203. doi: 10.1042/BJ20091612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, Coulthard A, Jackson MJ, Jackson AP, McHale DP, Hay D, Barker WA, Markham AF, Bates D, Curtis A, Burn J. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- Darnton-Hill I, Webb P, Harvey PW, Hunt JM, Dalmiya N, Chopra M, Ball MJ, Bloem MW, de BB. Micronutrient deficiencies and gender: social and economic costs. Am JClin Nutr. 2005;81:1198S–1205S. doi: 10.1093/ajcn/81.5.1198. [DOI] [PubMed] [Google Scholar]
- Dencher NA, Frenzel M, Reifschneider NH, Sugawa M, Krause F. Proteome alterations inrat mitochondria caused by aging. Ann NYAcad Sci. 2007;1100:291–298. doi: 10.1196/annals.1395.030. [DOI] [PubMed] [Google Scholar]
- Doulias PT, Vlachou C, Boudouri C, Kanavaros P, Siamopoulos KC, Galaris D. Flow cytometric estimation of ‘labile iron pool’ in human white blood cells reveals a positive association with ageing. Free Radic Res. 2008;42:253–259. doi: 10.1080/10715760801911649. [DOI] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J, Arosio P, Invernizzi R, Cazzola M, Volz A, Corsi B, Biasiotto G, Levi S. Mitochondrial ferritin: a new player in iron metabolism. Blood Cells Mol Dis. 2002;29:376–383. doi: 10.1006/bcmd.2002.0577. [DOI] [PubMed] [Google Scholar]
- Dunaief JL. Iron induced oxidative damage as a potential factor in age-related macular degeneration: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47:4660–4664. doi: 10.1167/iovs.06-0568. [DOI] [PubMed] [Google Scholar]
- Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Duvigneau JC, Piskernik C, Haindl S, Kloesch B, Hartl RT, Huttemann M, Lee I, Ebel T, Moldzio R, Gemeiner M, Redl H, Kozlov AV. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab Invest. 2008;88:70–77. doi: 10.1038/labinvest.3700691. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Stout AK. Assessment of mitochondrial membrane potential in situ using single potentiometric dyes and a novel fluorescence resonance energy transfer technique. Methods Cell Biol. 2001;65:285–309. doi: 10.1016/s0091-679x(01)65018-0. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Allen DL, Monti RJ. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am JPhys Med Rehabil. 2002;81:S127–S147. doi: 10.1097/00002060-200211001-00014. [DOI] [PubMed] [Google Scholar]
- Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal Biochem. 1997;248:31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- Gakh O, Park S, Liu G, Macomber L, Imlay JA, Ferreira GC, Isaya G. Mitochondrial iron detoxification is a primary function of frataxin that limits oxidative damage and preserves cell longevity. Hum Mol Genet. 2006;15:467–479. doi: 10.1093/hmg/ddi461. [DOI] [PubMed] [Google Scholar]
- Gakh O, Smith DY, Isaya G. Assembly of the iron-binding protein frataxin in Saccharomyces cerevisiae responds to dynamic changes in mitochondrial iron influx and stress level. JBiol Chem. 2008;283:31500–31510. doi: 10.1074/jbc.M805415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau RJ, Yang HL, Suen JL, Lu FJ. Induction of oxidative stress by humic acid through increasing intracellular iron: a possible mechanism leading to atherothrombotic vascular disorder in blackfoot disease. Biochem Biophys Res Commun. 2001;283:743–749. doi: 10.1006/bbrc.2001.4832. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Antonio T, Reith ME, Dutta AK. Discovery of 4-(4-(2-((5-Hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)ethyl) piperazin-1-yl)quinolin-8-ol and Its Analogues as Highly Potent Dopamine D2/D3 Agonists and as Iron Chelator: In Vivo Activity Indicates Potential Application in Symptomatic and Neuroprotective Therapy for Parkinson’s Disease. JMed Chem. 2010;53:2114–2125. doi: 10.1021/jm901618d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves S, Paupe V, Dassa EP, Rustin P. Deferiprone targets aconitase: implication for Friedreich’s ataxia treatment. BMCNeurol. 2008;8:20. doi: 10.1186/1471-2377-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Hayflick SJ. Neurodegeneration with brain iron accumulation. Folia Neuropathol. 2005;43:286–296. [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. JMed Genet. 