Abstract
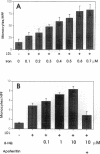
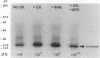
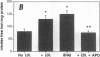
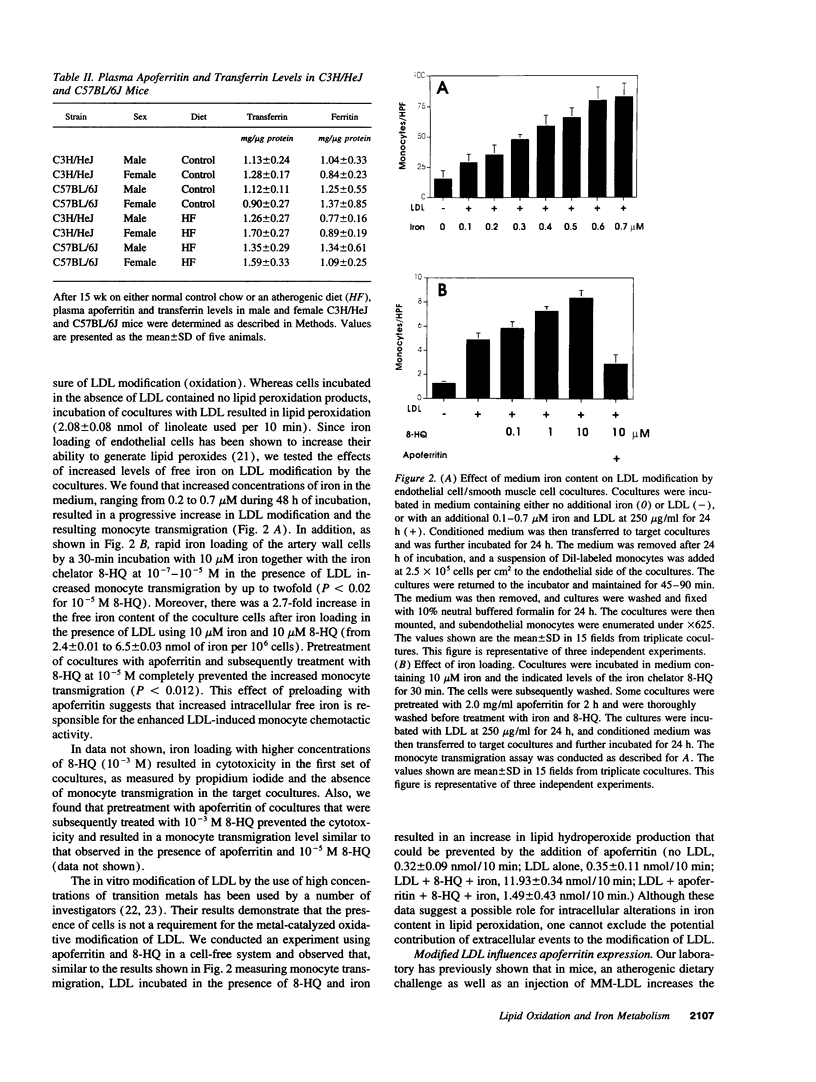
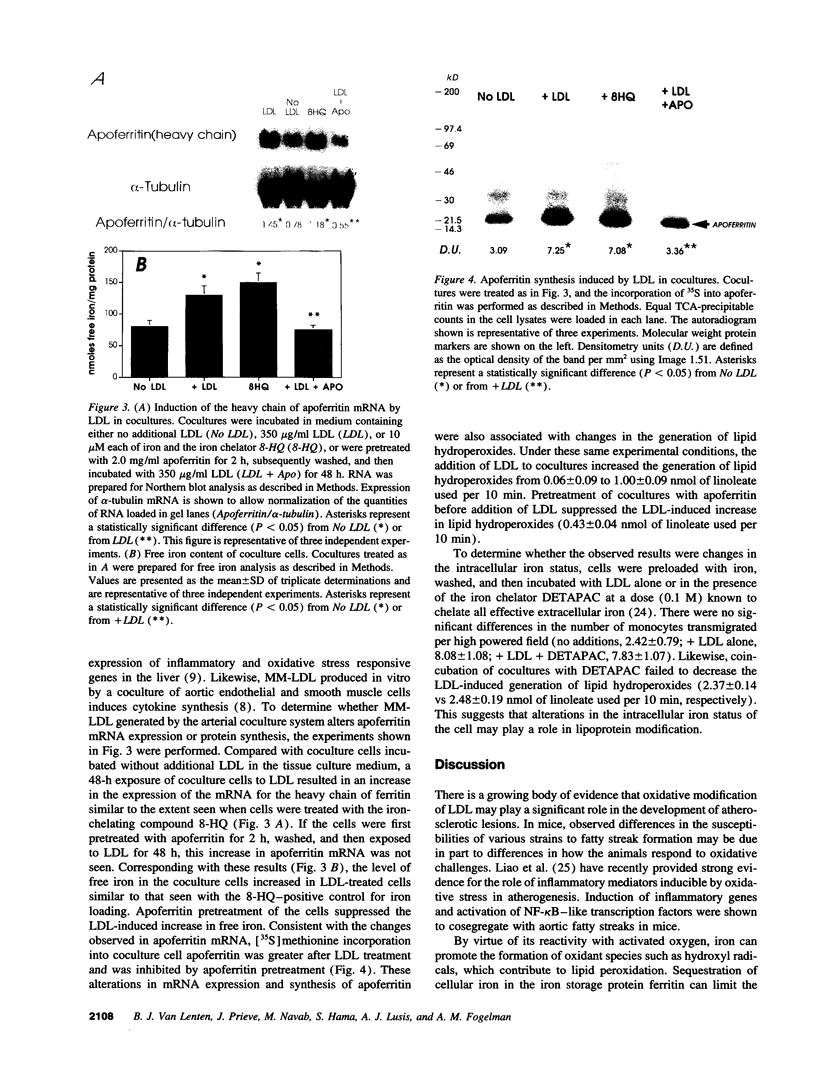
Iron promotes cellular damage via its capacity to catalyze hydroxyl radical formation and by peroxidation of unsaturated lipids. The major cellular iron storage depot, ferritin, acts as a critical antioxidant defense by sequestering unbound or "free" iron, limiting its participation in damaging oxidative reactions. In this study, we investigated the relationship between LDL modified by artery wall cells and the regulation of intracellular free iron levels in the mouse model and in a human aortic endothelial and smooth muscle cell coculture system. We found in response to an atherogenic diet, fatty streak-resistant C3H/HeJ mice exhibited higher levels of liver apoferritin and lower intracellular concentrations of free iron than did fatty streak-susceptible C57 BL/6J mice. Also, ferritin repressor protein mRNA was not significantly suppressed after 15 wk on the atherogenic diet in female C57BL/6J mice, which exhibit the most extensive fatty streak formation, but was significantly reduced in C3H/HeJ mice. Iron loading of coculture cells resulted in elevations of cellular free iron and enhanced LDL-induced monocyte transmigration. Pretreatment of cells with apoferritin completely abolished iron-induced LDL modification. Addition of LDL to cocultures resulted in elevations in lipid peroxidation products, intracellular free iron, apoferritin mRNA expression, and apoferritin synthesis, suggesting a possible relationship between the oxidative modification of LDL and iron metabolism.
