Abstract
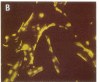
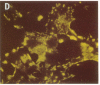
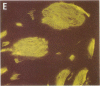
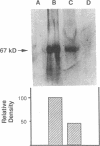
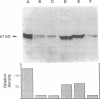
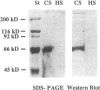
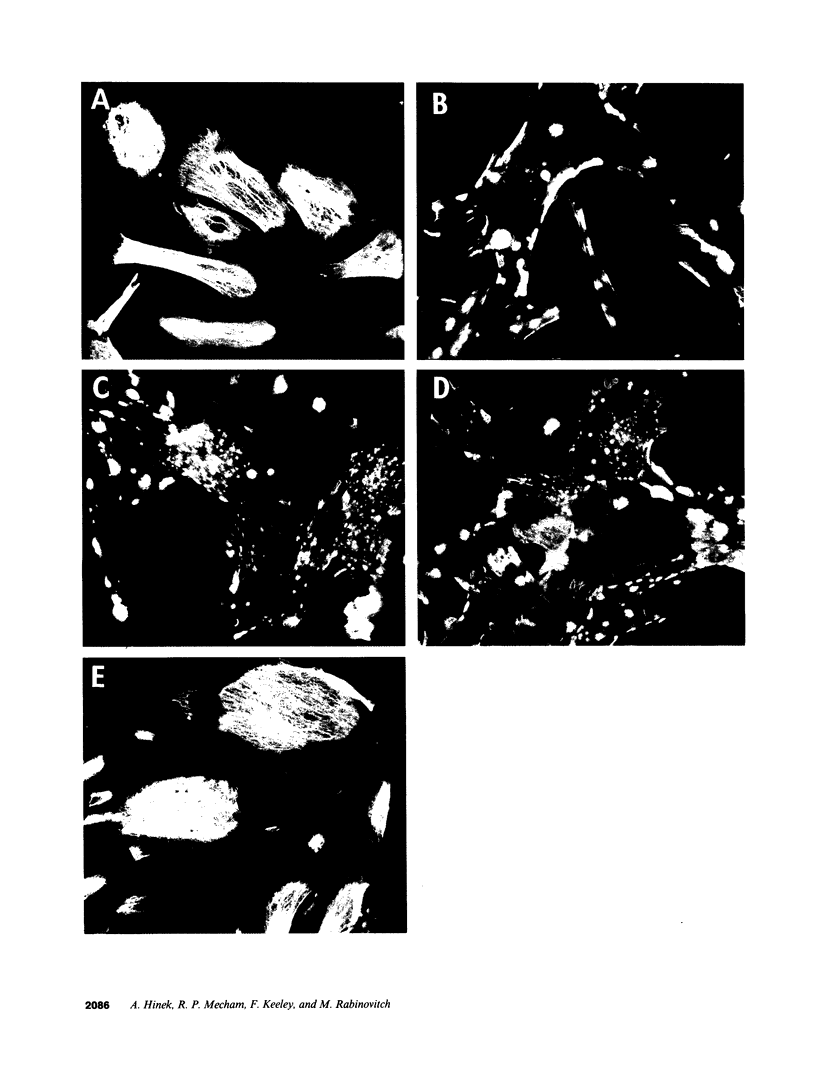
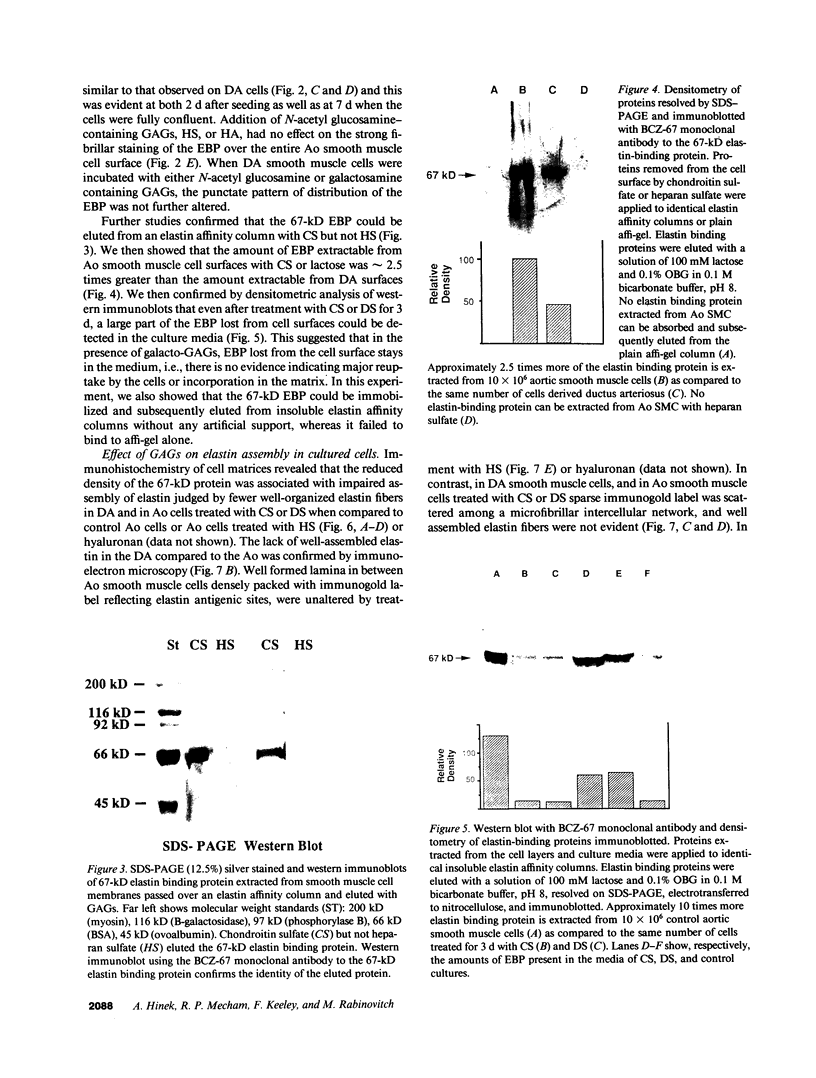
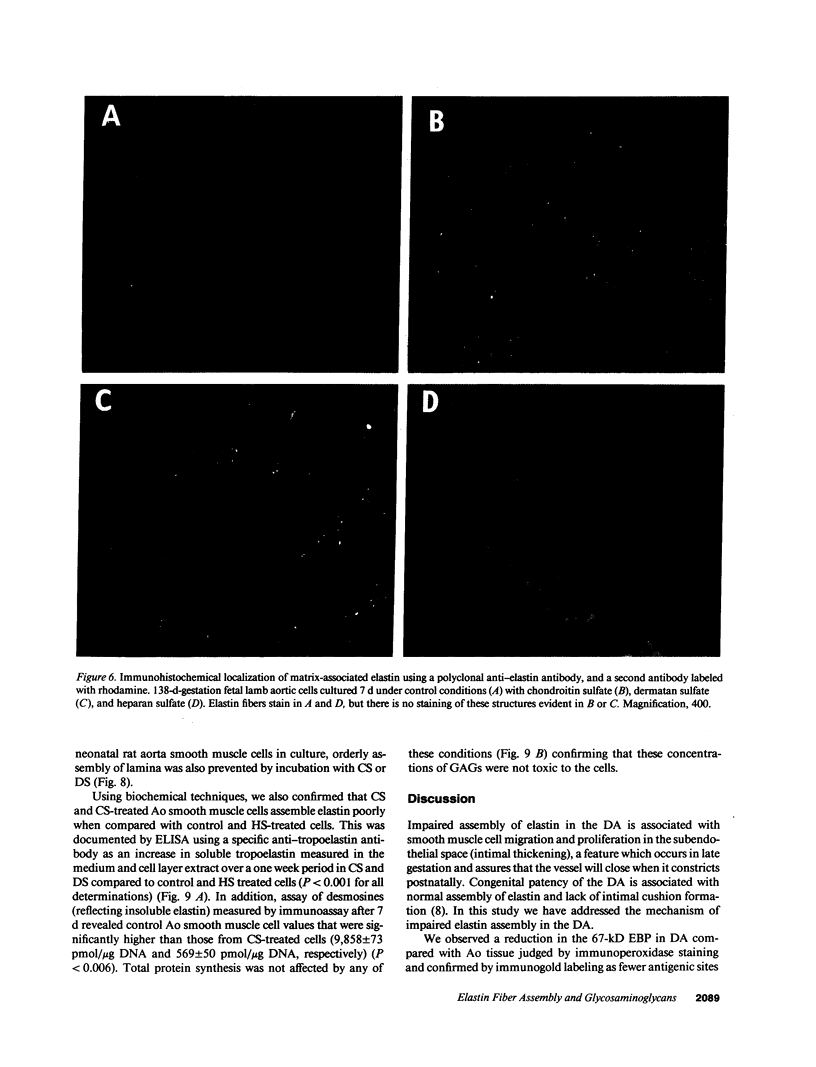
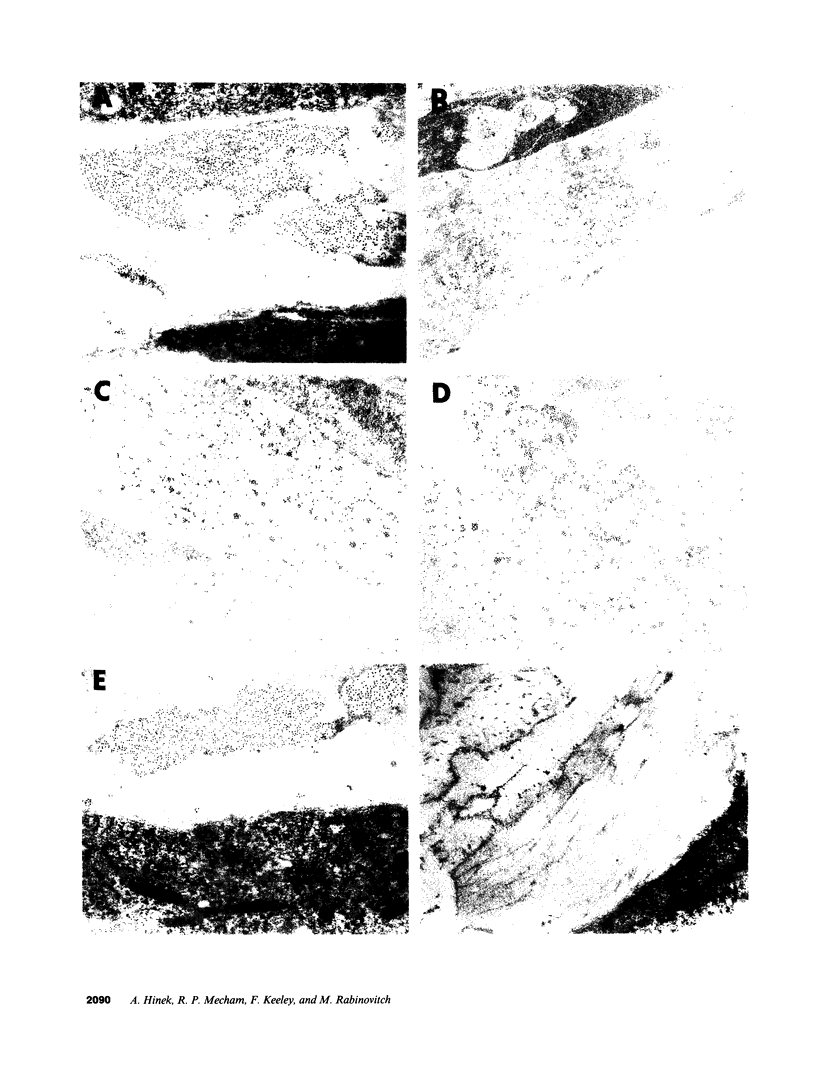
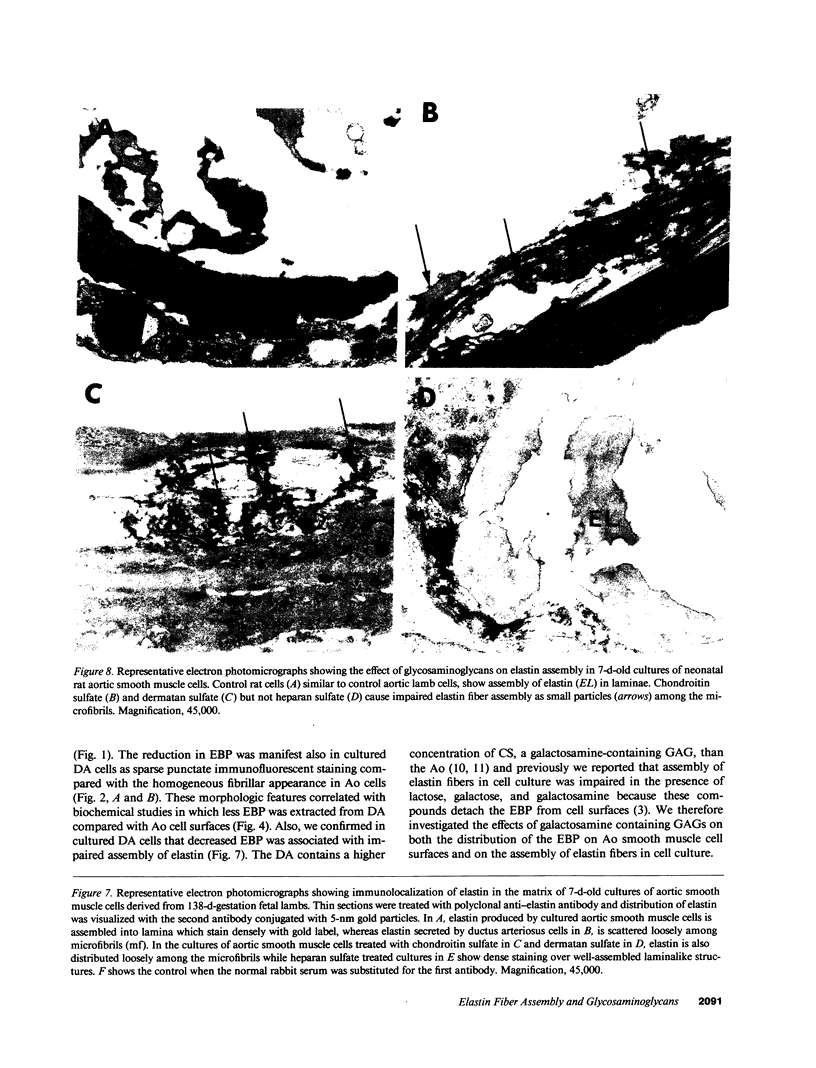
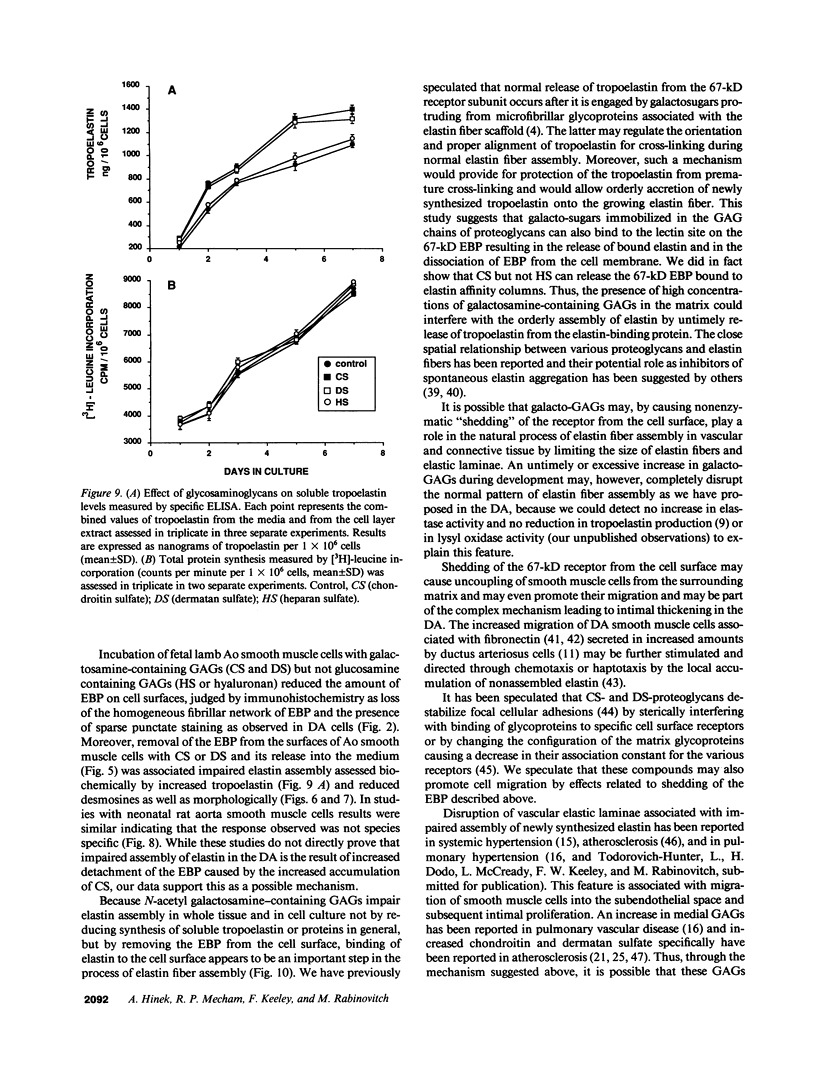
In the fetal ductus arteriosus (DA) disruption in the assembly of elastin fibers is associated with intimal thickening and we previously reported that fetal lamb DA smooth muscle cells incubated with endothelial conditioned medium produce two-fold more chondroitin sulfate (CS) compared with aorta (Ao) cells (Boudreau, N., and M. Rabinovitch. 1991. Lab. Invest. 64:187-199). We hypothesized that CS or dermatan sulfate (DS), both N-acetylgalactosamine glycosaminoglycans (GAGs), may be similar to free galactosugars in causing release of the 67-kD elastin binding protein (EBP) from the smooth muscle cell surfaces and impaired elastin fiber assembly. Using immunohistochemistry, immunoelectron microscopy, and western immunoblot we demonstrated a reduction in the 67-kD EBP in fetal lamb DA smooth muscle in tissue and in cultured cells. Also, reduced EBP was observed in fetal lamb and neonatal rat Ao smooth muscle cells incubated with N-acetylgalactosamine