ABSTRACT
Purpose: The purpose of this study was to explore the effect of modified constraint-induced movement therapy (CIMT) in a real-world clinical setting on spasticity and functional use of the affected arm and hand in patients with spastic chronic hemiplegia.
Method: A prospective consecutive quasi-experimental study design was used. Twenty patients with spastic hemiplegia (aged 22–67 years) were tested before and after 2-week modified CIMT in an outpatient rehabilitation clinic and at 6 months. The Modified Ashworth Scale (MAS), active range of motion (AROM), grip strength, Motor Activity Log (MAL), Sollerman hand function test, and Box and Block Test (BBT) were used as outcome measures.
Results: Reductions (p<0.05–0.001) in spasticity (MAS) were seen both after the 2-week training period and at 6-month follow-up. Improvements were also seen in AROM (median change of elbow extension 5°, dorsiflexion of hand 10°), grip strength (20 Newton), and functional use after the 2-week training period (MAL: 1 point; Sollerman test: 8 points; BBT: 4 blocks). The improvements persisted at 6-month follow-up, except for scores on the Sollerman hand function test, which improved further.
Conclusion: Our study suggests that modified CIMT in an outpatient clinic may reduce spasticity and increase functional use of the affected arm in spastic chronic hemiplegia, with improvements persisting at 6 months.
Key Words: exercise programme, motor control, spasticity, stroke, upper extremity (arm)
RÉSUMÉ
Objectif : L'objectif de cette étude est de se pencher sur les effets d'une thérapie du mouvement induit par la contrainte (CIMT) dans un cadre clinique réel sur la spasticité et l'utilisation fonctionnelle du bras ou de la main affectés chez les patients avec hémiplégie spastique chronique.
Méthode : Un modèle d'étude consécutive quasi expérimentale a été utilisé. Un échantillon de 20 patients avec hémiplégie spastique (de 22 à 67 ans) a été testé avant et après une thérapie du mouvement induit de deux semaines dans une clinique de réadaptation externe, et six mois plus tard. L'échelle modifiée d'Ashworth (EMA), le mouvement actif des articulations (AROM), la force de préhension, la mesure de l'activité motrice, le test de Sollerman pour la fonction de la main et le Box and Block Test (BBT) ont été utilisés comme mesures de résultats.
Résultats : Des réductions (p<0,05-0,001) de la spasticité (MAS) ont été observées tant après une période d'entraînement de deux semaines que lors d'un suivi après six mois. Des améliorations ont également été notées dans le mouvement actif des articulations (changement médian de 5° de l'extension du coude, flexion dorsale de la main de 10°), force de préhension (20 newtons) et utilisation fonctionnelle après la période d'entraînement de deux semaines (EMA : 1 point; test de Sollerman : 8 points; BBT : 4 blocs). Ces améliorations étaient encore présentes lors du suivi après six mois, sauf pour la note obtenue lors du test de Sollerman, qui a continué de s'améliorer.
Conclusion : Notre étude semble indiquer qu'une CIMT modifiée dans une clinique externe peut réduire la spasticité et accroître l'utilisation fonctionnelle du bras affecté dans les cas d'hémiplégie spastique chronique, et que ces améliorations peuvent persister après six mois.
Mots clés : accident vasculaire cérébral, contrôle moteur, membres supérieurs (bras), programme d'exercices, spasticité
INTRODUCTION
Motor deficits are common following stroke. Approximately 43% to 69% of people suffering from a stroke have upper-extremity impairment,1,2 and 4 years after a stroke, 67% still experience non-use of the affected arm as a major problem.1 One of the features of motor deficits after a stroke is spasticity. Spasticity develops slowly, peaking 1 to 4 months after onset of stroke.3 Watkins et al.4 found that 1 year after stroke, 38% of patients demonstrated spasticity.
The most widely accepted definition of spasticity is “a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (‘muscle tone’) with exaggerated tendon jerks, resulting from hyperexcitablility of the stretch reflex, as one component of the upper motor neuron syndrome.”5(p.606) Spasticity is thought to influence post-stroke body function, activity limitations, and participation restrictions.6 Sommerfeld et al. found that 3 months after stroke, patients with spasticity had lower motor and activity performance than patients who were non-spastic.3
There is no consensus on which interventions best reduce spasticity and improve functional use of the affected arm in the chronic stage after stroke. Many interventions traditionally used to treat spasticity are directed toward the positive features of spasticity—hyperreflexia, resistance to passive movement, and clonus.7,8 However, these interventions are not always associated with clear gains in functional use.7 Both Bhakta7 and Ada et al.8 have argued that the negative features of spasticity (i.e., weakness and loss of skill) are the major barriers to improved function. Neuro-rehabilitation interventions have moved in recent years toward therapies that engage the patient in some form of voluntary practice with the impaired side.9 Exercise training programmes directed toward negative features of spasticity, with enhanced frequency and repetitive training of one movement, reduce spasticity and improve functional use of the arm and hand after the training period.10,11 Butefisch et al.10 used repetitive movements for 30 minutes per day for 2 weeks, and Diserens et al.11 for 30 minutes per day, 5 days a week, for 3 weeks. Neither study included follow-up testing, however, so it is not clear whether the treatment effects persisted. Bhakta7 argued that interventions directed at single-motor symptoms are not likely to result in functional benefit. Therefore, an intensive exercise training programme that consists of repetitive training of varied movements and functional tasks might be used to reduce spasticity and improve functional use in the affected arm.