2009;46:73–80. doi: 10.1136/jmg.2008.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Song Y, Ying GS, He X, Beard J, Dunaief JL. Age-dependent and gender-specific changes in mouse tissue iron by strain. Exp Gerontol. 2009;44:594–600. doi: 10.1016/j.exger.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. JNeurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM, Hattier T, Harris PL, Siedlak SL, Fujioka H, Liu Q, Moreira PI, Miller FP, Nunomura A, Shimohama S, Perry G. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. JBiol Chem. 2005;280:20978–20986. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- Irazusta V, Obis E, Moreno-Cermeno A, Cabiscol E, Ros J, Tamarit J. Yeast frataxin mutants display decreased superoxide dismutase activities crucial to promote protein oxidative damage. Free Radic Biol Med. 2009;48:411–420. doi: 10.1016/j.freeradbiomed.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Jung SH, DeRuisseau LR, Kavazis AN, Deruisseau KC. Plantaris muscle of aged rats demonstrates iron accumulation and altered expression of iron regulation proteins. Exp Physiol. 2008;93:407–414. doi: 10.1113/expphysiol.2007.039453. [DOI] [PubMed] [Google Scholar]
- Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free Radic Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in Parkinson’s disease? Ageing Res Rev. 2004;3:327–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Killilea DW, Atamna H, Liao C, Ames BN. Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid Redox Signal. 2003;5:507–516. doi: 10.1089/152308603770310158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Wong SL, Cahaya HS, Atamna H, Ames BN. Iron accumulation during cellular senescence. Ann NYAcad Sci. 2004;1019:365–367. doi: 10.1196/annals.1297.063. [DOI] [PubMed] [Google Scholar]
- Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Miura M, Kodama J, Ahmed SM, Itokawa Y. Role of iron in oxidative stress in skeletal muscle atrophied by immobilization. Pflugers Arch. 1992;421:295–297. doi: 10.1007/BF00374844. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Yegorov DY, Vladimirov YA, Azizova OA. Intracellular free iron in liver tissue and liver homogenate: studies with electron paramagnetic resonance on the formation of paramagnetic complexes with desferal and nitric oxide. Free Radic Biol Med. 1992;13:9–16. doi: 10.1016/0891-5849(92)90159-e. [DOI] [PubMed] [Google Scholar]
- Kurz T, Gustafsson B, Brunk UT. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006;273:3106–3117. doi: 10.1111/j.1742-4658.2006.05321.x. [DOI] [PubMed] [Google Scholar]
- Lange H, Kispal G, Lill R. Mechanism of iron transport to the site of heme synthesis inside yeast mitochondria. JBiol Chem. 1999;274:18989–18996. doi: 10.1074/jbc.274.27.18989. [DOI] [PubMed] [Google Scholar]
- Lee DW, Andersen JK. Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? JNeurochem. 2010;112:332–339. doi: 10.1111/j.1471-4159.2009.06470.x. [DOI] [PubMed] [Google Scholar]
- Levenson CW. Trace metal regulation of neuronal apoptosis: from genes to behavior. Physiol Behav. 2005;86:399–406. doi: 10.1016/j.physbeh.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Levi S, Arosio P. Mitochondrial ferritin. Int JBiochem Cell Biol. 2004;36:1887–1889. doi: 10.1016/j.biocel.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Levi S, Rovida E. The role of iron in mitochondrial function. Biochim Biophys Acta. 2009;1790:629–636. doi: 10.1016/j.bbagen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Li YM, Chan HY, Huang Y, Chen ZY. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol Nutr Food Res. 2007;51:546–554. doi: 10.1002/mnfr.200600238. [DOI] [PubMed] [Google Scholar]
