Abstract
Objective
This study assesses whether the relationship of lipoprotein(a) (Lp(a)) with cardiovascular risk may be modified by concurrent hormone therapy (HT).
Background
Prior studies indicate that hormone therapy (HT) decreases plasma levels of Lp(a), but few have been powered to assess whether it modifies the relationship of Lp(a) with cardiovascular disease (CVD).
Methods
Lp(a) at baseline was measured among 27,736 initially healthy women, of whom 12,075 indicated active HT use at the time of blood draw at study initiation, and 15,661 did not. The risk of first-ever major cardiovascular event (nonfatal myocardial infarction, nonfatal cerebrovascular event, coronary revascularization or cardiovascular deaths) over a ten-year period was assessed in Cox-proportional hazard models according to Lp(a) levels and HT status, and adjusted for potential confounding variables.
Results
As anticipated, Lp(a) values were lower among women taking HT (median 9.4 vs. 11.6 mg/dL, P<0.0001). In women not taking HT, the hazard ratio of future CVD for the highest Lp(a) quintile compared to the lowest was 1.8 (P-trend <0.0001), after adjusting for age, smoking, blood pressure, diabetes, body mass index, total cholesterol, HDL, C-reactive protein and treatment arms of aspirin and Vitamin E. In contrast, among women taking HT, there was little evidence of association with CVD [hazard ratio 1.1, P-trend =0.18; interaction P-value 0.0009 between Lp(a) quintiles and HT on incident CVD].
Conclusion
The relationship of high Lp(a) levels with increased CVD is modified by hormone therapy. These data suggest that the predictive utility of Lp(a) is markedly attenuated among women taking HT, and may inform clinicians’ interpretation of Lp(a) values in such patients.
Keywords: Lipoprotein(a), Hormone Replacement Therapy, Cardiac risk stratification, Prevention, Women
INTRODUCTION
Lipoprotein(a) is a lipoprotein that differs from LDL cholesterol (LDL-C) by the presence of an apo(a) component that has marked size heterogeneity(1). It has been hypothesized that its structural homology to plasminogen(2) may result in its contribution to a thrombogenic milieu(3,4). Using an assay independent of apo(a) isoform size(5,6), we have recently shown that women with high levels of Lp(a) have increased incidence of cardiovascular disease, particularly if they also have high LDL-C levels (7), in concordance with work by other groups(8–14). However, data about what modifies the relationship of Lp(a) with cardiovascular disease is scarce. In terms of lifestyle, changes in diet and exercise are not known to decrease Lp(a) levels (15). In terms of medications, statin therapy may not decrease Lp(a) levels, but by lowering LDL-C, may modify the cardiovascular risk associated with Lp(a)(16); niacin, though difficult to tolerate in doses needed to decrease Lp(a) (17–19) does decrease Lp(a) levels. The data about the relationship between hormone replacement therapy and Lp(a) levels are conflicting and controversial, in part, because HT use in women itself is controversial(20–25), and because studies that show decreases in Lp(a) levels with HT use (26–31) have rarely been powered to assess for impact on cardiovascular disease (32).
To explore this issue in detail, we undertook a study in which we assessed whether the relationship of Lp(a) levels with cardiovascular disease is modified by concurrent use of hormone therapy among 27,736 women.
METHODS
Study population
Lp(a) was measured among 12,075 women taking hormone replacement therapy, and 15,661 not taking HT at the initiation of the study, and assessed for future cardiovascular events. Study participants were enrolled in the Women’s Health Study (WHS), a recently completed randomized, double-blinded, placebo-controlled clinical trial of low dose aspirin and vitamin E, in the primary prevention of CVD and cancer in U.S. female healthcare individuals(33–35). Eligible participants were apparently healthy women, aged 45 years or older, who were free of self-reported CVD or cancer at study entry (1992–1998) with follow-up for incident CVD through February 2005. At the time of enrollment between 1992 and 1995, participants provided information on whether they were currently taking HT and provided blood samples from which Lp(a) levels were assayed. They also provided demographic, medical history, medication and lifestyle data, as well as consent for blood based analyses related to the risk of incident chronic diseases. In total, 27,736 women who provided blood samples for successful Lp(a) analysis, and who also responded to questions about hormone therapy, constituted the study population for this analysis.
The study was approved by the institutional review board of the Brigham and Women’s Hospital (Boston, MA). The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Baseline Plasma Measurements
Among WHS participants, 27,736 provided data about hormone therapy at baseline and also provided baseline blood samples that were stored in liquid nitrogen (−150 to −180°C) until the time of analysis. These samples underwent Lp(a) and lipid analysis in a core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program. Due to poor agreement of Lp(a) values obtained by different methods(5), we utilized the only commercially available assay shown by the NHLBI and International Federation of Clinical Chemistry Lp(a) standardization groups to not be affected by Kringle IV-Type 2 repeats(5). We determined the concentration of Lp(a) using a turbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics - Indianapolis, IN), using reagents and calibrators from Denka Seiken (Niigata, Japan). The interassay coefficient of variation of Lp(a) concentrations of 17.6 and 58.1 mg/dL were 3.6 and 1.5%, respectively.
High-sensitivity CRP was measured using a validated immunoturbidometric method (Denka Seiken, Tokyo, Japan)(36). Total cholesterol was measured enzymatically. HDL (Roche Diagnostics, Basel, Switzerland) and LDL-C (Genzyme Corporation, Cambridge, Mass) were measured by a homogenous direct method. All lipid determinations were performed on the Hitachi 917 autoanalyzer. 98% of the samples received underwent successful evaluation for each biomarker.