Constraint-induced movement therapy (CIMT),12 which includes intensive training with various exercises and can be used with chronic patients with stroke, involves intensive training of the impaired upper extremity and restricting movements of the unaffected limb for 6 hours per day, 5 days a week, for 2 weeks.12,13 The principles of this method are derived from theories in behavioural psychology, motor learning, and skills acquisition.9 CIMT increases motor skill and use of the affected arm and hand in daily activities after stroke, and the increased daily use persists for at least 2 years.12–15 However, the studies referred to here were performed in a laboratory setting and did not investigate the effect of CIMT on spasticity.
Dettmers et al.16 studied the effect of a distributed form of CIMT in chronic patients with stroke at a neuro-rehabilitation clinic. As well as improved functional outcome and quality of life, Dettmers et al. observed reduced spasticity, and the improvements persisted 6 months later. Levy et al.17 and Sun et al.18 found that CIMT in combination with Botulinum toxin A tended to improve function for patients with spasticity problems. Blanton et al.19 and Bonaiuti et al.20 have argued in recent reviews that further studies of the effectiveness of CIMT and how it may be translated into clinical contexts are needed. Furthermore, the effect of CIMT on spasticity warrants investigation.
The purpose of the present study was to explore the effect of modified CIMT, using intensive and varied exercise training, on spasticity and functional use of the affected arm and hand in patients of working age with spastic hemiplegia more than 6 months after stroke. Another goal was to study the effectiveness of modified CIMT in a real-world clinical setting. The study addressed the following research question: Does modified CIMT in an outpatient rehabilitation clinic reduce spasticity and increase functional use of the affected arm and hand?
METHODS
Design
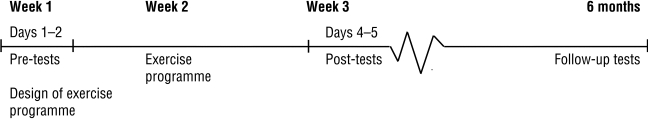
The study used a prospective consecutive quasi-experimental design. Patients were tested before and after a 2-week training period and again 6 months later. Taub et al.'s model for CIMT in post-stroke rehabilitation13 was modified slightly for use in a multi-disciplinary outpatient rehabilitation clinic.21 One occupational therapist and one physiotherapist organized the exercises; only one CIMT patient was treated at a time, but these patients carried out the CIMT exercises among other outpatients in the clinic. The programme modifications also included intensive and varied exercise training geared toward the negative symptoms of spasticity—exercises of strength, coordination, and speed. The exercises were arranged according to each patient's phase of motor learning. The Motor Activity Log (MAL) was assessed before and after the training period, but not every day.
Figure 1.
Study design
Participants
Patients with stroke were referred to the rehabilitation clinic where the present study was conducted if they were of working age (between 18 and 67 years old). Of these patients, all those who fulfilled the inclusion criteria for the training model (see below) between August 2000 and September 2004 (n=20) were included in the study. The inclusion criteria were similar to those specified by Taub et al.13 All patients completed the 2-week training programme and the 6-month follow-up. These patients had previously received primary rehabilitation at the same clinic or had been referred from other clinics.
The inclusion criteria were the following:
Reduced ability to use the hemi-paretic arm
Six months or more since stroke (patient had completed primary rehabilitation and was currently living at home)
Ability to actively extend the wrist at least 20° and to extend the metacarpophalangeal and the interphalangeal joints 10° (ROM was measured from the resting position of the hand for each patient)
Ability to walk and balance safely, without using the non-affected hand, with or without gait aid (patients who used a wheelchair had to be able to operate the wheelchair with their feet)
Absence of any serious cognitive deficit or uncontrolled medical problem believed to negatively affect participation during the training period (this criterion was evaluated after consultation with the therapists and the doctor in charge)
Ability to understand the content of the training period and motivation to participate
An additional inclusion criterion relating to spasticity was added: a minimum spasticity score of 1/5 on the Modified Ashworth Scale (MAS) for wrist flexors and/or elbow flexors.
Patients were excluded from the study if they were experiencing arm pain that was believed to affect exercise intensity.
Measurements
Spasticity in elbow and wrist flexors was measured using the MAS,22 which measures the resistance to passive movement of a relaxed group of muscles and characterizes change in muscle tone. The Ashworth scale was originally developed with five grades (0–4) as a tool to measure tone of the limbs and was modified by Bohannon and Smith to six grades to make it more sensitive (0=no increase in spasticity, 5=affected part is rigid in flexion or extension).6,22 The MAS has been tested for reliability with stroke patients; the scale's inter- and intrarater reliability for elbow and wrist flexors is good to very good (κw=0.73–0.96 for interrater reliability; κw=0.77–0.94 for intrarater reliability).23 For the present study, tests were performed with the patient in supine position on a treatment plinth. The examiner moved the patient's forearm passively from maximum flexion to maximum extension when testing the elbow flexors. For testing of the wrist flexors, the wrist was held in neutral position, with the elbow flexed 90°; the wrist was moved passively from maximum flexion to maximum extension. The movement in the test of elbow and wrist was performed for about 1 second. Four passive extensions were performed, and a mode was taken to score the MAS for each muscle group.6
Active range of motion (AROM) was tested using conventional goniometric technique.24 Reliability has previously been shown to be high both for elbow extension and for wrist extension (intraclass correlation coefficient (ICC)>0.9 for both motions).25,26 Elbow-joint extension was tested with the patient sitting on a chair with the arm hanging down freely; the humerus and radius were used as references. Maximum active extension was measured, with full extension defined as 0°;24 degrees lacking from 0° were recorded as extension deficit. Wrist dorsiflexion was measured with the patient sitting with the forearm and hand resting on a table, elbow flexed, forearm in neutral position between pronation and supination, and wrist in neutral position between flexion and extension. Maximum active dorsiflexion was measured and recorded in degrees. Radius and metacarpal III were used as references. No compensatory movements were allowed.