- Liang LP, Jarrett SG, Patel M. Chelation of mitochondrial iron prevents seizure-induced mitochondrial dysfunction and neuronal injury. JNeurosci. 2008;28:11550–11556. doi: 10.1523/JNEUROSCI.3016-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- Lim JE, Jin O, Bennett C, Morgan K, Wang F, Trenor CC, III, Fleming MD, Andrews NC. A mutation in Sec15l1 causes anemia in hemoglobin deficit (hbd) mice. Nat Genet. 2005;37:1270–1273. doi: 10.1038/ng1659. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu G, Men P, Perry G, Smith MA. Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol Biol. 2010;610:123–144. doi: 10.1007/978-1-60327-029-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukinova N, Iacovelli J, Dentchev T, Wolkow N, Hunter A, Amado D, Ying GS, Sparrow JR, Dunaief JL. Iron chelation protects the retinal pigment epithelial cell line ARPE-19 against cell death triggered by diverse stimuli. Invest Ophthalmol Vis Sci. 2009;50:1440–1447. doi: 10.1167/iovs.08-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, de GH, Liu Z, Hider RC, Petrat F. Chelation and determination of labile iron in primary hepatocytes by pyridinone fluorescent probes. Biochem J. 2006;395:49–55. doi: 10.1042/BJ20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: Relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2010;1800:235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors. 2009b;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Williams TR. Inhibition of iron absorption prolongs the life span of Drosophila. Mech Ageing Dev. 1993;67:227–237. doi: 10.1016/0047-6374(93)90001-8. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- Miyajima H, Takahashi Y, Kamata T, Shimizu H, Sakai N, Gitlin JD. Use of desferrioxamine in the treatment of aceruloplasminemia. Ann Neurol. 1997;41:404–407. doi: 10.1002/ana.410410318. [DOI] [PubMed] [Google Scholar]
- Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann NYAcad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- Muhlenhoff U, Richhardt N, Ristow M, Kispal G, Lill R. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum Mol Genet. 2002;11:2025–2036. doi: 10.1093/hmg/11.17.2025. [DOI] [PubMed] [Google Scholar]
- Napoli E, Taroni F, Cortopassi GA. Frataxin, iron-sulfur clusters, heme, ROS, and aging. Antioxid Redox Signal. 2006;8:506–516. doi: 10.1089/ars.2006.8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell K, Gannon J, Doran P, Ohlendieck K. Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. Int JMol Med. 2007;20:145–153. [PubMed] [Google Scholar]
- Pandolfo M, Pastore A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J Neurol. 2009;256(Suppl 1):9–17. doi: 10.1007/s00415-009-1003-2. [DOI] [PubMed] [Google Scholar]
- Peng C, Chan HY, Li YM, Huang Y, Chen ZY. Black tea theaflavins extend the lifespan of fruit flies. Exp Gerontol. 2009;44:773–783. doi: 10.1016/j.exger.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Peran P, Cherubini A, Luccichenti G, Hagberg G, Demonet JF, Rascol O, Celsis P, Caltagirone C, Spalletta G, Sabatini U. Volume and iron content in basal ganglia and thalamus. Hum Brain Mapp. 2009;30:2667–2675. doi: 10.1002/hbm.20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrat F, de GH, Rauen U. Subcellular distribution of chelatable iron: a laser scanning microscopic study in isolated hepatocytes and liver endothelial cells. Biochem J. 2001;356:61–69. doi: 10.1042/0264-6021:3560061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrat F, de GH, Sustmann R, Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biol Chem. 2002a;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- Petrat F, Weisheit D, Lensen M, de GH, Sustmann R, Rauen U. Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent sensor. Biochem J. 2002b;362:137–147. doi: 10.1042/0264-6021:3620137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage. 2009;47:493–500. doi: 10.1016/j.neuroimage.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla AS, Polla LL, Polla BS. Iron as the malignant spirit in successful ageing. Ageing Res Rev. 2003;2:25–37. doi: 10.1016/s1568-1637(02)00048-x. [DOI] [PubMed] [Google Scholar]
- Prokai L, Yan LJ, Vera-Serrano JL, Stevens SM, Jr, Forster MJ. Mass spectrometry-based survey of age-associated protein carbonylation in rat brain mitochondria. JMass Spectrom. 2007;42:1583–1589. doi: 10.1002/jms.1345. [DOI] [PubMed] [Google Scholar]
