ABSTRACT
Purpose: This paper explores ethical issues related to the involvement of children in health-related research through the application of a conceptual model (the Miller and Kenny framework) to a current clinical trial on casting protocols for equinus gait of children with cerebral palsy (CP).
Summary of key points: The direct involvement of children in health-related research is important for maintaining and improving standards of paediatric clinical care. Ethical considerations around investigations involving this highly vulnerable population are complex, however, requiring the involvement of many levels of decision makers—government, research ethics boards (REBs), health care providers, parents, and children. The Miller and Kenny framework is useful in distinguishing these levels and heightening awareness of the complexities of the issues around engaging children in research. Considerations include the role of parents/caregivers in decision making, individual assessment of the child's decisional capacities, close attention to the child's context and life experience, provision of developmentally appropriate information about the research study, and careful assessment of dissent prior to withdrawing the child from the study.
Recommendations: Physical therapists involved in paediatric clinical practice and/or research must be knowledgeable about ethical principles, policies, and REB requirements. The Miller and Kenny framework is a helpful guide to clarify decision-making roles around children's participation in research.
Key Words: assent, children, dissent, ethical issues, REB requirements, research participation
RÉSUMÉ
Objectif : Cet article se penche sur les questions qui entourent la participation des enfants à la recherche en santé par l'application d'un modèle conceptuel (la structure dite de Miller et Kenny) à un essai clinique en cours sur les méthodes de moulage de plâtres dans les cas de pied bot équin chez les enfants qui souffrent de paralysie cérébrale (PC).
Résumé des principaux points : La participation directe des enfants à la recherche en santé est importante pour le maintien et l'amélioration des normes liées à leurs soins cliniques. Les considérations éthiques en ce qui a trait aux recherches qui mettent à contribution ce segment très vulnérable de la population sont complexes et elles exigent l'engagement de décideurs à plusieurs échelons—gouvernements, comités d'éthique en recherche, fournisseurs de soins de santé, parents et enfants eux-mêmes. La structure de Miller et Kenny est utile pour établir une distinction entre ces divers paliers et pour accroître la sensibilisation aux complexités des enjeux liés à la participation des jeunes à la recherche. Parmi les considérations éthiques à prendre en compte, mentionnons le rôle des parents ou des personnes qui s'occupent de l'enfant dans la prise de décision, l'évaluation individuelle des capacités de l'enfant à prendre des décisions, une plus grande attention accordée au contexte dans lequel l'enfant se trouve et à son vécu, la communication d'informations appropriées sur le plan du développement concernant la recherche et une évaluation minutieuse des refus avant de retirer les enfants du projet.
Recommandations : Les physiothérapeutes en pratique clinique pédiatrique ou en recherche, ou les deux à la fois, devraient bien connaître les principes éthiques, les politiques et les exigences des comités d'éthique. La structure de Miller et Kenny constitue un guide utile pour clarifier les rôles dans la prise de décision entourant la participation des enfants à la recherche.
Mots clés : consentement, enfants, enjeux éthiques, exigences des comités d'éthique, participation à la recherche, refus
Introduction
Adherence to ethical principles is one of the most critical aspects of conducting clinical research. Physical therapy researchers should be well versed in national guidelines for ethical conduct and should be knowledgeable about guidelines in other countries when carrying out research involving international collaboration.1 Ethical considerations around investigations that involve children as subjects are arguably more complex than deliberations about adult involvement. The main dilemma is that children represent a highly vulnerable population because of their dependence on adults for care and protection, their relative lack of autonomy in decision making, and the real or perceived influence of authority figures on their decisions.2 In addition, harms inflicted on child research participants may have long-term consequences for their growth and development.3
Direct involvement of children in health-related research is essential to maintaining and improving standards of paediatric care. It is imprudent, and possibly dangerous, to presume that interventions studied in adults will be effective in paediatric populations, given the marked differences in biology, psychology, behaviour, and disease presentation between children and adults. In fact, a statement issued by the US National Institutes of Health encourages all researchers to address the inclusion or exclusion of children in grant proposals.4 Whenever possible, treatments that have been adequately evaluated in children should be the interventions of choice for child patients. Thus, in keeping with the principle of justice with respect to fairness, equity, and inclusiveness,1 there is an expectation that children share the burdens associated with participation in research.5 At the same time, it is necessary to protect children from inappropriate involvement in experimental procedures.