Ascertainment of Incident Cardiovascular Events
All participants were followed prospectively for the occurrence of the composite endpoint of first ever major cardiovascular event (nonfatal myocardial infarction, nonfatal ischemic stroke, coronary revascularization or cardiovascular death). Medical records were obtained and reviewed for confirmation of events by two cardiologists. Deaths from cardiovascular causes were confirmed by autopsy reports, death certificates, medical records and contact with family members.
Statistical Analysis
Baseline characteristics such as age, body mass index, smoking status, presence of diabetes, hypertension, family history of premature myocardial infarction, lipid profiles, CRP levels and postmenopausal status in women on hormone replacement therapy, and measures of socioeconomic status such as income and education, were compared to those of women free of HT.
Next, Lp(a) was divided into quintiles on the basis of their distribution in the entire Women’s Health study cohort, and these quintile cut points were then used to assess for evidence of interaction between overall Lp(a) quintiles and hormone therapy status in Cox proportional hazard models that regressed cardiovascular disease on Lp(a) quintiles, HT status and an interaction term of Lp(a) quintiles multiplied by HT status.
Because of evidence of interaction from this evaluation, we recalculated Lp(a) quintile cutoffs separately within each HT strata and then applied these cutoffs to subsequent analysis separately within HT strata. Hazard ratios (HR), comparing Lp(a) quintiles 2 through 5 were compared to the lowest (referent) quintile, were calculated by Cox proportional hazard models, in models that adjusted first for age (years), then for Framingham covariates as suggested by the most recent NCEPIII, ATPIII guidelines(37,38), and finally in fully-adjusted models that adjusted for age, blood pressure (as defined by Framingham risk models (<120/<75, 120 to 129/75 to 84, 130 to 139/85 to 89, 140 to 159/90 to 94 and ≥160/≥95 mmHg), diabetes, current smoking status, body mass index (linear continuous), total cholesterol, HDL, C-reactive protein and treatment arms. These variables were chosen a priori for their impact on cardiovascular disease. Tests for trend across quintiles of Lp(a) were addressed by entering a single ordinal term for each quintile based on the median value for Lp(a) within each quintile.
After stratification by HT, Kaplan-Meier curves were constructed to illustrate cumulative event rates of CVD as a function of Lp(a) quintiles over an average of 10 years of follow up.
Because prior evidence has pointed to increased associations of Lp(a) with CVD in women with concomitant elevations of LDL-C, we assessed for joint associations of Lp(a), LDL-C and HT with CVD by calculating hazard ratios for CVD by increasing quintiles of Lp(a) in: 1. women with LDL-C ≥ median (121.4 mg/dL) who were taking HT, 2. with LDL-C ≥ median not taking HT, 3. with LDL-C < median taking HT 4. with LDL-C < median not taking HT.
To test the robustness of our findings, we performed sensitivity analysis, using alternative models. Alternative models included those that replaced total cholesterol with LDL, BMI with waist circumference, models analyzed within whites only, and then models that further adjusted for income (categories <=19,999/yr, 20–29.9k/yr, 30–39.9k, 40–49.9k/yr, 50–99.9k/yr, >=100k/yr) and education (categories LPN,LVN, RN2yr, RN3 yr, BS, Master or Doctorate/MD). Finally, we divided women taking HT into those taking estrogen only and those taking estrogen plus progesterone combinations, and performed the Cox-proportional hazard models for CVD to assess whether the type of HT affected the relationship between Lp(a) and CVD.
All p-values are two-tailed, with a value of <0.05 considered significant. Data analysis was conducted using SAS statistical software version 9.1 (SAS Institute Inc, Cary, NC) and SPLUS v 6.0 (Insightful Corp., Seattle, WA).
RESULTS
Women taking HT at study initiation were older, had higher total cholesterol, HDL and CRP levels, and were more likely to be hypertensive compared to women not on hormone replacement therapy. They were also more likely to be in higher income brackets and to report longer years of education, but no relationships were seen between income or education and Lp(a) levels. Women on HT also had lower mean LDL levels, and were less likely to be diabetic or to be current smokers (Table 1).
Table 1.