GAGs, CS, and DS, but not with N-acetylglucosamine containing GAGs, heparan sulfate (HS), or hyaluronan. Reduction in EBP was related to shedding from cell surfaces into the conditioned medium. This was associated with impaired elastin fiber assembly in cultured cells, assessed both morphologically and by a relative increase in tropoelastin and decrease in desmosines. The EBP extracted from smooth muscle cell membranes binds to an elastin affinity gel and can be eluted from it with CS but not with HS. Moreover, the amount of EBP extractable from smooth muscle cell membranes correlated with the morphologic assessment. We propose that increased CS or DS, may impair assembly of newly synthesized elastin in the media of the ductus arteriosus associated with the development of intimal thickening.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benitz W. E., Bernfield M. Endothelial cell proteoglycans: possible mediators of vascular responses to injury. Am J Respir Cell Mol Biol. 1990 May;2(5):407–408. doi: 10.1165/ajrcmb/2.5.407. [DOI] [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy B., Srinivasan S. R., Vijayagopal P., Dalferes E. R., Jr Arterial wall injury and proteoglycan changes in atherosclerosis. Arch Pathol Lab Med. 1988 Oct;112(10):1002–1010. [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy B., Srinivasan S. R., Vijayagopal P., Dalferes E. R. Proteoglycans and potential mechanisms related to atherosclerosis. Ann N Y Acad Sci. 1985;454:69–78. doi: 10.1111/j.1749-6632.1985.tb11845.x. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Rabinovitch M. Developmentally regulated changes in extracellular matrix in endothelial and smooth muscle cells in the ductus arteriosus may be related to intimal proliferation. Lab Invest. 1991 Feb;64(2):187–199. [PubMed] [Google Scholar]
- Cleary E. G., Gibson M. A. Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tissue Res. 1983;10:97–209. doi: 10.1016/b978-0-12-363710-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Contri M. B., Fornieri C. C., Ronchetti I. P. Elastin-proteoglycans association revealed by cytochemical methods. Connect Tissue Res. 1985;13(3):237–249. doi: 10.3109/03008208509152403. [DOI] [PubMed] [Google Scholar]
- De Reeder E. G., Girard N., Poelmann R. E., Van Munsteren J. C., Patterson D. F., Gittenberger-De Groot A. C. Hyaluronic acid accumulation and endothelial cell detachment in intimal thickening of the vessel wall. The normal and genetically defective ductus arteriosus. Am J Pathol. 1988 Sep;132(3):574–585. [PMC free article] [PubMed] [Google Scholar]
- Fornieri C., Baccarani-Contri M., Quaglino D., Jr, Pasquali-Ronchetti I. Lysyl oxidase activity and elastin/glycosaminoglycan interactions in growing chick and rat aortas. J Cell Biol. 1987 Sep;105(3):1463–1469. doi: 10.1083/jcb.105.3.