Abstract
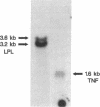
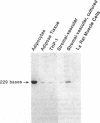
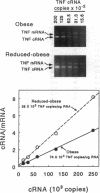
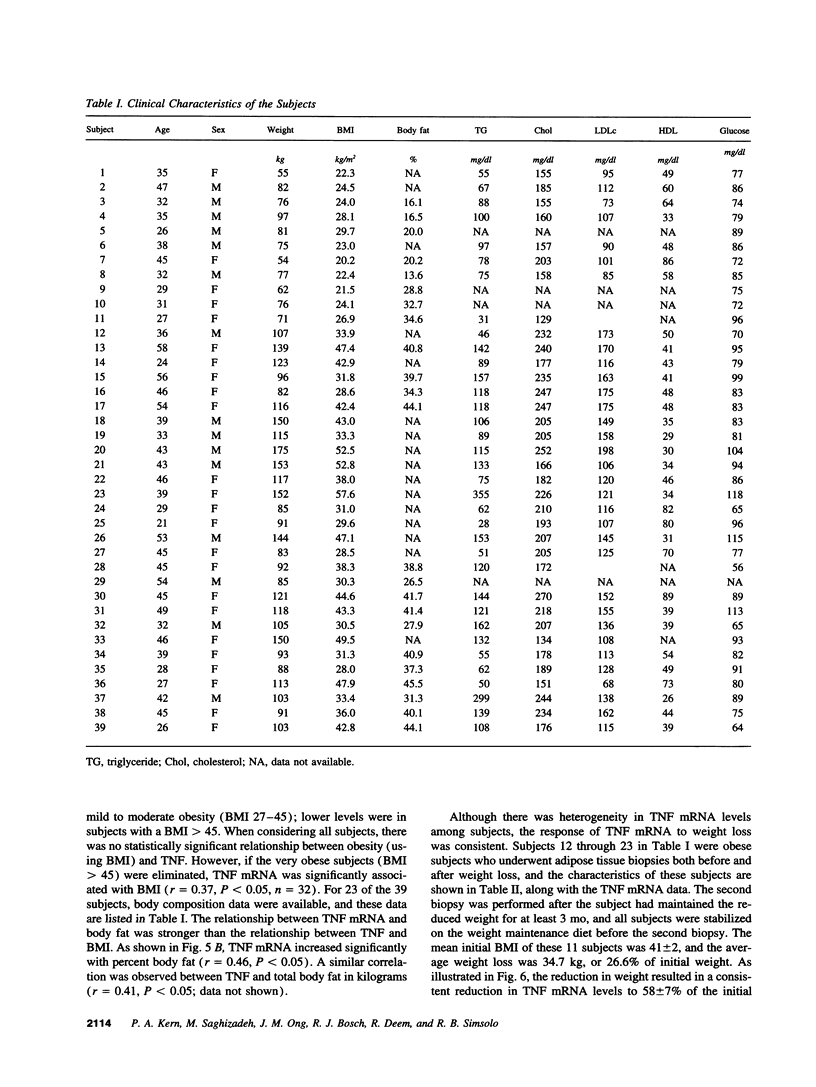
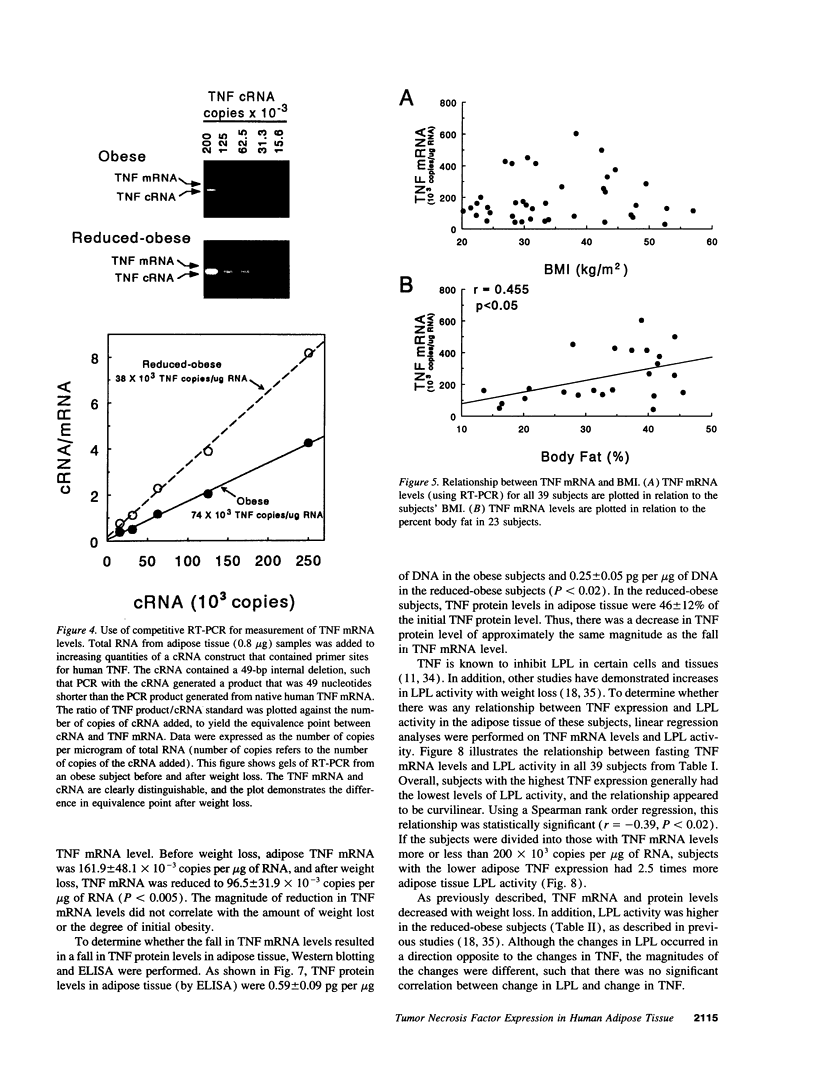
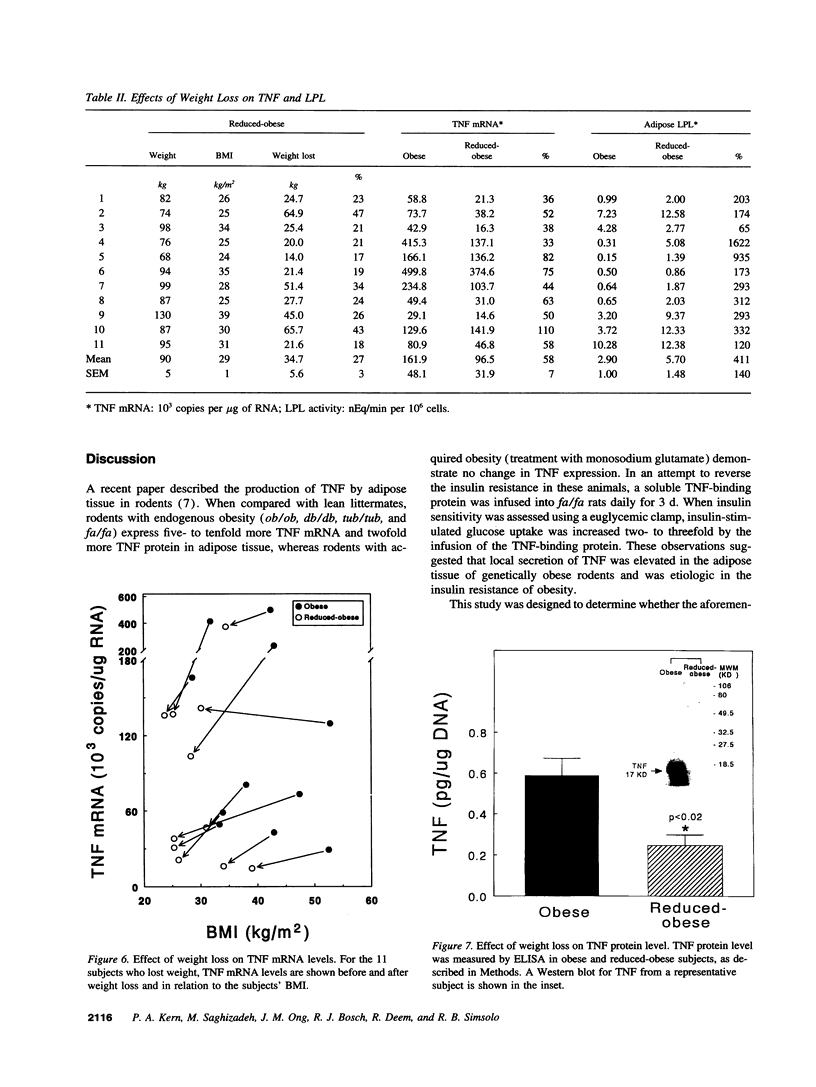
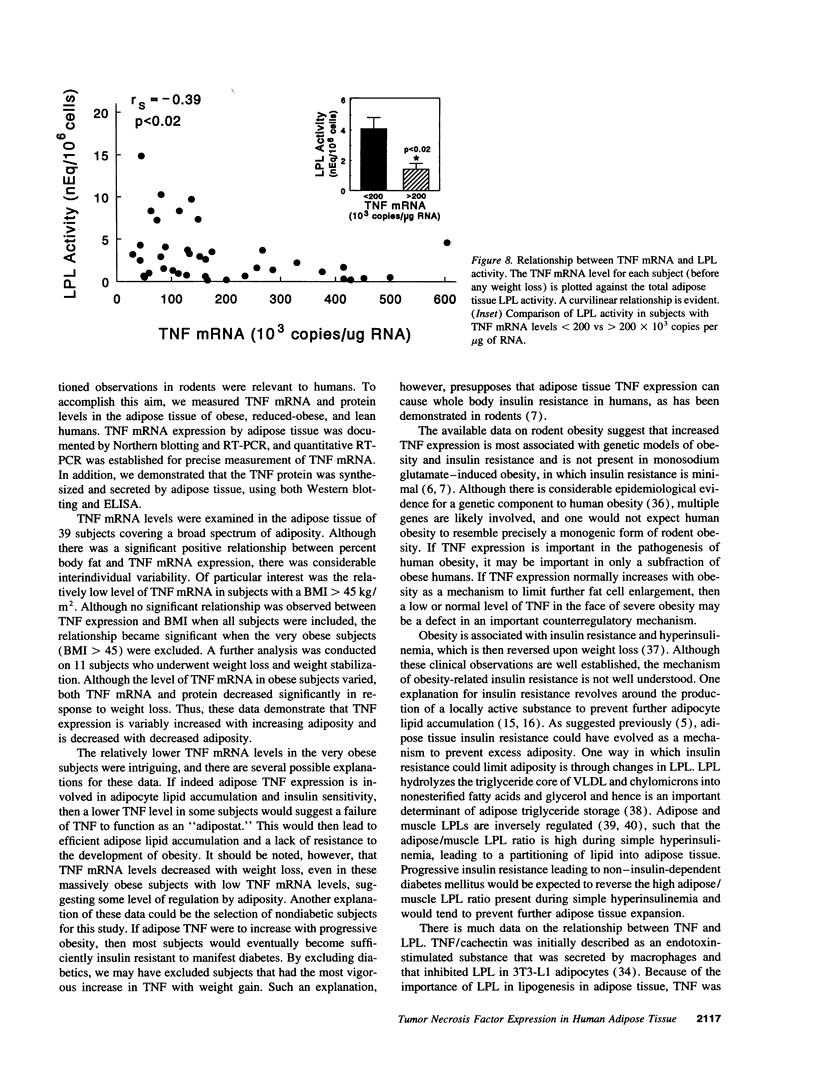
A previous study reported the increased expression of the cytokine TNF in the adipose tissue of genetically obese rodents. To examine this paradigm in humans, we studied TNF expression in lean, obese, and reduced-obese human subjects. TNF mRNA was demonstrated in human adipocytes and adipose tissue by Northern blotting and PCR. TNF protein was quantitated by Western blotting and ELISA in both adipose tissue and the medium surrounding adipose tissue. Using quantitative reverse transcriptase PCR (RT-PCR), TNF mRNA levels were examined in the adipose tissue of 39 nondiabetic subjects, spanning a broad range of body mass index (BMI). There was a significant increase in adipose TNF mRNA levels with increasing adiposity. There was a significant correlation between TNF mRNA and percent body fat (r = 0.46, P < 0.05, n = 23). TNF mRNA tended to decrease in very obese subjects, but when subjects with a BMI > 45 kg/m2 were excluded, there was a significant correlation between TNF mRNA and BMI (r = 0.37, P < 0.05, n = 32). In addition, there was a significant decrease in adipose TNF with weight loss. In 11 obese subjects who lost between 14 and 66 kg (mean 34.7 kg, or 26.6% of initial weight), TNF mRNA levels decreased to 58% of initial levels after weight loss (P < 0.005), and TNF protein decreased to 46% of initial levels (P < 0.02). TNF is known to inhibit LPL activity. When fasting adipose LPL activity was measured in these subjects, there was a significant inverse relationship between TNF expression and LPL activity (r = -0.39, P < 0.02, n = 39). With weight loss, LPL activity increased to 411% of initial levels. However, the magnitude of the increase in LPL did not correlate with the decrease in TNF. Thus, TNF is expressed in human adipocytes. TNF is elevated in most obese subjects and is decreased by weight loss. In addition, there is an inverse relationship between TNF and LPL expression. These data suggest that endogenous TNF expression in adipose tissue may help limit obesity in some subjects, perhaps by increasing insulin resistance and decreasing LPL.