Baseline Characteristics Among Women According to Active Hormone Replacement Therapy Status At Time of Blood Draw
| Active HT Users (n=12,075) |
No HT Use (n=15,661) |
P value | |
|---|---|---|---|
| Age, y | 55.0 ± 6.2 | 53.5 ± 7.6 | <0.0001 |
| Body mass index, kg/m2† | 25.4 ± 4.5 | 26.3 ± 5.3 | <0.0001 |
| Body Mass Index (WHO categories) kg/m2, % | |||
| < 25 | 55.0 | 49.2 | |
| 25–29.9 | 30.7 | 30.7 | |
| ≥ 30 | 14.4 | 20.2 | <0.0001 |
| Smoker, % | |||
| Never | 50.9 | 52.3 | |
| Past | 38.3 | 35.5 | |
| Current | 10.8 | 12.2 | <0.0001 |
| Diabetes, % | 2.1 | 3.4 | <0.0001 |
| History of hypertension, % | 25.7 | 24.6 | 0.03 |
| Family history of myocardial infarction | |||
| In a parent before age 60 years, % | 12.7 | 13.0 | 0.54 |
| Lp(a), mg/dL [median (IQR)] |
9.4 (3.9–30.4) | 11.6 (4.9–34.3) | <0.0001 |
| Total Cholesterol | 214.3 ± 41.1 | 209.9 ± 42.2 | <0.0001 |
| HDL | 57.3 ± 15.6 | 51.0 ± 14.0 | <0.0001 |
| LDL | 121.0 ± 33.2 | 126.6 ± 34.8 | <0.0001 |
| Triglycerides [median (IQR)] |
132.0 (92.0–188.0) | 111.0 (77.0–165.0) | <0.0001 |
| C-Reactive Protein [median (IQR)] |
2.8 (1.3–5.4) | 1.5 (0.6–3.5) | <0.0001 |
| Income, % | |||
| ≤19,999/yr | 3.8 | 6.2 | |
| 20–29,999/yr | 8.7 | 10.5 | |
| 30–39,999/yr | 13.6 | 14.2 | |
| 40–49,999/yr | 17.0 | 16.3 | |
| 50–99,999/yr | 43.0 | 40.3 | |
| ≥100,000/yr | 14.0 | 12.5 | <0.0001 |
| Education, % | |||
| LPN, LVN | 11.5 | 13.2 | |
| RN, 2 year | 11.8 | 10.8 | |
| RN, 3 year | 32.3 | 32.0 | |
| BS | 23.8 | 23.5 | |
| Master, Doctorate, MD | 20.7 | 20.5 | 0.0001 |
| % Caucasian | 95.9 | 94.9 | 0.0054 |
Values shown for continuous variables are mean ± SD unless otherwise indicated. IQR is the range between the 25th to 75th percentile. Ranked tests of significance (the Wilxocon rank sum test) were used to assess for differences in continuous variables that were not normally distributed, such as Lp(a), triglycerides and C-reactive protein. Age, body mass index, total cholesterol, HDL and LDL were compared using a non-paired t-test. Differences in frequencies among groups were assessed by chi-square tests.
Overall assessment of the relationship between Lp(a), hormone therapy and cardiovascular disease revealed significant interaction between Lp(a) quintiles and HT status on CVD (P - interaction = 0.0009),
The different relationships of Lp(a) with CVD, therefore, stratifying for HT therapy status are shown in Tables 2 and 3. In women free of HT (Table 2a), women in the highest quintile of Lp(a) (≥ 45.4 mg/dL in HT- strata) were 1.77 times more likely to develop cardiovascular events than those in the lowest quintile (≤ 3.9 mg/dL; P trend <0.0001), in analyses adjusting for age, smoking, blood pressure, body mass index, cholesterol, high density lipoprotein, diabetes, hormone use, C-reactive protein and randomization treatment arms. A threshold effect is seen such that risk increased predominantly among women with Lp(a) levels in the highest quintile (66.35 mg/dL), and particularly in women with high LDL (Table 2b), as noted previously (7).
Table 2.
| 2a. Association of Lipoprotein(a) with Incident Cardiovascular Disease in Women Not Taking Hormone Replacement Therapy | ||||||
|---|---|---|---|---|---|---|
| Quintiles | ||||||
| Lipoprotein (a) | 1 | 2 | 3 | 4 | 5 | P trend |
| Lp(a) median (range), mg/dL* | 2.10 (0.10–3.90) | 6.00 (4.00–8.30) | 11.70 (8.40–16.70) | 25.90 (16.80–45.30) | 66.35 (45.40–239.60) | |
| Women Not taking Hormone Replacement Therapy n=15,661 | ||||||
| No of events | 95 (3.0%) | 86 (2.8%) | 81 (2.6%) | 97 (3.1%) | 166 (5.3%) | |
| Person-years of follow-up | 31 395 | 30 877 | 30 999 | 30 723 | 30 549 | |
| HR (95% CI) (age-adjusted) | 1 | 0.93 (0.70, 1.25) | 0.82 (0.61, 1.10) | 0.95 (0.72, 1.26) | 1.67 (1.30, 2.15) | <0.0001 |
| HR (Framingham-adjusted)† | 1 | 0.93 (0.69, 1.24) | 0.82 (0.60, 1.10) | 0.90 (0.67, 1.20) | 1.64 (1.27, 2.12) | <0.0001 |
| HR (Fully-adjusted)‡ | 1 | 0.97 (0.72, 1.31) | 0.92 (0.68, 1.26) | 0.97 (0.72, 1.30) | 1.77 (1.36, 2.30) | <0.0001 |
| 2b. Lipoprotein (a), LDL-C and Hazard Ratios of Future Cardiovascular Events Among Women Not Taking Hormone Replacement Therapy | ||||||
|---|---|---|---|---|---|---|
| Quintiles | ||||||
| Lipoprotein (a) | 1 | 2 | 3 | 4 | 5 | P trend |
| Lp(a) median (range), mg/dL* | 2.10 (0.10–3.90) | 6.00 (4.00–8.30) | 11.70 (8.40–16.70) | 25.90 (16.80–45.30) | 66.35 (45.40–239.60) | |
| Women Not Taking Hormone Replacement Therapy with LDL-C≥ median†n=8269 | ||||||
| No. of CV Events | 55 (3.8%) | 50 (3.4%) | 58 (3.6%) | 67 (3.8%) | 139 (6.9%) | |
| Person-years of follow-up | 14 184 | 14 501 | 15 766 | 17 370 | 19 540 | |
| HR (95% CI) (age-adjusted) | 1 | 0.91 (0.62, 1.34) | 0.93 (0.64, 1.35) | 0.94 (0.66, 1.35) | 1.80 (1.32, 2.46) | <0.0001 |
| HR (Framingham-adjusted)‡ | 1 | 0.89 (0.61, 1.31) | 0.91 (0.62, 1.31) | 0.90 (0.62, 1.29) | 1.87 (1.36, 2.56) | <0.0001 |
| HR (fully-adjusted)§ | 1 | 0.99 (0.67, 1.48) | 1.04 (0.71, 1.53) | 1.03 (0.71, 1.50) | 2.06 (1.49, 2.86) | <0.0001 |
| Women Not Taking Hormone Replacement Therapy with LDL-C< median† n=7392 | ||||||
| No. of CV Events | 40 (2.3%) | 36 (2.2%) | 23 (1.5%) | 30 (2.2%) | 27 (2.4%) | |
| Person-years of follow-up | 17 211 | 16 376 | 15 233 | 13 353 | 11 010 | |
| HR (95% CI) (age-adjusted) | 1 | 0.95 (0.60, 1.49) | 0.60 (0.36, 1.00) | 0.91 (0.57, 1.46) | 1.07 (0.66, 1.74) | 0.45 |
| HR (Framingham-adjusted) | 1 | 0.99 (0.63, 1.56) | 0.67 (0.40, 1.13) | 0.98 (0.60, 1.58) | 1.18 (0.72, 1.93) | 0.30 |
| HR (fully-adjusted)§ | 1 | 0.92 (0.58, 1.47) | 0.77 (0.46, 1.30) | 0.89 (0.54, 1.48) | 1.25 (0.76, 2.06) | 0.23 |
Lp(a) quintile levels were derived from the stratum of women not taking hormone replacement therapy.