- Rauen U, Springer A, Weisheit D, Petrat F, Korth HG, de GH, Sustmann R. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. Chembiochem. 2007;8:341–352. doi: 10.1002/cbic.200600311. [DOI] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoSBiol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossander-Hulthen L, Hallberg L. Dietary factor influencing iron absorption - an overview. In: Hallberg L, Asp N-G, editors. Iron Nutrition in Health and Disease. London: John Libbey; 1996. pp. 105–115. [Google Scholar]
- Rothman RJ, Serroni A, Farber JL. Cellular pool of transient ferric iron, chelatable by deferoxamine and distinct from ferritin, that is involved in oxidative cell injury. Mol Pharmacol. 1992;42:703–710. [PubMed] [Google Scholar]
- Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- Rouault TA, Tong WH. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runko AP, Griswold AJ, Min KT. Overexpression of frataxin in the mitochondria increases resistance to oxidative stress and extends lifespan in Drosophila. FEBS Lett. 2008;582:715–719. doi: 10.1016/j.febslet.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Saito K, Ishizaka N, Mitani H, Ohno M, Nagai R. Iron chelation and a free radical scavenger suppress angiotensin II-induced downregulation of klotho, an anti-aging gene, in rat. FEBS Lett. 2003;551:58–62. doi: 10.1016/s0014-5793(03)00894-9. [DOI] [PubMed] [Google Scholar]
- Santambrogio P, Biasiotto G, Sanvito F, Olivieri S, Arosio P, Levi S. Mitochondrial ferritin expression in adult mouse tissues. JHistochem Cytochem. 2007;55:1129–1137. doi: 10.1369/jhc.7A7273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Cisse S. Mitochondrial constituents of corpora amylacea and autofluorescent astrocytic inclusions in senescent human brain. Glia. 1995;14:55–64. doi: 10.1002/glia.440140108. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Vininsky R, Brull R, Small L, Brawer JR. Astrocyte mitochondria: a substrate for iron deposition in the aging rat substantia nigra. Exp Neurol. 1998;152:188–196. doi: 10.1006/exnr.1998.6854. [DOI] [PubMed] [Google Scholar]
- Seguin A, Bayot A, Dancis A, Rogowska-Wrzesinska A, Auchere F, Camadro JM, Bulteau AL, Lesuisse E. Overexpression of the yeast frataxin homolog (Yfh1): contrasting effects on iron-sulfur cluster assembly, heme synthesis and resistance to oxidative stress. Mitochondrion. 2009;9:130–138. doi: 10.1016/j.mito.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Sen T, Jana S, Sreetama S, Chatterjee U, Chakrabarti S. Gene-specific oxidative lesions in aged rat brain detected by polymerase chain reaction inhibition assay. Free Radic Res. 2007;41:288–294. doi: 10.1080/10715760601083722. [DOI] [PubMed] [Google Scholar]
- Seo AY, Xu J, Servais S, Hofer T, Marzetti E, Wohlgemuth SE, Knutson MD, Chung HY, Leeuwenburgh C. Mitochondrial iron accumulation with age and functional consequences. Aging Cell. 2008;7:706–716. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel AD, Zhang AS, Brown C, Shirihai OS, Ponka P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood. 2007;110:125–132. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- Shirihai OS, Gregory T, Yu C, Orkin SH, Weiss MJ. ABC-me: a novel mitochondrial transporter induced by GATA-1 during erythroid differentiation. EMBO J. 2000;19:2492–2502. doi: 10.1093/emboj/19.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Casale M, Alcon B, Pham N, Narayan N, Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia. 2007;55:1074–1084. doi: 10.1002/glia.20526. [DOI] [PubMed] [Google Scholar]
- Simunek T, Boer C, Bouwman RA, Vlasblom R, Versteilen AM, Sterba M, Gersl V, Hrdina R, Ponka P, de Lange JJ, Paulus WJ, Musters RJ. SIH--a novel lipophilic iron chelator--protects H9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. JMol Cell Cardiol. 2005;39:345–354. doi: 10.1016/j.yjmcc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Wennberg-Kirch E, Jaiswal K, Kwong LK, Forster MJ. Effect of age and caloric restriction on bleomycin-chelatable and nonheme iron in different tissues of C57BL/6 mice. Free Radic Biol Med. 1999;27:287–293. doi: 10.1016/s0891-5849(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Stankiewicz JM, Brass SD. Role of iron in neurotoxicity: a cause for concern in the elderly? Curr Opin Clin Nutr Metab Care. 2009;12:22–29. doi: 10.1097/MCO.0b013e32831ba07c. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Iron in arterial plaque: modifiable risk factor for atherosclerosis. Biochim Biophys Acta. 2009;1790:718–723. doi: 10.1016/j.bbagen.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Texel SJ, Xu X, Harris ZL. Ceruloplasmin in neurodegenerative diseases. Biochem Soc Trans. 2008;36:1277–1281. doi: 10.1042/BST0361277. [DOI] [PubMed] [Google Scholar]