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredette B. J., Landmesser L. T. Relationship of primary and secondary myogenesis to fiber type development in embryonic chick muscle. Dev Biol. 1991 Jan;143(1):1–18. doi: 10.1016/0012-1606(91)90050-d. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot A. C., Strengers J. L., Mentink M., Poelmann R. E., Patterson D. F. Histologic studies on normal and persistent ductus arteriosus in the dog. J Am Coll Cardiol. 1985 Aug;6(2):394–404. doi: 10.1016/s0735-1097(85)80178-9. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot A. C., van Ertbruggen I., Moulaert A. J., Harinck E. The ductus arteriosus in the preterm infant: histologic and clinical observations. J Pediatr. 1980 Jan;96(1):88–93. doi: 10.1016/s0022-3476(80)80337-4. [DOI] [PubMed] [Google Scholar]
- Hinek A., Kawiak J., Czarnowska E., Barcew B. The effect of agarose and dexamethasone on the nature and production of extracellular matrix components by elastic cartilage chondrocytes. Acta Biol Hung. 1984;35(2-4):245–258. [PubMed] [Google Scholar]
- Hinek A., Thyberg J., Friberg U. Heterotopic allotransplantation of isolated aortic cells. An electron microscopic study. Cell Tissue Res. 1976 Sep 6;172(1):59–79. doi: 10.1007/BF00226049. [DOI] [PubMed] [Google Scholar]
- Hinek A., Wrenn D. S., Mecham R. P., Barondes S. H. The elastin receptor: a galactoside-binding protein. Science. 1988 Mar 25;239(4847):1539–1541. doi: 10.1126/science.2832941. [DOI] [PubMed] [Google Scholar]