Framingham-adjusted model refers to adjustments for covariates age, blood pressure categories, current smoking status, total cholesterol and high-density lipoprotein values.
Fully-adjusted models refer to Framingham covariates of age, blood pressure categories, current smoking status, total cholesterol and high density lipoprotein values as well as body mass index, diabetes, C-reactive protein and treatment arms.
Lp(a) quintile levels were derived from women not taking hormone replacement therapy.
Median LDL-C value was 121.4 mg/dL.
Framingham-adjusted model refers to adjustments for covariates age, blood pressure categories, current smoking status, total cholesterol and high-density lipoprotein values.
Fully-adjusted models refer to Framingham covariates of age, blood pressure categories, current smoking status, total cholesterol and high density lipoprotein values as well as body mass index, diabetes, C-reactive protein and treatment arms.
Table 3.
| 3a. Lipoprotein(a) and Incident Cardiovascular Disease in Women Taking Hormone Replacement Therapy | ||||||
|---|---|---|---|---|---|---|
| Quintiles | ||||||
| Lipoprotein (a) | 1 | 2 | 3 | 4 | 5 | P trend |
| Lp(a) median (range), mg/dL* | 1.70 (0.10–3.00) | 4.90 (3.10–6.80) | 9.50 (6.90–13.60) | 22.20 (13.70–41.90) | 64.40 (42.00–221.90) | |
| Women Taking Hormone Replacement Therapy n=12,075 | ||||||
| No of events | 86 (3.5%) | 78 (3.3%) | 57 (2.4%) | 58 (2.4%) | 94 (3.9%) | |
| Person-years of follow-up | 24 593 | 23 620 | 23 537 | 23 877 | 23 864 | |
| HR (95% CI) (age-adjusted) | 1 | 0.96 (0.71, 1.30) | 0.69 (0.49, 0.96) | 0.69 (0.50, 0.97) | 1.11 (0.83, 1.49) | 0.11 |
| HR (Framingham-adjusted)† | 1 | 1.02 (0.75, 1.38) | 0.70 (0.50, 0.98) | 0.72 (0.52, 1.01) | 1.07 (0.80, 1.44) | 0.27 |
| HR (Fully-adjusted)‡ | 1 | 1.04 (0.76, 1.42) | 0.74 (0.52, 1.04) | 0.79 (0.56, 1.11) | 1.13 (0.84, 1.53) | 0.18 |
| 3b. Lipoprotein (a), LDL-C and Hazard Ratios of Future Cardiovascular Events Among Women Taking Hormone Replacement Therapy | ||||||
|---|---|---|---|---|---|---|
| Quintiles | ||||||
| Lipoprotein (a) | 1 | 2 | 3 | 4 | 5 | P trend |
| Lp(a) median (range), mg/dL* | 1.70 (0.10–3.00) | 4.90 (3.10–6.80) | 9.50 (6.90–13.60) | 22.20 (13.70–41.90) | 64.40 (42.00–221.90) | |
| Women Taking Hormone Replacement Therapy with LDL-C≥ median†n=5614 | ||||||
| No. of CV Events | 43 (4.5%) | 36 (3.5%) | 26 (2.5%) | 38 (3.1%) | 64 (4.7%) | |
| Person-years of follow-up | 9 451 | 10 159 | 10 247 | 12 313 | 13 341 | |
| HR (95% CI) (age-adjusted) | 1 | 0.76 (0.49, 1.18) | 0.54 (0.33, 0.88) | 0.65 (0.42, 1.01) | 1.04 (0.71, 1.54) | 0.06 |
| HR (Framingham-adjusted)‡ | 1 | 0.83 (0.53, 1.29) | 0.56 (0.34, 0.91) | 0.71 (0.46, 1.09) | 1.08 (0.73, 1.60) | 0.07 |
| HR (fully-adjusted)§ | 1 | 0.79 (0.50, 1.25) | 0.62 (0.37, 1.01) | 0.78 (0.50, 1.22) | 1.14 (0.77, 1.70) | 0.04 |
| Women Taking Hormone Replacement Therapy with LDL-C< median† n=6461 | ||||||
| No. of CV Events | 43 (2.8%) | 42 (3.1%) | 31 (2.3%) | 20 (1.7%) | 30 (2.8%) | |
| Person-years of follow-up | 15 142 | 13 460 | 13 291 | 11 564 | 10 523 | |
| HR (95% CI) (age-adjusted) | 1 | 1.16 (0.76, 1.78) | 0.84 (0.53, 1.33) | 0.64 (0.38, 1.10) | 0.99 (0.62, 1.57) | 0.76 |
| HR (Framingham-adjusted) ‡ | 1 | 1.26 (0.82, 1.93) | 0.89 (0.55, 1.41) | 0.71 (0.42, 1.21) | 1.00 (0.62, 1.60) | 0.67 |
| HR (fully-adjusted) § | 1 | 1.36 (0.89, 2.09) | 0.90 (0.55, 1.45) | 0.74 (0.43, 1.27) | 1.04 (0.64, 1.67) | 0.73 |
Lp(a) quintile levels were derived from the stratum of women taking hormone replacement therapy.