Grip strength was measured by isometric muscle contraction with the Grippit instrument (AB Detektor, Göteborg, Sweden), an electronic device that registers grip force in Newtons.27 Grippit consists of an elliptical handle 12.5 cm in circumference, an electronic unit, and a power adapter; the grip handle and a forearm support are fixed on a wooden board, which enables the test position to be standardized. In a previous study, the within-session reliability for stroke patients CVwithin was 11% and the test–retest reliability CVwithin was 10%.27 The test was performed three times, and the mean of the three trials, measured in Newtons, was recorded. Patients were asked to squeeze the bars as strongly as possible.
Daily hand use was measured using the Motor Activity Log (MAL),28 a semi-structured interview relating to 30 common daily tasks and consisting of two assessment sub-scales for rating the affected upper extremity. The how well sub-scale addresses the quality of movement; the amount sub-scale addresses the amount of use. A six-point rating scale is used in each case (0=no use of the affected extremity, 5=normal use). The MAL has shown reliability and convergent validity (r=0.68) among adult patients with stroke.12 The how well sub-scale was used in this study; the result is presented as a median of the sub-scale items.
Functional change in dexterity was measured using the Sollerman hand function test.29 This test is standardized and based on eight of the most common hand grips that are essential for normal function; it consists of 20 items measuring ability to grasp different objects used in daily life. The eight main grip types tested are pulp pinch, lateral pinch, tripod pinch and five-finger pinch, diagonal volar grip, transverse volar grip, spherical volar grip, and extension grip. Points are assigned to each item on a five-point scale (0=The task can not be performed at all, 4=The task is completed without any difficulties within 20 seconds with prescribed hand-grip of normal quality). The test includes a time factor: the time to complete each task is measured, and the upper limit for each item is 60 seconds. The final score is the sum of all items (0–80).29 The Sollerman test has been shown to be a reliable test of hand function in patients with chronic stroke and mild to moderate impairment of hand function, using Spearman's rank correlation coefficient (Spearman's rho) and intraclass correlation coefficient (ρ>0.96, ICC>0.96 for reliability within examiners; ρ>0.96, ICC>0.92 for reliability between examiners).30
The Box and Block Test (BBT)31 is a measure of gross manual dexterity that consists of a wooden box with two compartments and 150 wooden blocks (1" cubes) collected in the compartment on the tested side. The person is asked to grasp the blocks one at a time with the affected hand and transfer them to the opposite compartment. The test score is the number of blocks transported during a 60-second period. The test has shown very high interrater and test–retest reliability (ICC>0.95, Pearson correlation coefficient>0.95).32
Training Programme
The non-affected arm was put in a restricting position belt for 90% of the patient's waking hours, 7 days per week, so as to restrict the use of the arm and hand in a comfortable position while permitting quick arm use in unsafe situations. Patients could use their non-affected hand when going to the toilet, bathing, and washing; in potentially unsafe situations; to prevent stiffening of the arm; and when it was impossible to perform a necessary task at home and there was no other person to help.
Patients signed a contract agreeing that the exercise activities would be done with the restraint on. On weekends, patients were told to wear the belt but not to do any specific exercises. Patients were encouraged to be active and to use the affected arm in daily activities at home. Shaping was used by giving strong reinforcement when patients succeeded in performing a functional activity; the activity was then increased in difficulty.33,34 An individualized training programme was performed 6 hours per day, Monday through Friday, for 2 weeks. Varied shaping, task practice, and exercises were designed on the basis of individual resources and problems; patient-specific tasks were chosen and practised. Each task was subdivided into sub-task practice; all aspects of the task were practised (strength, coordination, and speed). For example, a patient's training programme might include weight-bearing practice, moving articles as fast as possible, playing ball games, and writing or working in the kitchen. The intensity of the exercises was increased as the patient's functional level increased. Such alterations included increasing the number of repetitions per unit, increasing the resistance or load, changing the spatial domain or the level of complexity in which the task was undertaken, and introducing new and more difficult tasks. Training was organized on the basis of the theories of motor control, motor learning, and recovery of function.34–36 In the cognitive phase of motor learning, patients were stimulated to find the motor function; in the associative phase of learning, exercises were practised repetitively, varied, and done under different conditions and in different environments. The environment varied during the day between the physiotherapy gymnasium, the occupational therapy room, the kitchen, the dining room, the occupational workshop, and the rehabilitation garden. Short rest periods were included regularly during the training hours, and after lunch patients could take a half-hour rest. If a patient did not complete the 6 hours of training during the rehabilitation day (9:00 a.m.–3:30 p.m.), he or she was assigned further practice to do at home. There was no change in medication use during the training programme.