- King G. S., Mohan V. S., Starcher B. C. Radioimmunoassay for desmosine. Connect Tissue Res. 1980;7(4):263–267. doi: 10.3109/03008208009152362. [DOI] [PubMed] [Google Scholar]
- Lacovara J., Cramer E. B., Quigley J. P. Fibronectin enhancement of directed migration of B16 melanoma cells. Cancer Res. 1984 Apr;44(4):1657–1663. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Hinek A., Cleary E. G., Kucich U., Lee S. J., Rosenbloom J. Development of immunoreagents to ciliary zonules that react with protein components of elastic fiber microfibrils and with elastin-producing cells. Biochem Biophys Res Commun. 1988 Mar 15;151(2):822–826. doi: 10.1016/s0006-291x(88)80355-3. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Hinek A., Entwistle R., Wrenn D. S., Griffin G. L., Senior R. M. Elastin binds to a multifunctional 67-kilodalton peripheral membrane protein. Biochemistry. 1989 May 2;28(9):3716–3722. doi: 10.1021/bi00435a014. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Hinek A., Griffin G. L., Senior R. M., Liotta L. A. The elastin receptor shows structural and functional similarities to the 67-kDa tumor cell laminin receptor. J Biol Chem. 1989 Oct 5;264(28):16652–16657. [PubMed] [Google Scholar]
- Mecham R. P., Lange G. Antibodies to insoluble and solubilized elastin. Methods Enzymol. 1982;82(Pt A):744–759. doi: 10.1016/0076-6879(82)82099-5. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Scott L. J. Effects of endothelial removal and regeneration on smooth muscle glycosaminoglycan synthesis and growth in rat carotid artery in organ culture. Lab Invest. 1985 Apr;52(4):409–419. [PubMed] [Google Scholar]