Full text
PDF

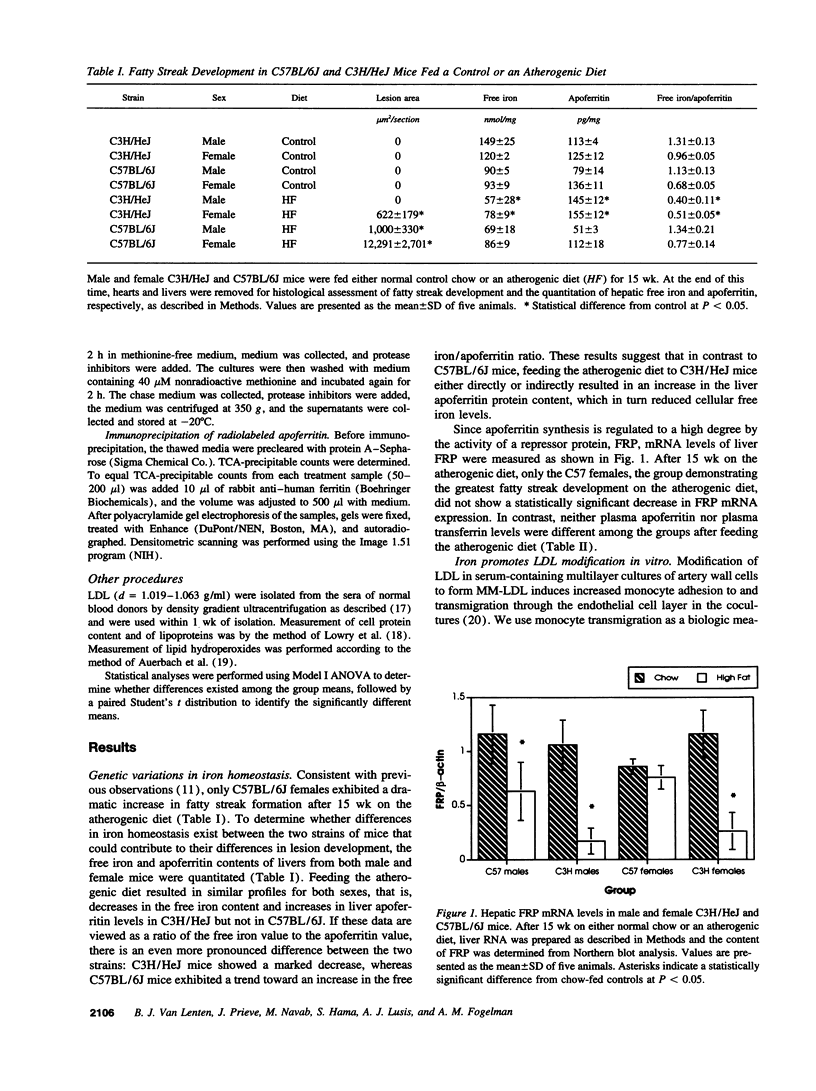


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach B. J., Kiely J. S., Cornicelli J. A. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem. 1992 Mar;201(2):375–380. doi: 10.1016/0003-2697(92)90354-a. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Bakker G. R., Boyer R. F. Iron incorporation into apoferritin. The role of apoferritin as a ferroxidase. J Biol Chem. 1986 Oct 5;261(28):13182–13185. [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Balla G., Vercellotti G. M., Eaton J. W., Jacob H. S. Iron loading of endothelial cells augments oxidant damage. J Lab Clin Med. 1990 Oct;116(4):546–554. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coccia E. M., Profita V., Fiorucci G., Romeo G., Affabris E., Testa U., Hentze M. W., Battistini A. Modulation of ferritin H-chain expression in Friend erythroleukemia cells: transcriptional and translational regulation by hemin. Mol Cell Biol. 1992 Jul;12(7):3015–3022. doi: 10.1128/mcb.12.7.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson R. M., Cleveland D. W. Ferritin synthesis is controlled by iron-dependent translational derepression and by changes in synthesis/transport of nuclear ferritin RNAs. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7613–7617. doi: 10.1073/pnas.90.16.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Elahi F., Sykes K., Van Lenten B. J., Territo M. C., Berliner J. A. Modification of the Recalde method for the isolation of human monocytes. J Lipid Res. 1988 Sep;29(9):1243–1247. [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao F., Andalibi A., Qiao J. H., Allayee H., Fogelman A. M., Lusis A. J. Genetic evidence for a common pathway mediating oxidative stress, inflammatory gene induction, and aortic fatty streak formation in mice. J Clin Invest. 1994 Aug;94(2):877–884. doi: 10.1172/JCI117409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F., Andalibi A., deBeer F. C., Fogelman A. M., Lusis A. J. Genetic control of inflammatory gene induction and NF-kappa B-like transcription factor activation in response to an atherogenic diet in mice. J Clin Invest. 1993 Jun;91(6):2572–2579. doi: 10.1172/JCI116495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F., Berliner J. A., Mehrabian M., Navab M., Demer L. L., Lusis A. J., Fogelman A. M. Minimally modified low density lipoprotein is biologically active in vivo in mice. J Clin Invest. 1991 Jun;87(6):2253–2257. doi: 10.1172/JCI115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau G., Chan L. M., Feng P. Increased ferritin gene expression is both promoted by cAMP and a marker of growth arrest in rabbit vascular smooth muscle cells. J Biol Chem. 1991 Oct 5;266(28):18819–18826. [PubMed] [Google Scholar]