Any discussion of the ethics of involving human subjects of any age in clinical research must consider the distinction between research and treatment. In comparing research and treatment, Alderson3 noted that while the main goal of treating a patient is to improve that individual's current health status, the principal goal of conducting a study is to acquire knowledge that may benefit future health care. Miller and Kenny referred to the “moral tension” between the obligation to encourage research participation to promote sharing of potential benefits and the obligation to protect against possible harms of such participation.5 To enhance our understanding of the ethical challenges of paediatric research, Miller and Kenny developed a framework of levels of decision making that work synergistically to ensure that children are involved respectfully in appropriate research and protected from undue harms.5 In this article, we explore key ethical issues related to the involvement of children in research by applying this framework to a clinical trial we recently initiated. For each decision-making level, we briefly address roles and responsibilities and related ethical issues. Using this trial as an example, we hope to elucidate the complexities of decision-making processes in studies involving children.
The Framework
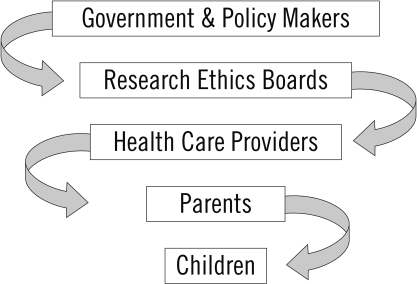
The Miller and Kenny framework (see Figure 1) guides researchers in distinguishing the roles and responsibilities at national, institutional, clinical, and individual levels for making decisions about the involvement of children in health-related research.5 Governments and policy makers, research ethics boards (REBs), and health care providers must work both independently of and in concert with parents or legal guardians (hereafter ‘parent(s)’) and children to safeguard the welfare of children involved in research. The distribution of responsibility differs by level—government and REBs are primarily concerned with protecting the collective well-being of their citizens, whereas health professionals, parents, and children themselves often focus their concerns on the individual child.
Figure 1.
Decision-making framework for the involvement of children in health-related research
The Clinical Trial
Recently we initiated a randomized controlled trial (RCT) in a tertiary-care children's hospital to compare the effectiveness of two casting protocols following botulinum toxin Type A (BTX-A) injections into the plantarflexors of children with cerebral palsy (CP). The design of this RCT was informed by a pilot feasibility study involving 10 children with CP.6 Criteria for participation in this ongoing trial include the following: 2–7 years of age, diagnosis of CP, Gross Motor Function Classification System level I or II,7 and equinus gait. One week after administration of BTX-A, participants are randomized to receive either a single cast for 3 weeks or a series of 3 casts applied at weekly intervals. Comprehensive assessments are carried out across domains of the International Classification of Functioning, Disability and Health (ICF)8 at baseline; directly post-casting; and at 1-month, 2-month, and 6-month follow-up. During the development of the assessment and intervention protocols, we struggled with the complexity of ethical issues related to children's participation in research. The Miller and Kenny framework helped us to clarify many of these issues, as will become evident in the following discussion.
The Role of Government as Policy Maker
Government, as the first decision-making level in the framework, oversees the development of public research policies in its role as guardian of its citizens. Particular emphasis is placed on protecting the most vulnerable citizens, including children. In Canada, the Tri-Council Policy Statement (TCPS) on Ethical Conduct for Research Involving Humans provides standards and procedures for conducting research involving human subjects.1 This joint policy was developed by three agencies created by acts of Parliament—the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Social Sciences and Humanities Research Council of Canada—to promote the ethical conduct of research involving Canadians.1 Key ethical principles underlying this policy include justice, respect, non-maleficence, beneficence, autonomy, and capacity. Justice, as mentioned above, requires the equitable distribution of study benefits and harms. Respect requires that (1) the dignity of participants be honoured, (2) those who can consent be given the opportunity to do so, (2) those with developing or diminished decisional capacity be protected, and (4) the privacy and confidentiality of participants be safeguarded.1 Non-maleficence refers to the intent to do no harm, whereas beneficence refers to the duty to do good.1
Autonomy and capacity are constructs closely associated with the consent process. Autonomy is the individual's independence in making ethical decisions and acting on them, whereas capacity is the ability to understand the information provided and the possible consequences of a decision, such as deciding whether to participate in a research study.1 The term “informed consent” is related to autonomy and capacity: consent refers to making a decision in a volitional, knowing, and rational manner, and without coercion, while informed means that the decision is based on knowledge of the situation and its potential consequences.9 In other words, the decision maker must demonstrate capacity in order to exercise the autonomy needed to provide informed consent. In the case of individuals who lack the capacity for autonomous decision making, the term “assent” (simply agreeing to participate), rather than “consent,” is used. Importantly, absence of dissent (unwillingness to participate) cannot be construed as assent.