Framingham-adjusted model refers to adjustments for covariates age, blood pressure categories, current smoking status, total cholesterol and high-density lipoprotein values.
Fully-adjusted models refer to Framingham covariates of age, blood pressure categories, current smoking status, total cholesterol and high density lipoprotein values as well as body mass index, diabetes, C-reactive protein and treatment arms.
Lp(a) quintile levels were derived from women taking hormone replacement therapy.
Median LDL-C value was 121.4 mg/dL.
Framingham-adjusted model refers to adjustments for covariates age, blood pressure categories, current smoking status, total cholesterol and high-density lipoprotein values.
Fully-adjusted models refer to Framingham covariates of age, blood pressure categories, current smoking status, total cholesterol and high density lipoprotein values as well as body mass index, diabetes, C-reactive protein and treatment arms.
In contrast, in women taking HT (Table 3a), the relationship of Lp(a) levels to cardiovascular disease was attenuated, with loss of significance of the relationship between the highest quintile of Lp(a) (≥ 42.0 mg/dL in women taking hormone therapy, with HR of 1.13, top vs bottom quintile) and CVD (P trend 0.18 in fully adjusted model), and even modified among women with elevated LDL, in whom synergistic elevations of CVD risk are usually seen (Table 3b).
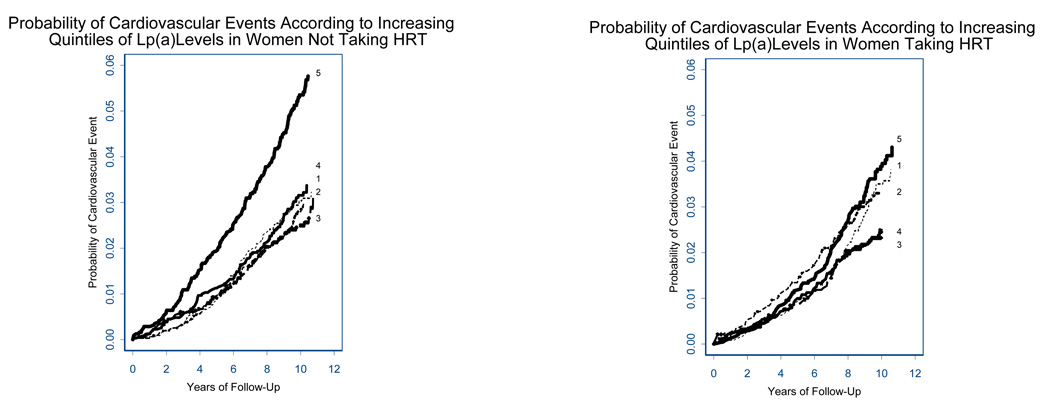
In sensitivity analyses, further adjustment for income and education, and replacement of total cholesterol with LDL, and then of BMI with waist circumference did not change the effect modification shown graphically in Figure 1, where high levels of Lp(a) predict CVD in women free of HT, but not in women on HT. Same relationships are seen in analyses limited to Caucasians only.
Figure 1.
Cumulative Incidence of Major Cardiovascular Disease According to Lipoprotein(a) Quintiles and Hormone Therapy Status
Lp(a) quintile levels were derived separately among women not taking hormone replacement therapy (left) and among women taking hormone replacement therapy at study initiation (right). Quintiles are labeled from 1–5, referring to 1st to 5th quintiles, and were defined separately within the two groups of women.
To address the potential for misclassification of exposure during follow-up, we repeated these analyses censoring follow-up time whenever a change in participant hormone therapy status occurred. In this censored analysis, the adjusted hazard ratio comparing the highest to lowest quintiles of Lp(a) on risk of all CVD was 1.96 (95% CI 1.40–2.76), a value if anything larger than that of the uncensored analysis (HR 1.77, 95% CI 1.36–2.30). In contrast, among women taking hormone therapy, the relationship of Lp(a) levels to cardiovascular disease was attenuated, concordant with the uncensored analysis.
Last, assessing the relationship between Lp(a) and CVD in women taking estrogen vs estrogen plus progestin formulas did not show any differences from the data for hormone replacement therapy as a whole (Appendices 1 and 2).
