Abstract
We report a case of a patient with invasive breast carcinoma which demonstrated HER-2 gene amplification on core biopsy, who relapsed while on adjuvant Trastuzumab therapy following her mastectomy and ultimately died 15 months after diagnosis. Surprisingly, analysis of multiple metastases harvested at rapid autopsy demonstrated no evidence of HER-2 gene amplification. Retrospective examination of the carcinoma in the patient’s mastectomy specimen revealed only focal HER-2 amplification within the tumor, localized to the region of the prior core biopsy site. This case highlights several important issues in HER-2 testing of breast cancer, including core biopsy-excision specimen discordance, primary-metastasis discordance, and potential selection for unamplified portions of a heterogeneously-amplified tumors by Trastuzumab.
Keywords: Breast, Metastasis, HER-2, Autopsy
Introduction
Trastuzumab is a highly effective, relatively non-toxic therapy for the approximately 15% of breast cancers which overexpress Her-2 protein, typically as a result of HER-2 gene amplification. Previously, Her-2 status was almost always determined on the patient’s primary cancer resection specimen (lumpectomy or mastectomy), which would allow identification of intratumoral heterogeneity of HER-2 gene amplification and/or protein overexpression. Currently, Her-2 status is more often performed on core biopsy specimens, due to the more prevalent use of neoadjuvant chemotherapy. However, a core biopsy may not detect intratumoral heterogeneity of HER-2 gene amplification or protein expression. We present a distinctive case illustrating the clinical consequences of this dilemma and suggest possible approaches to address this potential problem.
Clinical Case
The patient was a 34-year-old female who presented with a 2.3 cm left breast mass. Core biopsy demonstrated an infiltrating ductal carcinoma positive for estrogen receptor (10% of cells labeled with moderate intensity) and progesterone receptor (10% of cells labeled strongly). Her-2 protein expression by immunohistochemistry was scored as 2+ (equivocal); however, HER-2 fluorescence in situ hybridization (FISH) analysis showed gene amplification (HER-2/Chromosome 17 centromere ratio=5.6). One month after initial presentation, the patient underwent bilateral mastectomy. The left breast revealed a 2.7-cm infiltrating ductal carcinoma, Elston grade III of III, in the lower outer quadrant. One of eleven axillary lymph nodes contained metastatic carcinoma. The right breast was free of cancer. One month later, she was started on a regimen of 4 cycles of Adriamycin/Cyclophosphamide chemotherapy followed by weekly Taxol plus Trastuzumab for 12 weeks followed by 9 more months of Trastuzumab. She was also placed on Tamoxifen.
Eleven months after beginning treatment, the patient experienced increased shortness of breath. A CT scan showed a large pleural effusion and pleural satellite lesions consistent with metastasis. She was thought to be refractory to Trastuzumab, and was started on Tykerb plus Xeloda as palliative therapy. Despite aggressive therapy, the patient continued to experience drainage from her right chest tube site, recurrent fevers, and increasing pain. She was admitted to hospice care two months later and expired within one month, a total of 15 months after diagnosis. Before death, the patient had consented to participate in a rapid autopsy study at Johns Hopkins. Upon her death, additional consent was obtained from her husband and a complete autopsy was performed 4 hours later. This post mortem interval is similar to that which usually occurs between removal of a surgical breast specimen, its dissection, and immersion in formalin, so that the autopsy specimens are comparable to those derived from a surgical pathology lab.
Results
At autopsy, on gross examination there was diffuse metastatic involvement of the right lung, diaphragm, and pleural cavity. Microscopic examination showed evidence of disease also involving her left lower lung lobe and mediastinal lymph nodes; however, no evidence of disease was found below the diaphragm. A tissue microarray (TMA) was constructed from formalin-fixed, paraffin-embedded tissue blocks of the metastatic cancers harvested at autopsy, along with the patient’s primary tumor samples from her prior core needle biopsy and from her mastectomy. The TMA consisted of 99 spots, each 1.4 mm in diameter. (Figure 1). These included 4 spots from the core biopsy, 8 spots from the mastectomy specimen, 50 spots from 10 different grossly-identified metastases (4 lung, 3 pleural, 2 mediastinal, 1 diaphragm), 28 spots from normal tissues (including normal breast from the cancer resection specimens), and 9 spots from unrelated control tissues. Multiple spots were taken from each lesion to account for intratumoral heterogeneity and for loss of individual spots on serial sections. Her-2 IHC and FISH analysis was performed as previously described, using the Dako Herceptest kit (Carpinteria, CA) and the Path Vysion FISH kit (Des Plains, IL)[1].