Because our trial is being conducted in Canada, the ethical guidelines outlined in the TCPS must be followed.1 To ensure our understanding of these guidelines, the co-principal investigators were required to obtain TCSP certification. An online tutorial that prepares the candidate for certification is available free of charge at the Web site of the Panel on Research Ethics.10
The Role of the REB as Enforcer
In many countries, including Canada, the government delegates responsibility for interpreting, implementing, and monitoring government research policies to institutional REBs. REBs are entrusted to make certain that research that has the potential to be beneficial can proceed while at the same time safeguarding participants from unnecessary risks. Because REBs operate at the institutional level, their roles and responsibilities vary, depending on the local environment's demographics, needs, and resources. In other words, REBs contextualize the policies and related decision-making processes. Nevertheless, there are commonalities in roles across REBs, as summarized in Table 1.1,5,11
Table 1.
| For all clinical trial protocols, REBs must ensure that |
|
The REB must separately consider the risks associated with therapeutic and non-therapeutic procedures.11 Therapeutic procedures are interventions that have the potential to bestow a direct health benefit; they must be justified in terms of clinical equipoise,12 which means genuine uncertainty about the relative merits of each treatment arm in a clinical trial.3 In other words, the REB submission must convey unequivocally that there is insufficient evidence to favour either the experimental or the alternative procedure and must demonstrate that the research design has the potential to affect clinical practice by disturbing this state of “equipoise.” Non-therapeutic procedures, administered solely for research purposes, must be restricted to those that are necessary.13 Freedman et al. recommended that in the case of paediatric research, any non-therapeutic elements should fall in the category of “minor increase over minimal risk.”14(p.16)
Once the potential benefits and risks of therapeutic and non-therapeutic procedures have been assessed, the REB turns its attention to consent documentation (see Table 1). Public policies refer to the REB's role in upholding ethical principles with respect to consent of children participating in research, including the right to observe the assent process. However, the REB is not, and should not be, involved in deciding whether the risks posed by participation are acceptable to a particular child. Freedman, a noted bioethicist, has raised a general concern about unclear wording of research policies pertaining to the developing child.12 This lack of clarity has adversely influenced interpretation15 and has resulted in inconsistent application of guidelines by REBs. Simpson has argued that the TCPS policy on free and informed consent (section 2) fails to recognize the child's developing decisional capacities and recommended that the role of children in decision making about research participation be reconsidered.16 Similarly, Joffe et al. claimed that US federal regulations governing paediatric research (1) fail to provide guidelines on what constitutes meaningful assent and dissent, (2) do not require developmentally appropriate information in the assessment of decisional capacity, and (3) fall short in considering the complexity of the research project (e.g., whether the study involves healthy, acutely ill, or chronically ill children).17
Our REB submission outlined the benefits that could be derived from investigating the therapeutic procedures (i.e., single-casting vs. serial-casting protocols) in terms of enhancing mobility of children with CP and established that risks would be minimized and that the potential benefits outweighed the risks. To verify the clinical equipoise of the protocols, we conducted a systematic review of the literature;18 we concluded that while both single and serial casting procedures appear to be effective, their relative effectiveness has not been established. Therefore, we chose a trial design with two intervention arms (single and serial casts) but no placebo; in so doing, we resolved the potential conflict between the principles of beneficence and non-maleficence. The rigour of the research design, sample-size justification, and detailed protocols provided assurance that the findings could potentially disturb the current state of equipoise. Should the single-cast intervention prove to be as effective as or more effective than the serial-cast intervention, this conclusion would have significant ethical implications in terms of reducing future costs and time commitment on the part of patients, parents, and health care providers.
The non-therapeutic procedures proposed in our submission consisted of assessments of impairment (e.g., gait pattern, range of motion, muscle tone), activity (e.g., gross motor function, functional skills), participation (e.g., caregiver assistance, environmental modifications), and quality of life, to be repeated several times (at baseline; directly post-casting; and at 1-month, 2-month, and 6-month follow-up). Although the assessment protocol and schedule exceeded those of usual care, the condition of “minor increase over minimal risk” was deemed to be satisfied.
After reviewing our original application, the REB requested additional information about our approach to seeking assent. The REB suggested two options: (1) that we provide an age-appropriate information form that would explain the research procedures in plain language, or (2) that we outline an explicit process to provide the child with an oral explanation of the study. To decide between these options, we reviewed relevant literature and policies. Sample assent forms (option 1) are available for children 6 to 9 years of age19 and for children 6 to 12 years of age,4 and the reading level of these forms can be adjusted for other age groups. Ford et al.20 described a process whereby 6- to 12-year-old children were invited to participate in decision making about the content and language of the research information sheet and assent form. In support of the second option, Joffe et al.17 claimed that an assent discussion with written documentation is preferable to a signed assent form because a spoken exchange provides more flexibility to particular circumstances and eliminates the need for the child's signature, which may be neither appropriate nor possible.17
Ultimately, we chose to provide the REB with details of an assent process designed to be both interactive and iterative, guided by each child's level of comprehension and cognitive/emotional abilities. The information would be presented orally, using two simple and succinct scripts, one for children aged 2 to 4 years and the other for those aged 5 to 7 years. Each script contained developmentally appropriate information, augmented with photographs and pictures, about what the children would be expected to do, and why, and about the possible benefits of participating. To date, the REB has not exercised its right to observe the assent process.