DISCUSSION
In this prospective study of 27,736 initially healthy women, we found that Lp(a) is a determinant of risk of CVD among women free of hormone replacement therapy, but that little such relationship is seen among HT users. This effect modification persists after adjustment for traditional cardiovascular risk factors, and socioeconomic variables such as income and education. Quantitatively, the hazard ratios are 1.77 in HT − women, and only 1.13 in HT + women, when comparing the top to bottom Lp(a) quintile in respective strata. This effect modification is seen even among women with concomitant elevations of Lp(a) and LDL-C, who have the highest incidence of cardiovascular disease.
We believe that there are at least two different explanations for the effect modification seen. For one, it could be due to biological interaction between plasma Lp(a) levels and concurrent HT use, which is why it was important to measure HT status at the same time blood draws were done. Alternatively, HT use may be a surrogate variable for healthy lifestyles, not fully captured by the covariates addressed here that ameliorate deleterious relationships of lipid biomarkers with CVD. As for potential direct biological effects, it is intriguing that recent data show 1. estrogen may induce increased uptake of Lp(a) by the LDL receptor(39,40), 2. that it may cause reduction of Lp(a) production by the liver(30), where Lp(a) production is likely modulated(41–43), and 3. that certain cholesterol metabolites may interact directly with HT in the vasculature (44).
Strengths of our study include the reliable measurement of Lp(a), using a reproducible apo(a) isoform-independent assay(5,6) that has complicated many prior studies of Lp(a), the large number of women studied, and cardiovascular endpoints. While we consider independence of the assay from apo(a) isoforms a particular strength, it is possible that additional information can be gained by measuring apo(a) isoforms reproducibly. The size of this cohort of women helps us to evaluate what a number of prior epidemiological studies(26–31,45) and randomized controlled trials(46,47), while reported the association of HT with lower Lp(a) levels, were not powered to assess in relationship to actual hard cardiovascular outcomes. Our data addresses this question in a primary prevention cohort of women, and confirm trends reported previously in mostly secondary prevention cohorts (32).
Limitations include the predominantly Caucasian nature of our cohort, which does not allow us to assess potentially different relationships of plasma Lp(a) levels and CVD in different ethnic groups(48–50). Also, HT use in women is controversial, and since the publication of the Women’s Health Initiative(20), HERS I(21) and HERS II trials(22), the American Heart Association(23), the U.S. Food and Drug Administration (FDA)(24) and the U.S. Preventive Services Task Force(25) recommend against the use of estrogen and progestin or progesterone for prevention of chronic conditions nor for the primary or secondary prevention of CVD (51). However, treatment of menopausal symptoms remains an indication for personalized estrogen use, and new practice standards are emerging to help tailor HRT for menopausal symptoms for short-term use(24,52). The ongoing Kronos Early Estrogen Protection Study (KEEPs)(53) and Early Versus Late Intervention Trial with Estradiol (ELITE) trial(54) will add more information about the role of HT therapy.
In summary, these data suggest that the predictive utility of Lp(a) is attenuated among women taking HT. The data may inform clinicians’ interpretation of Lp(a) values in their patients who are concurrently taking HT.
Supplementary Material
Acknowledgments
The authors wish to thank the women who participate in the Women’s Health Study and Drs. Robert J. Glynn, Kathryn Rexrode and Emily G. Kurtz for their input into the analysis in this study.
This work was supported by grants from the Donald W. Reynolds Foundation (Las Vegas, NV) and the Leducq Foundation (Paris, France). The Women’s Health Study is supported by grants from the National Heart, Lung and Blood Institute (HL-43851) and the National Cancer Institute (CA-47988) and can be identified by ClinicalTrials.gov identifier NCT00000479.
Dr. Jacqueline Suk Danik had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Danik receives support from the National Heart, Lung and Blood Institute (HL-076443) and the Michael Lerner Foundation.
Dr. Nader Rifai reports that he receives grant support from Merck Research Laboratories, and has served as a consultant to Sanofi Aventis. He has also received honorarium from Ortho Diagnostics.
Dr. Julie E. Buring has received investigator-initiated research funding and support as a principal investigator from the National Institutes of Health (the National Heart, Lung, and Blood Institute, the National Cancer Institute, and the National Institute of Aging) and Dow Corning Corporation; research support for pills and/or packaging from Bayer Heath Care and the Natural Source Vitamin E Association; honoraria from Bayer for speaking engagements; and serves on an external scientific advisory committee for a study by Procter & Gamble.
Dr. Paul M Ridker reports that he currently or in the past five years has received research funding support from not-for-profit entities including the National Heart, Lung, and Blood Institute, the National Cancer Institute, the American Heart Association, the Doris Duke Charitable Foundation, the Leducq Foundation, the Donald W Reynolds Foundation, and the James and Polly Annenberg La Vea Charitable Trusts. Dr Ridker also reports that currently or in the past five years he has received investigator-initiated research support from for-profit entities including Astra-Zeneca, Bayer, Bristol-Myers Squibb, Dade-Behring, Novartis, Pharmacia, Roche, Sanofi-Aventis, and Variagenics. Dr Ridker reports being listed as a coinventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and has served as a consultant to Schering-Plough, Sanofi/Aventis, AstraZeneca, Isis Pharmaceutical, Dade-Behring, and Interleukin genetics.
The funding agencies played no role in the design, conduct, data management, analysis or manuscript preparation related to this project.