- Thomas LO, Boyko OB, Anthony DC, Burger PC. MR detection of brain iron. AJNR Am JNeuroradiol. 1993;14:1043–1048. [PMC free article] [PubMed] [Google Scholar]
- Tong WH, Rouault T. Distinct iron-sulfur cluster assembly complexes exist in the cytosol and mitochondria of human cells. EMBO J. 2000;19:5692–5700. doi: 10.1093/emboj/19.21.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Manrique RP, Gonzalez-Cabo P, Ros S, Aziz H, Baylis HA, Palau F. Reduction of Caenorhabditis elegans frataxin increases sensitivity to oxidative stress, reduces lifespan, and causes lethality in a mitochondrial complex II mutant. FASEB J. 2006;20:172–174. doi: 10.1096/fj.05-4212fje. [DOI] [PubMed] [Google Scholar]
- Veatch JR, McMurray MA, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. 2009;137:1247–1258. doi: 10.1016/j.cell.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura N, Rea S, Henderson ST, Condo I, Johnson TE, Testi R. Reduced expression of frataxin extends the lifespan of Caenorhabditis elegans. Aging Cell. 2005;4:109–112. doi: 10.1111/j.1474-9726.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Weinberg ED, Miklossy J. Iron withholding: a defense against disease. JAlzheimers Dis. 2008;13:451–463. doi: 10.3233/jad-2008-13409. [DOI] [PubMed] [Google Scholar]
- White RA, Boydston LA, Brookshier TR, McNulty SG, Nsumu NN, Brewer BP, Blackmore K. Iron metabolism mutant hbd mice have a deletion in Sec15l1, which has homology to a yeast gene for vesicle docking. Genomics. 2005;86:668–673. doi: 10.1016/j.ygeno.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Whitnall M, Rahmanto YS, Sutak R, Xu X, Becker EM, Mikhael MR, Ponka P, Richardson DR. The MCK mouse heart model of Friedreich’s ataxia: Alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc Natl Acad Sci USA. 2008;105:9757–9762. doi: 10.1073/pnas.0804261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Knutson MD, Carter CS, Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoSONE. 2008;3:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Jeong SY, Ghosh MC, Kovtunovych G, Silvestri L, Ortillo D, Uchida N, Tisdale J, Camaschella C, Rouault TA. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. JClinInvest. 2010:40372. doi: 10.1172/JCI40372. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- Zanella I, Derosas M, Corrado M, Cocco E, Cavadini P, Biasiotto G, Poli M, Verardi R, Arosio P. The effects of frataxin silencing in HeLa cells are rescued by the expression of human mitochondrial ferritin. Biochim Biophys Acta. 2008;1782:90–98. doi: 10.1016/j.bbadis.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Zarse K, Schulz TJ, Birringer M, Ristow M. Impaired respiration is positively correlated with decreased life span in Caenorhabditis elegans models of Friedreich Ataxia. FASEB J. 2007;21:1271–1275. doi: 10.1096/fj.06-6994com. [DOI] [PubMed] [Google Scholar]
- Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp Gerontol. 2001;36:125–140. doi: 10.1016/s0531-5565(00)00189-3. [DOI] [PubMed] [Google Scholar]
- Zecca L, Gallorini M, Schunemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. JNeurochem. 2001;76:1766–1773. doi: 10.1046/j.1471-4159.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhang AS, Sheftel AD, Ponka P. Intracellular kinetics of iron in reticulocytes: evidence for endosome involvement in iron targeting to mitochondria. Blood. 2005;105:368–375. doi: 10.1182/blood-2004-06-2226. [DOI] [PubMed] [Google Scholar]
- Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet. 2001;28:345–349. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]
- Zutz A, Gompf S, Schagger H, Tampe R. Mitochondrial ABC proteins in health and disease. Biochim Biophys Acta. 2009;1787:681–690. doi: 10.1016/j.bbabio.2009.02.009. [DOI] [PubMed] [Google Scholar]