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Statt M. C., Welle S. L. Total and resting energy expenditure in obese women reduced to ideal body weight. J Clin Invest. 1993 Sep;92(3):1236–1242. doi: 10.1172/JCI116695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andus T., Targan S. R., Deem R., Toyoda H. Measurement of tumor necrosis factor alpha mRNA in small numbers of cells by quantitative polymerase chain reaction. Reg Immunol. 1993 Jan-Feb;5(1):11–17. [PubMed] [Google Scholar]
- Arner P. Control of lipolysis and its relevance to development of obesity in man. Diabetes Metab Rev. 1988 Aug;4(5):507–515. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Bouchard C. Current understanding of the etiology of obesity: genetic and nongenetic factors. Am J Clin Nutr. 1991 Jun;53(6 Suppl):1561S–1565S. doi: 10.1093/ajcn/53.6.1561S. [DOI] [PubMed] [Google Scholar]
- Bouchard C., Tremblay A., Després J. P., Nadeau A., Lupien P. J., Thériault G., Dussault J., Moorjani S., Pinault S., Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med. 1990 May 24;322(21):1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coiro V., Volpi R., Capretti L., Speroni G., Marchesi C., Vescovi P. P., Caffarri G., Colla R., Rossi G., Davoli C. Influence of thyroid status on the paradoxical growth hormone response to thyrotropin-releasing hormone in human obesity. Metabolism. 1994 Apr;43(4):514–517. doi: 10.1016/0026-0495(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- Eckel R. H. Insulin resistance: an adaptation for weight maintenance. Lancet. 1992 Dec 12;340(8833):1452–1453. doi: 10.1016/0140-6736(92)92633-q. [DOI] [PubMed] [Google Scholar]