The Role of Health Care Providers as Consultants
Only after the REB has approved the research protocol can individual children and their caregivers be approached about participating in the study. Health professionals play a consultative role, as opposed to a decisive role, with respect to the researcher by identifying children who meet the eligibility criteria rather than deciding whether a particular child should or will participate. Professionals involved in a child's care assist parents and families in deciding whether it is in the child's best interest to participate and, if so, the extent to which the child might participate in the decision-making process. Informed decisions about participation in research usually require the involvement of someone with a clear understanding of the research being proposed, particularly the important distinction between therapeutic and non-therapeutic procedures. Health care providers must also ensure that the parents and, if appropriate, the child understand the distinction as well.
Specific personal factors (e.g., physical, emotional, developmental, and cognitive status; personality; life experience) need to be considered in deciding whether a particular child's participation is warranted. Because attending health professionals often have rich knowledge of the particular child and family, they are regarded as invaluable resources in the decision-making process. Further, they are usually aware when a child (possibly unbeknownst to the research team) is participating in other studies. Since multiple enrolments can lead to cumulative risks and unreasonable expectations on the part of the participant and his or her family, careful consideration must be given to such situations.
Parents and children often make decisions based on the advice of their clinical caregivers. This trusting relationship can give rise to a conflict of interest on the part of the clinician who is conducting a study. Therefore, clinical researchers directly involved in the clinical care of a patient should not approach that patient to participate in their study. Furthermore, health professionals involved directly in research, as well as those who bear witness to the research process without direct involvement, have a responsibility to ensure that moral and procedural standards are upheld throughout the research process. In other words, any health professional who observes the research process unfolding has a role in monitoring the well-being of the participants and an obligation to report any concerns.
In the case of our trial, we recognized that an open, distributed recruitment strategy would be necessary to ensure fairness and to avoid real or perceived vulnerability or privilege in participant selection. We distributed the study protocol and eligibility criteria to the staff of the hospital's CP Clinic, who, in turn, began the process of identifying children who met the inclusion/exclusion criteria and whose families had expressed interest in the study and agreed to be contacted. A staff member not involved with the project continues to be responsible for carefully explaining the study's purpose and protocol to the parents and child and for answering all questions on the part of both child and parents. For those children and families who remain interested in participating, the processes of obtaining written consent from the parents and assent from the children are implemented, as discussed in the following sections.
The Role of Parents as Decision Makers
For children who are unable to provide informed consent, the ultimate decision for participation in research often rests with their parents. In such cases, parents are granted wide discretion in decision making because they are assumed to have the child's best interests in mind. The TCPS guidelines do not define the term “parent,” but reference is made in article 2.1 to “parents, guardians or authorized third parties.”1 If the parent, after carefully weighing the risks and potential benefits of participation, agrees that participation is appropriate, voluntary written consent must be obtained. Procuring informed consent is often construed as the hallmark of what makes clinical research ethical; however, as is apparent in Miller and Kenny's framework (see Figure 1), this procedure is only one step in the complex decision-making process.
Shared decision making is a developmental approach in which the parent guides the child in deciding whether or not to participate, thereby facilitating mutual agreement while allowing the child to act somewhat autonomously.21,22 It is worth noting, however, that whereas Western society tends to embrace the notion of an independent self, non-Western cultures may view decision making about research participation as more of a collective responsibility.17 The research team must therefore be sensitive to the cultural background of participants and their families and must adjust the consent and assent processes accordingly.
With respect to our study, we have implemented several procedures to ensure the ongoing integrity of the trial from the parents' perspective. First, parents are invited to ask questions during the process of obtaining written consent, in order to clarify their understanding of risks and benefits, alternatives to participation, and obligations subsumed by agreeing to participate. In addition, parents are encouraged to engage actively in shared decision making through open and frank discussion with their child. After completion of the baseline assessment, transparency of group allocation from the parent's perspective is achieved by having the parent open a sealed envelope containing the child's random assignment, as determined by a computerized random number generator. Further, to ease the burden of participating in the trial, parents' travel expenses are reimbursed and casting materials are provided free of charge; an institutional grant provided funding to cover these costs. Finally, parents are given an opportunity to provide formal feedback on the research process through a questionnaire consisting of eight Likert-type questions (e.g., “On a scale from 1–5, with 1 being very dissatisfied and 5 being very satisfied, how satisfied were you about the explanation about the research project?, the consent process?, quality of care that your child received?”) and four open-ended questions (e.g., “How convenient was it for you to come to the Centre for the casting procedure?”)