Statistical Analysis
Parametric tests were used to analyze changes in ROM and grip strength after the training period and at 6-month follow-up. Wilcoxon's non-parametric test was used to analyze change in functional use of the arm and hand in the Sollerman test and the BBT. The result of the paired ordinal data for the MAS and MAL was analyzed according to the method developed by Svensson.37 Changes from baseline to follow-up may be analyzed with McNemar's test; however, this test yields no information about the magnitude of the change or about individual or group changes. Svensson's method uses information on how many patients improve, how many remain unchanged, and how many become worse following the treatment (the test uses paired ranks, which are dependent data). When using paired ranks, one can distinguish between individual and group changes. For the present study, the values of relative position (RP), a systematic change in position on the scale between baseline and follow-up, and relative change (RC), a systematic change in concentration on the scale between baseline and follow-up, are reported with 95% confidence intervals. The data analysis for this paper was carried out using STATISTICA version 8 (StatSoft, Inc., Tulsa, OK). The calculations with Svensson's method were done with the help of a macro37 in Microsoft Excel. Statistical significance was set at p≤0.05.
Ethics
CIMT is more intense than ordinary rehabilitation programmes; during the rehabilitation period, the patient's ability to perform activities is impaired by restraint of the less-affected arm. Prospective participants were informed of these consequences and signed a contract stating their willingness and motivation to complete the training period. When invited to enter the study, patients were informed by a letter that the results would be compiled in a study and that they could decline participation in the study. All personal data were coded to preserve patient confidentiality. Ethical approval was received from the ethics committee of Linköping University.
RESULTS
Study participants were between 22 and 67 years of age (median age: 54 years). Of the 20 participants, 12 had brain injuries resulting from a cerebral vascular infarct, while the other 8 had experienced cerebral vascular haemorrhage. Time since injury varied from 6 months to 10 years (see Table 1).
Table 1.
Participant characteristics (n=20)
| Variable | n | |
|---|---|---|
| Gender | Male | 13 |
| Female | 7 | |
| Hemiplegic side | Right | 8 |
| Left | 12 | |
| Time since onset of hemiplegia | <1 year | 8 |
| 1–3 years | 9 | |
| >3 years | 3 | |
| Cause of brain injury | Infarct | 12 |
| Haemorrhage | 8 |
A total of 20 patients fulfilled the inclusion criteria, completed the training programme, and were assessed at 6-month follow-up. There were no dropouts.
Spasticity of Elbow Flexors
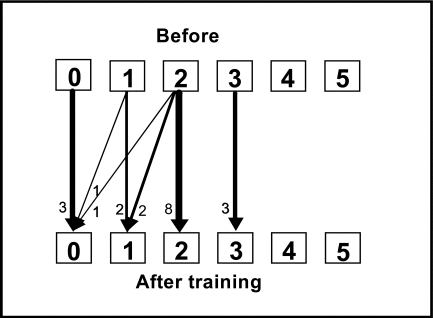
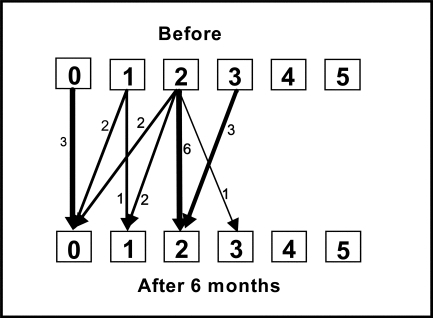
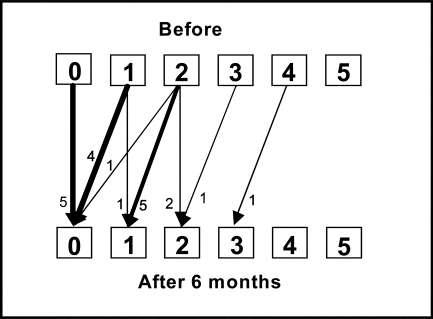
Three of the patients had no spasticity either before or after the training period; 17 showed spasticity of the elbow flexors before training. Four of the latter group showed improved scores for spasticity at the end of the 2-week training period (see Figure 2a); at 6-month follow-up, nine patients had better elbow-flexor scores, while one had worsened spasticity (see Figure 2b). The change in relative position from before the training period was significant at 6-month follow-up.
Figure 2a.
Change in spasticity in the elbow flexors, measured by MAS score, before and after the 2-week training period. RP=−0.14 (95% CI: −0.27–0.004, ns); RC=−0.09 (95% CI: −0.3–0.07, ns). Arrows indicate transfer between scores before and after training. RP=relative position; RC=relative change.
Figure 2b.
Change in spasticity in the elbow flexors, measured by MAS score, before the 2-week training period and at 6-month follow-up. RP=−0.27 (95% CI: -0.41–−0.13, p<0.05); RC=−0.025 (95% CI: −0.37–0.32, ns). RP=relative position; RC=relative change.