- Eckel R. H. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989 Apr 20;320(16):1060–1068. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Prasad J. E., Kern P. A., Marshall S. Insulin regulation of lipoprotein lipase in cultured isolated rat adipocytes. Endocrinology. 1984 May;114(5):1665–1671. doi: 10.1210/endo-114-5-1665. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Reichart D., Olefsky J. M., Henry R. R. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988 Oct;82(4):1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried S. K., Zechner R. Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J Lipid Res. 1989 Dec;30(12):1917–1923. [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Huecksteadt T. P., Birnbaum M. J., Molina J. M., Ciaraldi T. P. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991 Mar;87(3):1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C., Feingold K. R. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992 Jul 30;327(5):329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Gulli R., Moser A. H., Gavin L. A., Feingold K. R. Effect of tumor necrosis factor administration in vivo on lipoprotein lipase activity in various tissues of the rat. J Lipid Res. 1989 Apr;30(4):579–585. [PubMed] [Google Scholar]
- Grunfeld C., Wilking H., Neese R., Gavin L. A., Moser A. H., Gulli R., Serio M. K., Feingold K. R. Persistence of the hypertriglyceridemic effect of tumor necrosis factor despite development of tachyphylaxis to its anorectic/cachectic effects in rats. Cancer Res. 1989 May 15;49(10):2554–2560. [PubMed] [Google Scholar]
- Hofmann C., Lorenz K., Braithwaite S. S., Colca J. R., Palazuk B. J., Hotamisligil G. S., Spiegelman B. M. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994 Jan;134(1):264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. H. The origin and glyceride distribution of fatty acids in rat adipose tissue. J Clin Invest. 1966 Feb;45(2):205–216. doi: 10.1172/JCI105333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S., Budavari A., Murray D., Spiegelman B. M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994 Oct;94(4):1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993 Jan 1;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Larsson A., Smith U., Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992 May;89(5):1610–1617. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P. A., Marshall S., Eckel R. H. Regulation of lipoprotein lipase in primary cultures of isolated human adipocytes. J Clin Invest. 