Figure 1.
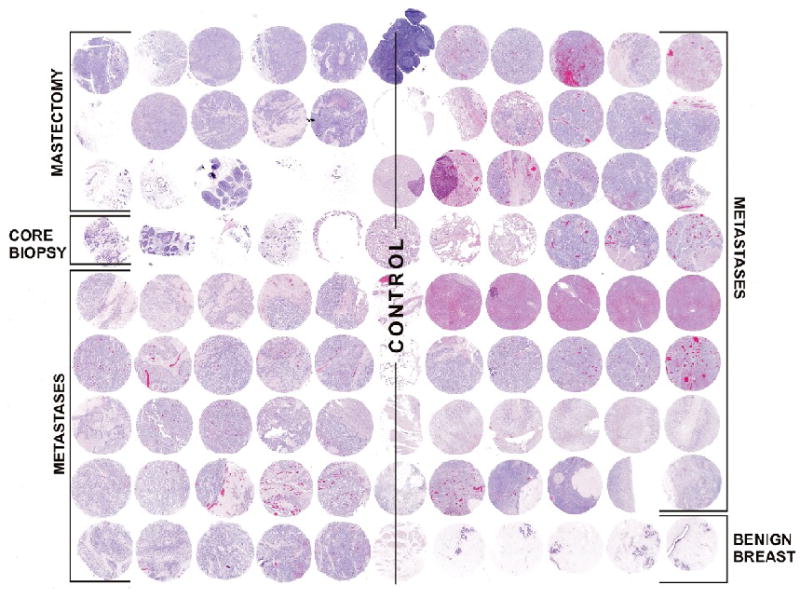
Single Patient Tissue Microarray Constructed from Patient’s Primary Tumor Core Biopsy Specimen, Mastectomy Specimen, and Multiple Metastases harvested at Autopsy.
FISH analysis of the TMA revealed HER-2 gene amplification in the initial core biopsy in 2 of 2 evaluable spots (ratios 4.07 and 4.43) (Figure 2A). Analysis of the excision specimen showed HER-2 gene amplification in 1 of 4 evaluable spots (ratio, 3.03) (Figure 2B), but the others (Figure 2C) were non-amplified . Surprisingly, none of the 35 evaluable spots from the 10 metastatic lesions (Figure 2D) showed HER-2 amplification. IHC for Her-2 protein paralleled the FISH results; the two spots from the core biopsy and the one spot from the mastectomy were scored as 2+, the rest were scored as 0 (negative). FISH and IHC were then performed on whole sections of the mastectomy specimen. FISH demonstrated amplification only in the area of previous core biopsy site (ratio=2.90), which constituted approximately 5% of the total tumor area. IHC demonstrated heterogeneity of labeling for Her-2 protein which paralleled the FISH results (Figure 3).
Figure 2.