Spasticity of Wrist Flexors
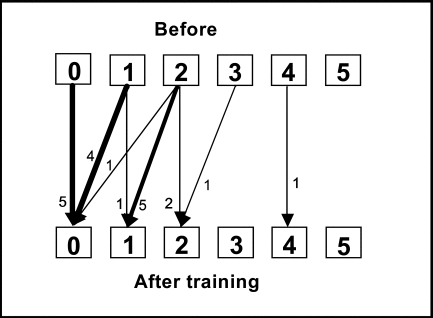
Five patients had no spasticity either before or after the training period. The other 15 showed spasticity in the wrist flexors before training; 11 of these showed improved scores for spasticity at the end of the 2-week training period (see Figure 3a). At 6-month follow-up, 12 patients had improved wrist flexor scores (see Figure 3b). The change in relative position after the training period, as well as at 6-month follow-up, was also significant.
Figure 3a.
Change in spasticity in the wrist flexors, measured by MAS score, before and after the 2-week training period. RP=−0.34 (95% CI: −0.48–−0.20, p<0.05); RC=0.10 (95% CI: −0.3–0.50, ns). RP=relative position; RC=relative change.
Figure 3b.
Change in spasticity in the wrist flexors, measured by MAS score, before the 2-week training period and at 6-month follow-up. RP=−0.37 (95% CI: -0.51–−0.23, p<0.05); RC=−0.02 (95% CI: −0.41–0.38, ns). RP=relative position; RC=relative change.
Active Range of Motion
Elbow-extension deficit decreased after the 2-week training period (p=0.002); this increased elbow extension persisted at 6-month follow-up (see Table 2).
Table 2.
Median, change in median, and lower and upper quartiles before and after the 2-week training period and at 6-month follow-up (vs. before training)*
| Before Md (Q1–Q3) |
After Md (Q1–Q3) |
Change in Median (Before–After) ΔMd (Q1–Q3) |
Follow-Up Md (Q1–Q3) |
Change in Median (Before–6-Month Follow-Up) ΔMd (Q1–Q3) |
|
|---|---|---|---|---|---|
| AROM—elbow extension deficit (n=20) | 20 (8–28) | 12 (5–22) | 5 (0–10) | 12 (2–22) | 5 (0–10) |
| AROM—wrist dorsiflexion (n=20) | 35 (22–50) | 45 (40–65) | 10 (5–15) | 48 (40–68) | 10 (2–20) |
| Grippit Newton (n=20) | 101 (71–126) | 126 (92–150) | 20 (8–27) | 128 (96–166) | 24 (12–40) |
| MAL (n=20) | 0 (0–1) | 2 (0–3) | 1 (0–2) | 2.5 (0–3) | 2 (0–3) |
| Sollerman (n=20) | 36 (22–50) | 48 (28–59) | 8 (6–10) | 51 (31–64) | 12 (6–18) |
| BBT (n=17**) | 9 (6–28) | 16 (10–30) | 4 (2–6) | 22 (10–34) | 6 (5–8) |
Md=median; ΔMd=change in median; Q1–Q3=lower/upper quartile; AROM=active range of motion; MAL=Motor Activity Log; BBT=Box and Block Test; Sollerman=Sollerman hand function test
Statistically significant changes are shown in boldface.
Three patients who scored ≥52 on the Sollerman hand function test did not complete the BBT; they were tested with the nine-hole peg test instead, and their scores are not included in the results.
Wrist dorsiflexion also showed improvement after the training period (p<0.001), and this improvement persisted at 6-month follow-up (see Table 2).
Grip Strength
Grip strength improved after training (p<0.001), and the improvement persisted at 6-month follow-up (see Table 2).
Functional Use
The MAL pre-treatment score varied between 0/5 and 2.5/5. Daily hand use and functional use, measured with MAL, the Sollerman test, and the BBT, increased after the 2-week training period (p<0.05). Functional use improved further between the end of the training period and 6-month follow-up; for the Sollerman test, the change was significant (p<0.05; see Table 2).
DISCUSSION
The results show both reduced spasticity and improved function, measured as AROM, grip strength, increased daily hand use, and functional use of the affected arm, after 2 weeks of modified CIMT with intensive and varied exercise training in an outpatient rehabilitation clinic. Some previous studies have also shown that daily repetitive training enhances recovery and reduces spasticity in stroke patients.10,11,16 The various outcome measures in this study showed improvements after the 2-week training period in all health-related domains according to the World Health Organization's International Classification of Functioning, Disability, and Health (ICF),38 and these improvements closely paralleled one another. These results are in line with those reported by Dettmers et al.,16 Butefisch et al.,10 and Diserens et al.11 As the different measurements show parallel improvements in this study, we believe that our results attest to the clinical relevance of these improvements for the patients.
Prior studies showed improvements in motor skill and in use of the affected arm and hand in daily activities after CIMT.12,13,15,28 However, these studies were carried out in laboratory settings with one therapist per patient, which is not always possible in a rehabilitation clinic; clinics do not normally have the resources for this amount of therapeutic effort. There is concern about how to carry out the intensive therapist supervision required by CIMT in a clinical or hospital setting.34,39 A strength of our study is that it replicated previous laboratory results in an outpatient clinical setting where CIMT was modified to be carried out using the available resources.