1985 Jan;75(1):199–208. doi: 10.1172/JCI111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P. A., Ong J. M., Goers J. W., Pedersen M. E. Regulation of lipoprotein lipase immunoreactive mass in isolated human adipocytes. J Clin Invest. 1988 Feb;81(2):398–406. doi: 10.1172/JCI113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P. A., Ong J. M., Saffari B., Carty J. The effects of weight loss on the activity and expression of adipose-tissue lipoprotein lipase in very obese humans. N Engl J Med. 1990 Apr 12;322(15):1053–1059. doi: 10.1056/NEJM199004123221506. [DOI] [PubMed] [Google Scholar]
- Kern P. A. Recombinant human tumor necrosis factor does not inhibit lipoprotein lipase in primary cultures of isolated human adipocytes. J Lipid Res. 1988 Jul;29(7):909–914. [PubMed] [Google Scholar]
- Kern P. A., Svoboda M. E., Eckel R. H., Van Wyk J. J. Insulinlike growth factor action and production in adipocytes and endothelial cells from human adipose tissue. Diabetes. 1989 Jun;38(6):710–717. doi: 10.2337/diab.38.6.710. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Nishida T., Ameis D., Stahnke G., Schotz M. C., Hashimoto H., Fukamachi I., Shirai K., Saito Y., Yoshida S. A heterozygous mutation (the codon for Ser447----a stop codon) in lipoprotein lipase contributes to a defect in lipid interface recognition in a case with type I hyperlipidemia. Biochem Biophys Res Commun. 1992 Jan 15;182(1):70–77. doi: 10.1016/s0006-291x(05)80113-5. [DOI] [PubMed] [Google Scholar]
- Mackay A. G., Oliver J. D., Rogers M. P. Regulation of lipoprotein lipase activity and mRNA content in rat epididymal adipose tissue in vitro by recombinant tumour necrosis factor. Biochem J. 1990 Jul 1;269(1):123–126. doi: 10.1042/bj2690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Sherman M. L., Spriggs D. R., Rounds J., Christie M., Wilmore D. W. Chronic TNF infusion causes anorexia but not accelerated nitrogen loss. Ann Surg. 1989 Jan;209(1):19–24. doi: 10.1097/00000658-198901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. C., Lindeman A. K., Wallace J., Niederpruem