Each of the eight parents approached thus far has been willing to participate and has provided written consent. The feedback received from them has been positive.
The Role of Children in Decision Making
The legitimate role of children in making decisions about involvement in research is becoming increasingly recognized.23 In fact, it is now standard practice to seek the child's agreement to participate (i.e., assent) when the child is deemed capable of making such a decision. Respect for the autonomy of vulnerable participants is clearly articulated in article 2.10 of the TCPS guidelines: “Where free and informed consent has been obtained from an authorized third party, and in those circumstances where the legally incompetent individual understands the nature and consequences of the research, the researcher shall seek to ascertain the wishes of the individual concerning participation.”1
Research policies and previous studies provide inconsistent guidance on the issue of children's capacity to assent.20 The TCPS mandates that assent be sought from vulnerable populations whose capacity for judgement and self-direction is maturing (e.g., most children) or only partially developed (e.g., those suffering permanent cognitive impairment).1 Disparity exists in recommendations of the minimum age to seek assent. Whittle et al. reported that 20% of American institutional review boards surveyed stipulate 7 years as the age of assent, whereas 47% leave determination of the minimum age to the judgement of the investigator.24 The criteria endorsed by various ethicists span several ages: 7 years,25 9 years,17 11 years,26 and 14 years.27 These discrepancies are due largely to differences in the interpretation of what constitutes assent. Proponents of a high minimum age believe that assent should be restricted to those capable of autonomous decision making; for example, in supporting an age limit of 14 years, Wendler and Shah argued that the assent process should be restricted to those able to make autonomous decisions and understand altruistic behaviour.27 In contrast, others regard the assent process as more of a continuum, with different levels of assent based on the individual child's capacities; for example, Vitiello contended that some children as young as 7 are capable of understanding the essential elements of research participation and of providing assent.25
Miller and Nelson22 favoured assessing each child individually rather than defining an age limit, stating that, unlike consent, “assent is not meant to be an autonomous decision but functions as a way to provide children with the opportunity to choose to the extent that they are able.”22(p.23) Consistent with this perspective, Simpson16 described five categories of children, not in terms of age ranges but, rather, in terms of their developing decisional capacity (see Appendix). This categorization helps to operationalize aspects of the Miller and Kenny framework by linking each category to specific roles for the REB, parent, and child with respect to the child's participation in research. Unlike the Miller and Kenny framework, however, Simpson's categorization also includes the role of the researcher.
Indicators of decisional capacity to assent, in addition to age and developmental level, include health status, emotional well-being, personality, social competence, emotional maturity, previous experience with research, and complexity of the research.28,29 An understanding of the family's cultural background is also helpful.15 In some cases, life experience is thought to have a greater impact than age on the child's ability to understand research participation.30 Chronicity of the disease process is another consideration; Alderson3 observed that children aged 3 and 4 years with chronic conditions such as Type 1 diabetes and cystic fibrosis can act responsibly when adults are not present. Indeed, it has been reported that many very young children with long-term conditions demonstrate altruism in deciding to participate in research.31
Honouring a child's unwillingness to participate (i.e., dissent) is as important a consideration as seeking assent. Neill contended that for the child who can give a reasoned argument not to participate, decision-making capacity is deemed sufficient for dissent, and this decision should be respected.30 Nevertheless, support for respecting children's dissent is not universal. Whereas the TCPS indicates that dissent is binding,1 it has also been argued that honouring the dissent of a child with diminished decisional capacity may undermine the parent's responsibilities as a third-party decision maker15 or may have a negative effect on a parent whose culture does not share the Western emphasis on autonomy.17 Wendler32 proposed that dissent by young children may reflect only temporary distress, and thus parents and researchers should “stop, assess and address” the situation rather than withdrawing the child from the study.32 Simpson16 proposed that “protest” be used instead of “dissent” when referring to children with some language comprehension but limited decisional capacity,16 reasoning that the parent, who is ultimately responsible for the child's research participation, should judge the protest based on the harm–benefit ratio before deciding whether to override the child's objections.16 Alternatively, an investigator may remove the child from the study despite the parent's objection if it is felt that more harm may come from ignoring the child's protests.16
Once recruited into the trial, participants must continue to be treated with respect. Conditions to maintain the integrity of the study include an open invitation to ask questions, protection of privacy, constant monitoring of the participant's well-being, and ongoing opportunities to withdraw.
Given the young age of the participants in our trial (2–7 years) and the possibility of cognitive and communication delays, we grappled with selecting criteria to assess the child's capacity to assent. All potential participants had a chronic disability (CP) and, as such, would be familiar with the hospital environment and would have “consented” previously to physiotherapy and occupational therapy interventions. Therefore, we concluded that, despite their young age, all children meeting the eligibility requirements should be given the opportunity to provide assent. The children recruited to date have been in the second category of Simpson's classification (Some language comprehension, Limited decisional capacity; see Appendix). Because their role is deemed to be “receptive” as opposed to “decisional,”16 we informed these participants about the project using one of the two REB-approved scripts and then asked them about their willingness to participate, making it very clear that they were not obliged to do so and that if they did decide to participate, they could withdraw at any time.