The programme in our study included varied shaping and task-specific training as well as modification of the CIMT model, including intensive and varied exercise training directed toward the negative symptoms of spasticity—exercises of strength, coordination, and speed in repetitive movements. Higher intensity in stroke rehabilitation has been shown to improve results;40,41 Kwakkel's review41 noted that augmented exercise therapy time may affect functional recovery. Different exercises included in the programme described here have been studied before. Some authors35,42 have noted that weakness and loss of skill should be addressed in task-specific rehabilitation practice; others have shown that training of grip strength and repetitive movements may lead to reduced spasticity and improved function in spastic hemiplegia.8,10,11,43,44 The effect of modified CIMT in reducing spasticity, as used in the present study, has not been investigated before.
The modifications adopted for the present study were aimed at implementing CIMT with the resources available in an outpatient clinic and at including intensive and varied exercise training geared toward the negative symptoms of spasticity (i.e., exercises of strength, coordination, and speed). A strength of the study is that improvements were seen in the MAL and that the results suggest significant effects on both positive and negative symptoms of spasticity and functional use following training with modified CIMT. It may be possible to improve results even further by including more techniques to transfer the gains made in functional use into the patients' real-world environment (e.g., the “transfer package” described by Morris et al.34); however, further research on methods of intensive rehabilitation in patients with chronic spastic hemiplegia is needed to verify this hypothesis.
Taub's laboratory study showed an improvement of two points on the MAL.13 One reason for the smaller (one-point) improvement in the present study may be that the original CIMT protocol was modified and performed in an outpatient clinic; another reason may be that the presence of spasticity impeded improvements in motor activity. Studies in which CIMT was combined with Botulinum toxin A for patients with spastic hemiplegia17,18 also showed MAL improvements of approximately one point; however, in studies by Rijntjes et al.45 and Park et al.,46 the presence of spasticity was not found to influence the effect of CIMT.45,46
Butefisch et al.10 and Diserens et al.11 measured results only immediately following the training period. In the present study, 6-month follow-up testing found that the reduced spasticity and improved function persisted (and even improved further, based on results of the Sollerman test). This suggests that there may be a long-lasting effect on spasticity and functional use of the affected arm and hand after 2 weeks of modified CIMT using intensive and varied exercise training. Other studies have shown that increased motor skill and daily use of the more affected extremity after CIMT have a long-lasting effect.15,16,21,28 Dettmers et al.16 showed reduced spasticity that persisted after 6 months in chronic patients with stroke after a distributed form of CIMT in a clinical setting. One explanation for the persistence of improvements in the present study may be that increased motor skill and functional improvement led to further use of the affected arm and hand in daily activities after the training period was over.
The patients in this study had experienced brain injury between 6 months and 10 years before the training period began, meaning that little or no further improvement was to be expected according to earlier studies.2,47 Nakayama et al.2 studied patients with stroke (n=421) in a prospective study investigating the time course and degree of recovery of an upper extremity; they found no further recovery of function after 11 weeks. Formisano et al.47 found that patients with spasticity after stroke reached a plateau in motor recovery in the first month after the stroke event. For the patients in the present study, no significant difference in improvement was seen between patients whose stroke had occurred less than 1 year before the training and those who undertook the training more than 1 year after stroke, which suggests that the reduction in spasticity seen in this study may be the result of the training.
LIMITATIONS
Participation in this study was restricted to patients of working age; more studies, including controlled studies with a larger sample that includes patients older than 67 years, are needed to verify the results and to further our understanding of optimal training intensity and exercise design.
The study was performed in a clinical outpatient setting, and assessors were not blinded. However, the lack of independent assessors was mitigated by the use of strictly prepared measurement criteria.
CONCLUSION
Following 2 weeks of modified CIMT, the present study found reduced spasticity, increased daily use of the affected arm, and functional improvement, as measured by the MAS, AROM (elbow extension and wrist dorsiflexion), grip strength, MAL, the Sollerman hand function test, and the BBT. The improvements persisted at 6-month follow-up, except in the case of the Sollerman test, on which scores improved further. These results suggest that modified CIMT using intensive and varied exercise training in an outpatient clinic can help to reduce spasticity and improve functional use in chronic stroke patients. However, the results need to be replicated using an experimental design with a control group.
KEY MESSAGES
What Is Already Known on This Topic
There is no consensus on which interventions best reduce spasticity and improve functional use of the affected arm in the chronic stage of hemiplegia following stroke. Exercise training programmes with enhanced frequency and repetitive training of one movement have been shown to reduce spasticity and improve functional use. Constraint-induced movement therapy (CIMT) is known to increase motor skill and use of the affected arm and hand in daily activities after stroke.
What This Study Adds
This study suggests that a 2-week modified CIMT programme, including intensive and varied exercises, delivered in an outpatient clinic may reduce spasticity and improve functional use of the affected arm and hand for patients of working age with chronic hemiplegia. Furthermore, the study suggests that these improvements persist 6 months after completion of the exercise programme.