- Miller L. L., Miller S. C., Torti S. V., Tsuji Y., Torti F. M. Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4946–4950. doi: 10.1073/pnas.88.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Stevenson L. W., Drinkwater D. C., Laks H., Fogelman A. M. Monocyte migration into the subendothelial space of a coculture of adult human aortic endothelial and smooth muscle cells. J Clin Invest. 1988 Dec;82(6):1853–1863. doi: 10.1172/JCI113802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Liao F., Hough G. P., Ross L. A., Van Lenten B. J., Rajavashisth T. B., Lusis A. J., Laks H., Drinkwater D. C., Fogelman A. M. Interaction of monocytes with cocultures of human aortic wall cells involves interleukins 1 and 6 with marked increases in connexin43 message. J Clin Invest. 1991 May;87(5):1763–1772. doi: 10.1172/JCI115195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter L. M., Sierra E. E., Ngo E. O. Heme oxygenase does not protect human cells against oxidant stress. J Lab Clin Med. 1994 Apr;123(4):506–514. [PubMed] [Google Scholar]
- Paigen B., Morrow A., Brandon C., Mitchell D., Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985 Oct;57(1):65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Puntarulo S., Cederbaum A. I. Stimulation of microsomal chemiluminescence by ferritin. Biochim Biophys Acta. 1993 May 7;1157(1):1–8. doi: 10.1016/0304-4165(93)90071-f. [DOI] [PubMed] [Google Scholar]
- Rogers J. T., Bridges K. R., Durmowicz G. P., Glass J., Auron P. E., Munro H. N. Translational control during the acute phase response. Ferritin synthesis in response to interleukin-1. J Biol Chem. 1990 Aug 25;265(24):14572–14578. [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Nyyssönen K., Korpela H., Tuomilehto J., Seppänen R., Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992 Sep;86(3):803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- Tang C. K., Chin J., Harford J. B., Klausner R. D., Rouault T. A. Iron regulates the activity of the iron-responsive element binding protein without changing its rate of synthesis or degradation. J Biol Chem. 1992 Dec 5;267(34):24466–24470. [PubMed] [Google Scholar]
- Tanner F. C., Noll G., Boulanger C. M., Lüscher T. F. Oxidized low density lipoproteins inhibit relaxations of porcine coronary arteries. Role of scavenger receptor and endothelium-derived nitric oxide. Circulation. 1991 Jun;83(6):2012–2020. doi: 10.1161/01.cir.83.6.2012. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goossen B., Doppler W., Fuchs D., Pantopoulos K., Werner-Felmayer G., Wachter H., Hentze M. W. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993 Sep;12(9):3651–3657. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk H. G., de Jong G. The physiology of iron, transferrin, and ferritin. Biol Trace Elem Res. 1992 Oct;35(1):13–24. doi: 10.1007/BF02786234. [DOI] [PubMed] [Google Scholar]
- van Lenten B. J., Fogelman A. M. Lipopolysaccharide-induced inhibition of scavenger receptor expression in human monocyte-macrophages is mediated through tumor necrosis factor-alpha. J Immunol. 1992 Jan 1;148(1):112–116. [PubMed] [Google Scholar]