To date, all children approached for the current trial have given their assent to participate. The information in the assent scripts has been geared to the level of comprehension of two age groups and is simple and concrete. We have found a need to use a combination of scripts on some occasions, depending on the individual child's level of understanding. In addition, we have used pictures and photographs to complement the information that is provided verbally. Thus far, the children have responded well to this combination. The pictures and photographs have served to keep them engaged in the process. Parents have been present during the assent process; where possible, they have remained at a distance, but within sight of the child, in order to minimize the effect of parental influence on the child.
The consent process, assessment protocol, and casting protocols have been well accepted, and there have been no adverse events. One 6-year-old participant with spastic diplegia, refused to complete the baseline gait analysis and became quite upset; rather than immediately withdrawing him from the study, we applied Wendler's “stop, assess and address” strategy,32 allowing time for him to calm down and discuss his concerns. Despite his continued resistance to the gait analysis, he agreed to cooperate with the rest of the baseline assessment. Therefore, his dissent was honoured and he remained in the study.
Future Directions and Research
There is a paucity of research in the field of bioethics pertaining specifically to physical rehabilitation. Probing ethical issues that underlie the unique interactions between the physical therapist and patient—paediatric or adult—would enhance the understanding of clinicians and researchers alike. Future directions for investigating involvement of children in physical therapy research could include an exploration of the degree to which potential participants (particularly young children or children with impaired communication) understand the assent processes used by physical therapy researchers. Another important contribution would be to compare levels of understanding between parents and paediatric research participants.
Conclusion
Using the Miller and Kenny framework1 as a conceptual model and a current clinical trial as an example, we have explored ethical considerations applicable to each level of decision making with respect to children's participation in research—from government to the individual child. Clinical investigators involved in research with children must be cognizant of ethical policies and REB requirements relevant to this vulnerable population. Although the ideal approach to obtaining the participant's assent remains unclear, there is agreement about the importance of involving children in research, the role of parents/caregivers in decision-making, assessment of the child's decisional capacities, attention to the child's context and life experience, provision of developmentally appropriate information about the research study, and assessment of dissent prior to withdrawing the child from the study.
KEY MESSAGES
What Is Already Known on This Topic
The direct involvement of children in health-related research is important for improving standards of paediatric clinical care. Ethical considerations relating to investigations that involve children as subjects are complex, and require the involvement of government, REBs, health care providers, parents, and children.
What This Study Adds
The Miller and Kenny framework heightens awareness of the complexity of issues around engaging children in research. The framework distinguishes roles and responsibilities at national, institutional, clinical, and individual levels for making decisions about children's participation in research.
Appendix: Decision-Making Rules with Respect to Children's Participation in Research
| Category of Children | REB's Role | Parent's Role | Researcher's Role | Child's Role |
|---|---|---|---|---|
|
(1) Protect children's interests by approving research that meets specified preconditions. (2) Require full disclosure to parents and their authorization. | (1) Exercise full decision-making authority based on benefit/harm ratio. (2) Be present/available during research or delegate responsibilities to another. (3) Withdraw child if benefit/harm ratio becomes unfavourable. | (1) Ensure full disclosure to parents about participation. (2) Withdraw child if benefit/harm ratio becomes unfavourable. (3) Can override parental decision re: issue of withdrawal. | (1) Has no decision-making authority. (2) May protest, but this does not preclude participation. |
|
(1) and (2)(3) Require that relevant information be shared with child. (4) Require discussion with and documented authorization by child and parents. | (1), (2), and (3)(4) Share information with child about participation and address questions. (5) Heed child's protest. | (1), (2), and (3)(4) Ensure relevant disclosure to child about participation and address child's questions. (5) Heed child's protest. | (1) and (2)(3) Has limited role in decision-making process (receptive). (4) May ask/answer questions. |