Siebers A, Öberg U, Skargren E. The effect of modified constraint-induced movement therapy on spasticity and motor function of the affected arm in patients with chronic stroke. Physiother Can. 2010;62:388–396
References
- 1.Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21:357–64. doi: 10.1080/096382899297459. doi: 10.1080/096382899297459. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–8. doi: 10.1016/0003-9993(94)90161-9. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 3.Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35:134–9. doi: 10.1161/01.STR.0000105386.05173.5E. doi: 10.1161/01.STR.0000105386.05173.5E. [DOI] [PubMed] [Google Scholar]
- 4.Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK. Prevalence of spasticity post stroke. Clin Rehabil. 2002;16:515–22. doi: 10.1191/0269215502cr512oa. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]
- 5.Lance JW. What is spasticity? Lancet. 1990;335:606. doi: 10.1016/0140-6736(90)90389-m. doi: 10.1016/0140-6736(90)90389-M. [DOI] [PubMed] [Google Scholar]
- 6.Gregson JM, Leathley M, Moore AP, Sharma AK, Smith TL, Watkins CL. Reliability of the Tone Assessment Scale and the Modified Ashworth Scale as clinical tools for assessing poststroke spasticity. Arch Phys Med Rehabil. 1999;80:1013–6. doi: 10.1016/s0003-9993(99)90053-9. doi: 10.1016/S0003-9993(99)90053-9. [DOI] [PubMed] [Google Scholar]
- 7.Bhakta BB. Management of spasticity in stroke. Brit Med Bull. 2000;56:476–85. doi: 10.1258/0007142001903111. doi: 10.1258/0007142001903111. [DOI] [PubMed] [Google Scholar]
- 8.Ada L, Dorsch S, Canning CG. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother. 2006;52:241–8. doi: 10.1016/s0004-9514(06)70003-4. [DOI] [PubMed] [Google Scholar]
- 9.Winstein CJ, Miller JP, Blanton S, Taub E, Uswatte G, Morris D, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–52. doi: 10.1177/0888439003255511. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 10.Bütefisch C, Hummelsheim H, Denzler P, Mauritz KH. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci. 1995;130:59–68. doi: 10.1016/0022-510x(95)00003-k. doi: 10.1016/0022-510X(95)00003-K. [DOI] [PubMed] [Google Scholar]
- 11.Diserens K, Perret N, Chatelain S, Bashir S, Ruegg D, Vuadens P, et al. The effect of repetitive arm cycling on post stroke spasticity and motor control: repetitive arm cycling and spasticity. J Neurol Sci. 2007;253:18–24. doi: 10.1016/j.jns.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. J Am Med Assoc. 2006;296:2095–104. doi: 10.1001/jama.296.17.2095. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 13.Taub E, Miller NE, Novack TA, Cook EW, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–54. [PubMed] [Google Scholar]
- 14.Sawaki L, Butler AJ, Xiaoyan L, Wassenaar PA, Mohammad YM, Blanton S, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22:505–13. doi: 10.1177/1545968308317531. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dettmers C, Teske U, Hamzei F, Uswatte G, Taub E, Weiller C. Distributed form of constraint-induced movement therapy improves functional outcome and quality of life after stroke. Arch Phys Med Rehabil. 2005;86:204–9. doi: 10.1016/j.apmr.2004.05.007. doi: 10.1016/j.apmr.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Levy CE, Giuffrida C, Richards L, Wu S, Davis S, Nadeau SE. Botulinum toxin A, evidence-based exercise therapy, and constraint-induced movement therapy for upper-limb hemiparesis attributable to stroke: a preliminary study. Am J Phys Med Rehabil. 2007;86:696–706. doi: 10.1097/PHM.0b013e31813e2b4d. [DOI] [PubMed] [Google Scholar]
- 18.Sun SF, Hsu CW, Hwang CW, Hsu PT, Wang JL, Yang CL. Application of combined Botulinum toxin type A and modified constraint-induced movement therapy for an individual with chronic upper-extremity spasticity after stroke. Phys Ther. 2006;86:1387–97. doi: 10.2522/ptj.20050262. doi: 10.2522/ptj.20050262. [DOI] [PubMed] [Google Scholar]
- 19.Blanton S, Wilsey H, Wolf SL. Constraint-induced movement therapy in stroke rehabilitation: perspectives on future clinical applications. NeuroRehabilitation. 2008;23:15–28. [PubMed] [Google Scholar]
- 20.Bonaiuti D, Rebasti L, Sioli P. The constraint induced movement therapy: a systematic review of randomised controlled trials on the adult stroke patients. Europa Medicophysica. 2007;43:139–46. [PubMed] [Google Scholar]
- 21.Siebers A, Öberg U, Skargren E. Improvement and impact of initial motor skill after intensive rehabilitation—CI-therapy in patients with chronic hemiplegia. A follow-up study. Adv Physiother. 2006;8:146–53. doi: 10.1080/14038190600921221. [Google Scholar]
- 22.Bohannon RW, Smith MB. Inter rater reliability of a modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 23.Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing. 2000;29:223–8. doi: 10.1093/ageing/29.3.223. doi: 10.1093/ageing/29.3.223. [DOI] [PubMed] [Google Scholar]
- 24.Hoppenfeld S. Physical examination of the spine and extremities. Norwalk, CT: Appleton & Lange; 1976. [Google Scholar]