Abbreviations
- Lp(a)
Lipoprotein (a)
- HT
Hormone replacement therapy
- CVD
Cardiovascular disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov identifier NCT00000479 Women’s Health Study (WHS)
REFERENCES
- 1.Lackner C, Cohen JC, Hobbs HH. Molecular definition of the extreme size polymorphism in apolipoprotein(a) Hum Mol Genet. 1993;2:933–940. doi: 10.1093/hmg/2.7.933. [DOI] [PubMed] [Google Scholar]
- 2.McLean JW, Tomlinson JE, Kuang WJ, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 3.Loscalzo J, Weinfeld M, Fless GM, Scanu AM. Lipoprotein(a), fibrin binding, and plasminogen activation. Arteriosclerosis. 1990;10:240–245. doi: 10.1161/01.atv.10.2.240. [DOI] [PubMed] [Google Scholar]
- 4.Ezratty A, Simon DI, Loscalzo J. Lipoprotein(a) binds to human platelets and attenuates plasminogen binding and activation. Biochemistry. 1993;32:4628–4633. doi: 10.1021/bi00068a021. [DOI] [PubMed] [Google Scholar]
- 5.Marcovina SM, Albers JJ, Scanu AM, et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 6.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 7.Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. Jama. 2006;296:1363–1370. doi: 10.1001/jama.296.11.1363. [DOI] [PubMed] [Google Scholar]
- 8.Sigurdsson G, Baldursdottir A, Sigvaldason H, Agnarsson U, Thorgeirsson G, Sigfusson N. Predictive value of apolipoproteins in a prospective survey of coronary artery disease in men. Am J Cardiol. 1992;69:1251–1254. doi: 10.1016/0002-9149(92)91215-p. [DOI] [PubMed] [Google Scholar]
- 9.Wald NJ, Law M, Watt HC, et al. Apolipoproteins and ischaemic heart disease: implications for screening. Lancet. 1994;343:75–79. doi: 10.1016/s0140-6736(94)90814-1. [DOI] [PubMed] [Google Scholar]
- 10.Shai I, Rimm EB, Hankinson SE, et al. Lipoprotein (a) and coronary heart disease among women: beyond a cholesterol carrier? Eur Heart J. 2005;26:1633–1639. doi: 10.1093/eurheartj/ehi222. [DOI] [PubMed] [Google Scholar]
- 11.Assmann G, Schulte H, von Eckardstein A. Hypertriglyceridemia and elevated lipoprotein(a) are risk factors for major coronary events in middle-aged men. Am J Cardiol. 1996;77:1179–1184. doi: 10.1016/s0002-9149(96)00159-2. [DOI] [PubMed] [Google Scholar]
- 12.Bostom AG, Gagnon DR, Cupples LA, et al. A prospective investigation of elevated lipoprotein (a) detected by electrophoresis and cardiovascular disease in women. The Framingham Heart Study. Circulation. 1994;90:1688–1695. doi: 10.1161/01.cir.90.4.1688. [DOI] [PubMed] [Google Scholar]
- 13.Bostom AG, Cupples LA, Jenner JL, et al. Elevated plasma lipoprotein(a) and coronary heart disease in men aged 55 years and younger. A prospective study. Jama. 1996;276:544–548. doi: 10.1001/jama.1996.03540070040028. [DOI] [PubMed] [Google Scholar]
- 14.Rosengren A, Wilhelmsen L, Eriksson E, Risberg B, Wedel H. Lipoprotein (a) and coronary heart disease: a prospective case-control study in a general population sample of middle aged men. Bmj. 1990;301:1248–1251. doi: 10.1136/bmj.301.6763.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berglund L, Ramakrishnan R. Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler Thromb Vasc Biol. 2004;24:2219–2226. doi: 10.1161/01.ATV.0000144010.55563.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a) Jama. 1995;274:1771–1774. [PubMed] [Google Scholar]
- 17.Rader DJ, Brewer HB., Jr Lipoprotein(a). Clinical approach to a unique atherogenic lipoprotein. Jama. 1992;267:1109–1112. doi: 10.1001/jama.267.8.1109. [DOI] [PubMed] [Google Scholar]
- 18.Pan J, Van JT, Chan E, Kesala RL, Lin M, Charles MA. Extended-release niacin treatment of the atherogenic lipid profile and lipoprotein(a) in diabetes. Metabolism. 2002;51:1120–1127. doi: 10.1053/meta.2002.34701. [DOI] [PubMed] [Google Scholar]
- 19.Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med. 1989;226:271–276. doi: 10.1111/j.1365-2796.1989.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 21.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 22.Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) Jama. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672–693. doi: 10.1161/01.CIR.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- 24.Stephenson J. FDA orders estrogen safety warnings: agency offers guidance for HRT use. Jama. 2003;289:537–538. doi: 10.1001/jama.289.5.537. [DOI] [PubMed] [Google Scholar]
- 25.Summaries for patients. Hormone therapy to prevent chronic conditions in postmenopausal women: recommendations from the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:I59. doi: 10.7326/0003-4819-142-10-200505170-00004. [DOI] [PubMed] [Google Scholar]
- 26.Vigna GB, Donega P, Zanca R, et al. Simvastatin, transdermal patch, and oral estrogen-progestogen preparation in early-postmenopausal hypercholesterolemic women: a randomized, placebo-controlled clinical trial. Metabolism. 2002;51:1463–1470. doi: 10.1053/meta.2002.35584. [DOI] [PubMed] [Google Scholar]
- 27.Smolders RG, Vogelvang TE, Mijatovic V, et al. A 2-year, randomized, comparative, placebo-controlled study on the effects of raloxifene on lipoprotein(a) and homocysteine. Maturitas. 2002;41:105–114. doi: 10.1016/s0378-5122(01)00280-8. [DOI] [PubMed] [Google Scholar]