|
(1), (2), (3), and (4)(5) Respect/defend that child's protest can be authoritative in some cases. | (2), (3), and (4)(6) Parental agreement is always required; in some instances, a child's protest is authoritative. (7) Give child opportunity to express wishes and take these into account in assessing benefit/harm ratio. (8) Limited decision-making authority | (1), (2), (3), and (4)(6) Ensure that child is given opportunity to express his/her wishes. (7) Ensure that parents are taking child's wishes into account. | (5) Has an increasing role in decision-making process, including limited decision-making authority. (6) Agreement to participate is necessary, but not sufficient. (7) Protest is authoritative, precluding participation except when parents judge that potential benefit outweighs potential harm and the additional harm of overriding child's preference(s). |
|
(6) Recognize children's decision-making ability. (7) Approve research that meets specified preconditions. (8) Ensure full disclosure to child and, when necessary, to parents. (9) Require documented consent of child and, when necessary, parental agreement. | (8)(9) Parental agreement is sometimes necessary for participation (e.g., when legally required, when participation has consequences for parents, or when there is more than minimal risk), but it is never sufficient. | (8) Ensure full disclosure to child about participation. (9) Determine if child's consent is sufficient or if parental agreement is also required. (10) Ensure relevant disclosure to parents about participation when legitimately involved in decision making. (11) Discuss withdrawal with child if benefit/harm ratio becomes unfavourable. | (8) Has significant decision-making authority. (9) Consent to participate is necessary and sometimes sufficient (parental agreement is sometimes necessary). (10) Refusal is always authoritative (no exceptions). |
|
(10) Obligations for research involving legally competent adults apply. | (10) Have no decision-making authority. | (12) Obligations for research involving legally competent adults apply. | (11) Has full decision-making authority (consent/refusal). (12) Is not obliged to share information with parents re: participation. |
Adapted from Simpson16
Kelly B, MacKay-Lyons MJ. Ethics of involving children in health-related research: applying a decision-making framework to a clinical trial. Physiother Can. 2010;62:338–346
References
- 1.Canadian Institutes of Health Research; Natural Sciences and Engineering Research Council of Canada; Social Sciences and Humanities Research Council of Canada. Tri-Council policy statement: ethical conduct for research involving humans. 1998 (with 2000, 2002 and 2005 amendments) [Internet] Ottawa: Public Works and Government Services Canada; 2005. [cited 2009 Oct 4]. Available from: http://www.pre.ethics.gc.ca/policy-politique/tcps-eptc/docs/TCPS%20October%202005_E.pdf. [Google Scholar]
- 2.Kipnis K. Seven vulnerabilities in the pediatric research subject. Theor Med Bioeth. 2003;24:107–20. doi: 10.1023/a:1024646912928. [DOI] [PubMed] [Google Scholar]
- 3.Alderson P. Competent children? minors' consent to health care treatment and research. Soc Sci Med. 2007;65:2272–83. doi: 10.1016/j.socscimed.2007.08.005. doi: 10.1016/j.socscimed.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Broome M. Consent (assent) for research with pediatric patients. Semin Oncol Nurs. 1999;15:96–103. doi: 10.1016/s0749-2081(99)80067-9. doi: 10.1016/S0749-2081(99)80067-9. [DOI] [PubMed] [Google Scholar]
- 5.Miller P, Kenny N. Walking the moral tightrope: respecting and protecting children in health-related research. Camb Q Healthc Ethics. 2002;11:217–29. doi: 10.1017/s096318010211303x. doi: 10.1017/S096318010211303X. [DOI] [PubMed] [Google Scholar]
- 6.Kelly B, MacKay-Lyons M, Berryman S, Hyndman J, Wood E. Assessment protocol for serial casting after Botulinum toxin injections to treat equinus gait. Pediatr Phys Ther. 2008;20:233–41. doi: 10.1097/PEP.0b013e3181825c1b. doi: 10.1097/PEP.0b013e3181825c1b. [DOI] [PubMed] [Google Scholar]
- 7.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 8.Unstün T, Chatterji S, Bickenback J, Kostanjsek N, Schneider M. The International Classification of Functioning, Disability and Health: a new tool for understanding disability and health. Disabil Rehabil. 2003;25:565–71. doi: 10.1080/0963828031000137063. doi: 10.1080/0963828031000137063. [DOI] [PubMed] [Google Scholar]
- 9.Scott E. Judgment and reasoning in adolescent decision making. Villanova Law Rev. 1992;37:1607–70. [PubMed] [Google Scholar]