- 25.Armstrong AD, MacDermid JC, Chinchalkar S, Stevens RS, King GJ. Reliability of range-of-motion measurement in the elbow and forearm. J Shoulder Elbow Surg. 1998;7:573–80. doi: 10.1016/s1058-2746(98)90003-9. doi: 10.1016/S1058-2746(98)90003-9. [DOI] [PubMed] [Google Scholar]
- 26.Horger MM. The reliability of goniometric measurements of active and passive wrist motions. Am J Occup Ther. 1990;44:342–8. doi: 10.5014/ajot.44.4.342. [DOI] [PubMed] [Google Scholar]
- 27.Hammer A, Lindmark B. Test–retest intra-rater reliability of grip force in patients with stroke. J Rehabil Med. 2003;35:189–94. doi: 10.1080/16501970306132. doi: 10.1080/16501970306132. [DOI] [PubMed] [Google Scholar]
- 28.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation—a clinical review. J Rehabil Res Dev. 1999;36:237–51. [PubMed] [Google Scholar]
- 29.Sollerman C, Ejeskar A. Sollerman hand function test: a standardised method and its use in tetraplegic patients. Scand J Plast Reconstr Surg Hand Surg. 1995;29:167–76. doi: 10.3109/02844319509034334. [DOI] [PubMed] [Google Scholar]
- 30.Brogardh C, Persson AL, Sjolund BH. Intra- and inter-rater reliability of the Sollerman hand function test in patients with chronic stroke. Disabil Rehabil. 2007;29:145–54. doi: 10.1080/09638280600747603. doi: 10.1080/09638280600747603. [DOI] [PubMed] [Google Scholar]
- 31.Lin FM, Sabbahi M. Correlation of spasticity with hyperactive stretch reflexes and motor dysfunction in hemiplegia. Arch Phys Med Rehabil. 1999;80:526–30. doi: 10.1016/S0003-9993(99)90193-4. [PubMed] [Google Scholar]
- 32.Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidlines for the Fugle-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil. 2005;19:404–11. doi: 10.1191/0269215505cr832oa. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 33.Kupfermann I. Learning and memory. In: Kandel ER, Schwartz J, Jessel TM, editors. Principles of neuroscience. New York: Elsevier; 1991. pp. 997–1008. [Google Scholar]
- 34.Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterizing the intervention protocol. Eura Medicophys. 2006;42:257–68. [PubMed] [Google Scholar]
- 35.Dean CM, Shepherd RB. Task-related training improves performance of seated reaching tasks after stroke: a randomized controlled trial. Stroke. 1997;28:722–8. doi: 10.1161/01.str.28.4.722. [DOI] [PubMed] [Google Scholar]
- 36.Shumway-Cook A, Woollacott M. Motor control: translating research into clinical practice. 3rd ed. Baltimore, MD: 2007. [Google Scholar]
- 37.Svensson E. Guidelines to statistical evaluation of data from rating scales and questionnaires. J Rehabil Med. 2001;33:47–8. doi: 10.1080/165019701300006542. doi: 10.1080/165019701300006542. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization [WHO] International classification of functioning, disability, and health. Geneva: The Organization; 2001. [cited 2010 Mar 15]. Available from: http://www.who.int/classifications/icf/en. [Google Scholar]
- 39.Brogardh C, Sjolund BH. Constraint-induced movement therapy in patients with stroke: a pilot study on effects of small group training and of extended mitt use. Clin Rehabil. 2006;20:218–27. doi: 10.1191/0269215506cr937oa. doi: 10.1191/0269215506cr937oa. [DOI] [PubMed] [Google Scholar]
- 40.Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–7. doi: 10.1053/apmr.2002.35108. doi: 10.1053/apmr.2002.35108. [DOI] [PubMed] [Google Scholar]
- 41.Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disabil Rehabil. 2006;28:823–30. doi: 10.1080/09638280500534861. doi: 10.1080/09638280500534861. [DOI] [PubMed] [Google Scholar]
- 42.Hesse S, Werner C. Poststroke motor dysfunction and spasticity. CNS Drug Rev. 2003;17:1093–107. doi: 10.2165/00023210-200317150-00004. doi: 10.2165/00023210-200317150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke: an evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatr. 1989;52:1267–72. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dombovy ML. Understanding stroke recovery and rehabilitation: current and emerging approaches. Curr Neurol Neurosci Rep. 2004;4:31–5. doi: 10.1007/s11910-004-0008-6. doi: 10.1007/s11910-004-0008-6. [DOI] [PubMed] [Google Scholar]
- 45.Rijntjes M, Hobbeling V, Hamzei F, Dohse S, Ketels G, Liepert J, et al. Individual factors in constraint-induced movement therapy after stroke. Neurorehabil Neural Repair. 2005;19:238–49. doi: 10.1177/1545968305279205. doi: 10.1177/1545968305279205. [DOI] [PubMed] [Google Scholar]
- 46.Park SW, Wolf SL, Blanton S, Winstein C, Nichols-Larsen DS. The EXCITE trial: predicting a clinically meaningful motor activity log outcome. Neurorehabil Neural Repair. 2008;22:486–93. doi: 10.1177/1545968308316906. doi: 10.1177/1545968308316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Formisano R, Pantano P, Buzzi MG, Vinicola V, Penta F, Barbanti P, et al. Late motor recovery is influenced by muscle tone changes after stroke. Arch Phys Med Rehabil. 2005;86:308–11. doi: 10.1016/j.apmr.2004.08.001. doi: 10.1016/j.apmr.2004.08.001. [DOI] [PubMed] [Google Scholar]