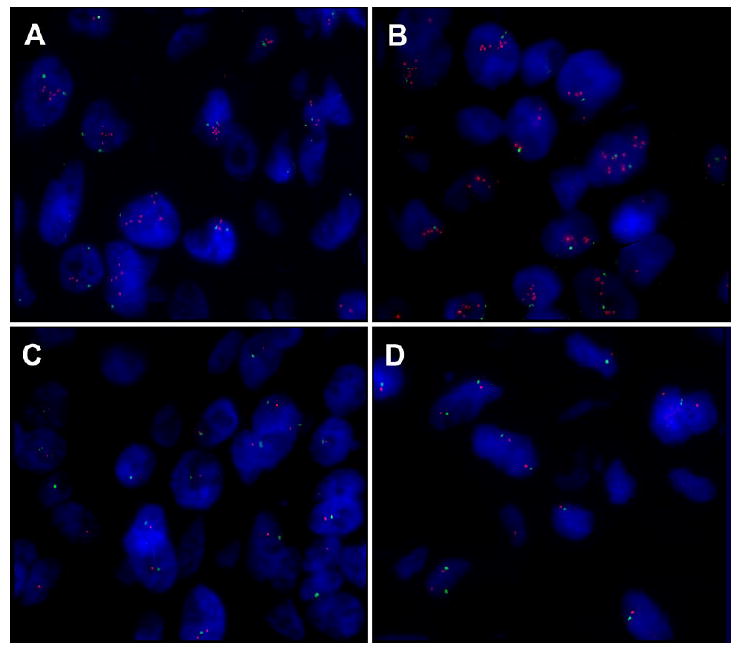
Fluorescence in Situ Hybridization (FISH) Analysis of HER-2 Gene Amplification in Primary Tumors and Metastases. Orange signal is HER-2 probe, green signal is centromere probe. A. Primary tumor in initial core biopsy shows HER-2 gene amplification. B. Focal area of primary tumor in mastectomy specimen shows HER-2 gene amplification. C. Other areas of primary tumor in mastectomy specimen do not show HER-2 gene amplification. D. No metastases showed evidence of HER-2 gene amplification.
Figure 3.
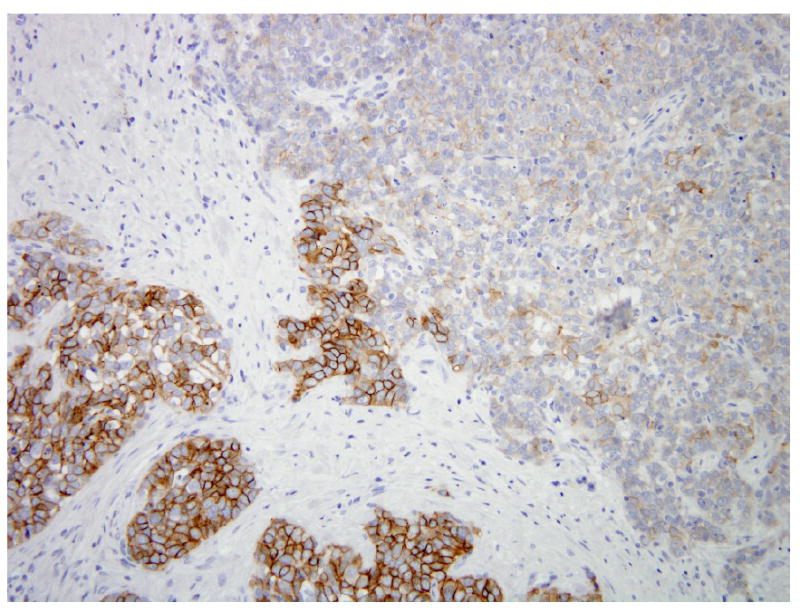
Heterogeneous HER-2 protein expression in primary tumor from mastectomy specimen by Immunohistochemistry (Dako Herceptest), ranging from 0 (upper right) to 3+ (lower left).
Discussion
This case highlights several important questions regarding Her-2 analysis of breast cancer. First, how accurately do core biopsies reflect the status of excision specimens? The consensus in the published literature (2, 3) is that the status is usually concordant. However, heterogeneity of HER-2 gene amplification in breast cancer is now well recognized, particularly in cases with equivocal IHC or FISH results (4,5,6). This can reflect distinct well demarcated clusters of amplified cells, or individual amplified cells scattered amongst unamplified cells. Variability in inclusion or exclusion of these cells in scoring between the observers can lead to differing results. A recent study suggested that FISH analysis of cases with equivocal 2+ IHC labeling on core biopsy “almost completely resolves the issue of heterogeneous expression of HER-2” (7). However, closer inspection of the data in that study indicates that 22% of the cases which demonstrated amplification on the core biopsy did not show amplification in the excision specimen. One wonders if these cases were similar to ours, where the core sampled a focus of HER-2 amplified cancer cells in a background of unamplified cells. We suggest that it may be wise to repeat the HER-2 IHC and FISH analysis in the excision specimens of cases which show either borderline HER-2 overexpression (2+ score) or gene amplification (ratio 1.8-2.2) on core biopsies. Regardless, at this time, the clinical consequences of HER-2 heterogeneity remain incompletely understood.