- 10.Interagency Advisory Panel on Research Ethics. Introductory tutorial for the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS) [Internet] Ottawa: The Panel; c2009. [updated 2009 Aug 26; cited 2010 Jul 9]. Available from: http://pre.ethics.gc.ca/english/tutorial/ [Google Scholar]
- 11.Freedman B, Fuks A, Weijer C. Demarcating research and treatment: a systematic approach for the analysis of the ethics of clinical research. Clin Res. 1992;40:653–60. [PubMed] [Google Scholar]
- 12.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–5. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 13.Weijer C. Thinking clearly about research risk: implications of the work of Benjamin Freedman. IRB: Ethics Hum Res. 1999;21:1–5. doi: 10.2307/3564450. [PubMed] [Google Scholar]
- 14.Freedman B, Fuks A, Weijer C. In loco parentis: minimal risk as an ethical threshold for research upon children. Hastings Cent Rep. 1993;23:13–9. doi: 10.2307/3562813. [PubMed] [Google Scholar]
- 15.Baylis F, Downie J, Kenny N. Children and decision making in health research. IRB: Ethics Hum Res. 1999;21:5–10. [PubMed] [Google Scholar]
- 16.Simpson C. Children and research participation: who makes what decisions? Health Law Rev. 2003;11:20–9. [PubMed] [Google Scholar]
- 17.Joffe S, Fernandez C, Pentz R, Ungar D, Matthew N, Turner C, et al. Involving children with cancer in decision-making about research participation. J Pediatr. 2006;149:862–8. doi: 10.1016/j.jpeds.2006.08.027. doi: 10.1016/j.jpeds.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Kelly B, MacKay-Lyons M, Ruggles T, Woodward J. Botulinum toxin type A and serial casting versus botulinum toxin type A or serial casting in treating equinus gait of children with cerebral palsy. Cochrane Db Syst Rev. 2008;3 doi: 10.1002/14651858.CD007343. [Google Scholar]
- 19.Lindeke L, Hauck M, Tanner M. Practical issues in obtaining child assent for research. J Pediatr Nurs. 2000;15:99–104. doi: 10.1053/jn.2000.5447. doi: 10.1053/jn.2000.5447. [DOI] [PubMed] [Google Scholar]
- 20.Ford K, Sankey J, Crisp J. Development of children's assent documents using a child-centred approach. J Child Health Care. 2007;11:19–28. doi: 10.1177/1367493507073058. doi: 10.1177/1367493507073058. [DOI] [PubMed] [Google Scholar]
- 21.Leikin S. Minors' assent, consent, or dissent to medical research. IRB: Ethics Hum Res. 1993;15:1–7. doi: 10.2307/3564579. [PubMed] [Google Scholar]
- 22.Miller V, Nelson R. A developmental approach to child assent for nontherapeutic research. J Pediatr. 2006;149:S25–30. doi: 10.1016/j.jpeds.2006.04.047. doi: 10.1016/j.jpeds.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Spencer G. Children's competency to consent: an ethical dilemma. J Child Health Care. 2000;4:117–22. doi: 10.1177/136749350000400305. doi: 10.1177/136749350000400305. [DOI] [PubMed] [Google Scholar]
- 24.Whittle A, Shah S, Wilfond B, Gensler G, Wendler D. Institutional review board practices regarding assent in pediatric research. Pediatrics. 2004;113:1747–52. doi: 10.1542/peds.113.6.1747. doi: 10.1542/peds.113.6.1747. [DOI] [PubMed] [Google Scholar]
- 25.Vitiello B. Ethical considerations in psychopharmacological research involving children and adolescents. Psychopharmacology (Berl) 2003;171:86–91. doi: 10.1007/s00213-003-1400-7. doi: 10.1007/s00213-003-1400-7. [DOI] [PubMed] [Google Scholar]
- 26.Tait A, Voepel-Lewis T, Malviya S. Do they understand? part II: assent of children participating in clinical anesthesia and surgery research. Anesthesiology. 2003;98:609–14. doi: 10.1097/00000542-200303000-00006. doi: 10.1097/01.sa.0000108447.59995.bb. [DOI] [PubMed] [Google Scholar]
- 27.Wendler D, Shah S. Should children decide whether they are enrolled in nonbeneficial research? Am J Bioeth. 2003;3:1–7. doi: 10.1162/152651603322614382. doi: 10.1162/152651603322614382. [DOI] [PubMed] [Google Scholar]
- 28.Meaux J, Bell P. Balancing recruitment and protection: children as research subjects. Iss Comp Pediatr Nurs. 2001;24:241–51. doi: 10.1080/014608601753260335. doi: 10.1080/014608601753260335. [DOI] [PubMed] [Google Scholar]
- 29.Broome M, Richards D, Hall J. Children in research: the experience of ill children and adolescents. J Family Nurs. 2001;7:32–49. doi: 10.1177/107484070100700103. [Google Scholar]
- 30.Neill S. Research with children: a critical review of the guidelines. J Child Health Care. 2005;9:46–58. doi: 10.1177/1367493505049646. doi: 10.1177/1367493505049646. [DOI] [PubMed] [Google Scholar]
- 31.Nelson R, Reynolds W. We should reject passive resignation in favor of requiring the assent of younger children for participation in non-beneficial research. Am J Bioethics. 2003;3:11–3. doi: 10.1162/152651603322614418. [DOI] [PubMed] [Google Scholar]
- 32.Wendler D. Assent in paediatric research: theoretical and practical considerations. J Med Ethics. 2006;32:229–34. doi: 10.1136/jme.2004.011114. doi: 10.1136/jme.2004.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]