- 28.Ossewaarde ME, Bots ML, Bak AA, et al. Effect of hormone replacement therapy on lipids in perimenopausal and early postmenopausal women. Maturitas. 2001;39:209–216. doi: 10.1016/s0378-5122(01)00224-9. [DOI] [PubMed] [Google Scholar]
- 29.Mijatovic V, Kenemans P, Netelenbos JC, et al. Oral 17 beta-estradiol continuously combined with dydrogesterone lowers serum lipoprotein(a) concentrations in healthy postmenopausal women. J Clin Endocrinol Metab. 1997;82:3543–3547. doi: 10.1210/jcem.82.11.4357. [DOI] [PubMed] [Google Scholar]
- 30.Taskinen MR, Puolakka J, Pyorala T, et al. Hormone replacement therapy lowers plasma Lp(a) concentrations. Comparison of cyclic transdermal and continuous estrogen-progestin regimens. Arterioscler Thromb Vasc Biol. 1996;16:1215–1221. doi: 10.1161/01.atv.16.10.1215. [DOI] [PubMed] [Google Scholar]
- 31.Godsland IF. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: analysis of studies published from 1974–2000. Fertil Steril. 2001;75:898–915. doi: 10.1016/s0015-0282(01)01699-5. [DOI] [PubMed] [Google Scholar]
- 32.Shlipak MG, Simon JA, Vittinghoff E, et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. Jama. 2000;283:1845–1852. doi: 10.1001/jama.283.14.1845. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 34.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 35.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 36.Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47:418–425. [PubMed] [Google Scholar]
- 37.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 38.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 39.Sacks FM, McPherson R, Walsh BW. Effect of postmenopausal estrogen replacement on plasma Lp(a) lipoprotein concentrations. Arch Intern Med. 1994;154:1106–1110. [PubMed] [Google Scholar]
- 40.Hofmann SL, Eaton DL, Brown MS, McConathy WJ, Goldstein JL, Hammer RE. Overexpression of human low density lipoprotein receptors leads to accelerated catabolism of Lp(a) lipoprotein in transgenic mice. J Clin Invest. 1990;85:1542–1547. doi: 10.1172/JCI114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuck CH, Holleran S, Berglund L. Hormonal regulation of lipoprotein(a) levels: effects of estrogen replacement therapy on lipoprotein(a) and acute phase reactants in postmenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:1822–1829. doi: 10.1161/01.atv.17.9.1822. [DOI] [PubMed] [Google Scholar]
- 42.Rader DJ, Cain W, Zech LA, Usher D, Brewer HB., Jr Variation in lipoprotein(a) concentrations among individuals with the same apolipoprotein (a) isoform is determined by the rate of lipoprotein(a) production. J Clin Invest. 1993;91:443–447. doi: 10.1172/JCI116221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rader DJ, Cain W, Ikewaki K, et al. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J Clin Invest. 1994;93:2758–2763. doi: 10.1172/JCI117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 45.Brown SA, Hutchinson R, Morrisett J, et al. Plasma lipid, lipoprotein cholesterol, and apoprotein distributions in selected US communities. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1993;13:1139–1158. doi: 10.1161/01.atv.13.8.1139. [DOI] [PubMed] [Google Scholar]
- 46.Meschia M, Bruschi F, Soma M, Amicarelli F, Paoletti R, Crosignani P. Effects of oral and transdermal hormone replacement therapy on lipoprotein(A) and lipids: a randomized controlled trial. Menopause. 1998;5:157–162. [PubMed] [Google Scholar]
- 47.Shewmon DA, Stock JL, Rosen CJ, et al. Tamoxifen and estrogen lower circulating lipoprotein(a) concentrations in healthy postmenopausal women. Arterioscler Thromb. 1994;14:1586–1593. doi: 10.1161/01.atv.14.10.1586. [DOI] [PubMed] [Google Scholar]
- 48.Christodoulakos GE, Lambrinoudaki IV, Panoulis CP, Papadias CA, Kouskouni EE, Creatsas GC. Effect of hormone replacement therapy, tibolone and raloxifene on serum lipids, apolipoprotein A1, apolipoprotein B and lipoprotein(a) in Greek postmenopausal women. Gynecol Endocrinol. 2004;18:244–257. doi: 10.1080/09513590410001715207. [DOI] [PubMed] [Google Scholar]
- 49.Kalogeropoulos S, Petrogiannopoulos C, Gagos S, Kampas N, Kalogeropoulos G. The influence of 5-year therapy with tibolone on the lipid profile in postmenopausal women with mild hypercholesterolemia. Gynecol Endocrinol. 2004;18:227–232. doi: 10.1080/09513590410001667238. [DOI] [PubMed] [Google Scholar]
- 50.Sumino H, Ichikawa S, Sakamoto H, et al. Effects of conjugated equine estrogen and medroxyprogesterone acetate on lipoprotein(a) and other lipoproteins in japanese postmenopausal women with and without dyslipidemia. Horm Res. 2004;62:1–9. doi: 10.1159/000077399. [DOI] [PubMed] [Google Scholar]
- 51.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 52.Nelson HD. Postmenopausal estrogen for treatment of hot flashes: clinical applications. Jama. 2004;291:1621–1625. doi: 10.1001/jama.291.13.1621. [DOI] [PubMed] [Google Scholar]
- 53.Harman SM, Brinton EA, Cedars M, et al. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 54.ClinicalTrials.gov identifier NCT00114517
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.