M. Diet composition, energy intake, and exercise in relation to body fat in men and women. Am J Clin Nutr. 1990 Sep;52(3):426–430. doi: 10.1093/ajcn/52.3.426. [DOI] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Ong J. M., Kern P. A. Effect of feeding and obesity on lipoprotein lipase activity, immunoreactive protein, and messenger RNA levels in human adipose tissue. J Clin Invest. 1989 Jul;84(1):305–311. doi: 10.1172/JCI114155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J. M., Kirchgessner T. G., Schotz M. C., Kern P. A. Insulin increases the synthetic rate and messenger RNA level of lipoprotein lipase in isolated rat adipocytes. J Biol Chem. 1988 Sep 15;263(26):12933–12938. [PubMed] [Google Scholar]
- Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Price S. R., Horn C. A., Hom B. E., Moss J., Cerami A. Model for cachexia in chronic disease: secretory products of endotoxin-stimulated macrophages induce a catabolic state in 3T3-L1 adipocytes. Trans Assoc Am Physicians. 1984;97:251–259. [PubMed] [Google Scholar]
- Powell E. E., Kroon P. A. Measurement of mRNA by quantitative PCR with a nonradioactive label. J Lipid Res. 1992 Apr;33(4):609–614. [PubMed] [Google Scholar]
- Pykälistö O. J., Smith P. H., Brunzell J. D. Determinants of human adipose tissue lipoprotein lipase. Effect of diabetes and obesity on basal- and diet-induced activity. J Clin Invest. 1975 Nov;56(5):1108–1117. doi: 10.1172/JCI108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Sadur C. N., Eckel R. H. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J Clin Invest. 1982 May;69(5):1119–1125. doi: 10.1172/JCI110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Brunzell J. D. Increase of adipose tissue lipoprotein lipase activity with weight loss. J Clin Invest. 1981 May;67(5):1425–1430. doi: 10.1172/JCI110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal K. R., Gutin B., Presta E., Wang J., Van Itallie T. B. Estimation of human body composition by electrical impedance methods: a comparative study. J Appl Physiol (1985) 1985 May;58(5):1565–1571. doi: 10.1152/jappl.1985.58.5.1565. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. Competitive PCR. Nature. 1992 Oct 8;359(6395):557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Sims E. A. Experimental obesity, dietary-induced thermogenesis, and their clinical implications. Clin Endocrinol Metab. 1976 Jul;5(2):377–395. doi: 10.1016/s0300-595x(76)80027-8. [DOI] [PubMed] [Google Scholar]