- Osterlund E., Eronen I., Osterlund K., Vuento M. Secondary structure of human plasma fibronectin: conformational change induced by calf alveolar heparan sulfates. Biochemistry. 1985 May 21;24(11):2661–2667. doi: 10.1021/bi00332a011. [DOI] [PubMed] [Google Scholar]
- Prosser I. W., Whitehouse L. A., Parks W. C., Stahle-Bäckdahl M., Hinek A., Park P. W., Mecham R. P. Polyclonal antibodies to tropoelastin and the specific detection and measurement of tropoelastin in vitro. Connect Tissue Res. 1991;25(3-4):265–279. doi: 10.3109/03008209109029162. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Beharry S., Bothwell T., Jackowski G. Qualitative and quantitative differences in protein synthesis comparing fetal lamb ductus arteriosus endothelium and smooth muscle with cells from adjacent vascular sites. Dev Biol. 1988 Nov;130(1):250–258. doi: 10.1016/0012-1606(88)90431-9. [DOI] [PubMed] [Google Scholar]
- Robbins R. A., Wagner W. D., Sawyer L. M., Caterson B. Immunolocalization of proteoglycan types in aortas of pigeons with spontaneous or diet-induced atherosclerosis. Am J Pathol. 1989 Mar;134(3):615–626. [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P., Wrenn D. S., Prasad K. U., Urry D. W. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984 Sep;99(3):870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovich-Hunter L., Johnson D. J., Ranger P., Keeley F. W., Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest. 1988 Feb;58(2):184–195. [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Wagner W. D. Proteoglycan structure and function as related to atherosclerosis. Ann N Y Acad Sci. 1985;454:52–68. doi: 10.1111/j.1749-6632.1985.tb11844.x. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Curwen K. D., Litrenta M. M., Alonso D. R., Minick C. R. Effect of endothelium on glycosaminoglycan accumulation in injured rabbit aorta. Am J Pathol. 1983 Nov;113(2):156–164. [PMC free article] [PubMed] [Google Scholar]
- Wight T. N. Proteoglycans in pathological conditions: atherosclerosis. Fed Proc. 1985 Feb;44(2):381–385. [PubMed] [Google Scholar]
- Wight T. N. Vessel proteoglycans and thrombogenesis. Prog Hemost Thromb. 1980;5:1–39. [PubMed] [Google Scholar]
- Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res. 1970 Apr;26(4):507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- Wrenn D. S., Hinek A., Mecham R. P. Kinetics of receptor-mediated binding of tropoelastin to ligament fibroblasts. J Biol Chem. 1988 Feb 15;263(5):2280–2284. [PubMed] [Google Scholar]
- Yoder M. J., Baumann F. G., Grover-Johnson N. M., Brick I., Imparato A. M. A morphological study of early cellular changes in the closure of the rabbit ductus arteriosus. Anat Rec. 1978 Sep;192(1):19–39. doi: 10.1002/ar.1091920103. [DOI] [PubMed] [Google Scholar]