- Simsolo R. B., Ong J. M., Kern P. A. The regulation of adipose tissue and muscle lipoprotein lipase in runners by detraining. J Clin Invest. 1993 Nov;92(5):2124–2130. doi: 10.1172/JCI116813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsolo R. B., Ong J. M., Saffari B., Kern P. A. Effect of improved diabetes control on the expression of lipoprotein lipase in human adipose tissue. J Lipid Res. 1992 Jan;33(1):89–95. [PubMed] [Google Scholar]
- Spiegelman B. M., Choy L., Hotamisligil G. S., Graves R. A., Tontonoz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J Biol Chem. 1993 Apr 5;268(10):6823–6826. [PubMed] [Google Scholar]
- Spiegelman B. M., Hotamisligil G. S. Through thick and thin: wasting, obesity, and TNF alpha. Cell. 1993 May 21;73(4):625–627. doi: 10.1016/0092-8674(93)90243-j. [DOI] [PubMed] [Google Scholar]
- Stephens J. M., Pekala P. H. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem. 1991 Nov 15;266(32):21839–21845. [PubMed] [Google Scholar]
- Taskinen M. R., Nikkilä E. A. Lipoprotein lipase activity of adipose tissue and skeletal muscle in insulin-deficient human diabetes. Relation to high-density and very-low-density lipoproteins and response to treatment. Diabetologia. 1979 Dec;17(6):351–356. doi: 10.1007/BF01236268. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wion K. L., Kirchgessner T. G., Lusis A. J., Schotz M. C., Lawn R. M. Human lipoprotein lipase complementary DNA sequence. Science. 1987 Mar 27;235(4796):1638–1641. doi: 10.1126/science.3823907. [DOI] [PubMed] [Google Scholar]
- Wong H., Davis R. C., Nikazy J., Seebart K. E., Schotz M. C. Domain exchange: characterization of a chimeric lipase of hepatic lipase and lipoprotein lipase. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11290–11294. doi: 10.1073/pnas.88.24.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]