Second, how often is Her-2 status different in metastases compared to the primary tumor? Some of the IHC data on this cites high percentages of discordance, but differences in fixation between primary and metastases likely account for some of this IHC discordance (8). FISH analysis of HER-2 gene amplification minimizes the confounding variable of fixation. FISH analyses have shown a discordance rate ranging from 0 to 22% (8,9), and the conclusions reached by the authors of these studies have been disparate. One study found a 7% discordance rate between metastases and primary tumors by FISH, leading the authors to state that retesting of metastases was largely unnecessary (10). Another found a lower (2.9%) discordance rate, yet advocated retesting all metastases (11). A reasonable approach may be to consider biopsy and retesting of metastases of patients whose primary tumors showed amplification but do not respond to Trastuzumab. If the metastases do not show protein overexpression and amplification, one could consider stopping Trastuzumab therapy, particularly if cardiac toxicity is a major clinical concern in that patient. Whether retesting of metastases of non-amplified primary tumors is appropriate is a separate issue, but one report demonstrating acquisition of amplification in 22% of evaluated metastases (4 of 18) would support this practice (8).
Third, does Trastuzumab therapy select for relapse by the unamplified portions of a patient’s tumor? In a heterogeneously amplified tumor, given that HER-2 activates multiple downstream growth pathways, one would expect the portions with HER-2 amplification to grow differently than the unamplified portions. While examination of the mastectomy specimen in our case revealed that only a minority of the primary tumor was HER-2 amplified, the fact that that this “clone” did not appear in any metastatic site suggests negative selection by Trastuzumab. Several prior publications suggest that Trastuzumab may select for Her-2 negative tumor cells in the metastatic setting. Kunitomo et al (12) reported a case of metastatic breast cancer in which the primary tumor showed heterogeneity of HER-2 amplification. The patient’s liver metastases (presumably HER-2 amplified) resolved on Trastuzumab therapy, while the pleural effusion which persisted contained unamplified cells. Salomon at al (13) reported a case of a relapsed axillary metastasis which showed heterogeneous Her-2 overexpression by IHC and responded completely to Trastuzumab therapy. A new axillary relapse 8 months later was Her-2 negative. Addition, Salomon et al reported a chest wall relapse which initially overexpressed Her-2 by IHC and partially responded to Trastuzumab; the residual tumor did not overexpress Her-2 by IHC. Pectasides et al (14) demonstrated that 6 of 16 metastatic carcinomas which were HER-2 FISH-positive before Trastuzumab therapy developed additional FISH-negative metastases. Finally, in the neoadjuvant setting, Burstein et al. (15) showed that 4 of 23 (17%) of residual cancers which had been strongly positive for Her-2 (3+) by IHC became negative (0 or 1+) after Trastuzumab therapy. How should this data affect clinical practice? One might consider retesting cancers which persist or relapse while on Trastuzumab therapy to be sure that they are still amplified; if not, one could consider stopping Trastuzumab and moving to other therapeutic agents
Finally, our case highlights the educational and research value of the rapid autopsy in the study of breast cancer metastases. Without the autopsy findings, this patient would have been erroneously classified as showing primary Trastuzumab resistance in the face of documented gene amplification. Instead, the autopsy demonstrated the otherwise unappreciated clinical significance of heterogeneous HER-2 amplification in her cancer.
Acknowledgments
Supported by DOD Center of Excellence W81XWH-04-1-0595, and NIH P50 CA88843
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu JM, Fackler MJ, Halushka MK, et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs TW, Siziopikou KP, Prileau JE, et al. Do prognostic marker studies on core needle biopsy specimens of breast carcinoma accurately reflect the marker status of the tumor? Mod Pathol. 1998;11:259–264. [PubMed] [Google Scholar]
- 3.Mueller-Holzer E, Fink V, Frede T, Marth C. Immunohistochemical determination of HER2 expression in breast cancer from ore biopsy specimens: a reliable predictor of HER2 status of the whole tumor. Breast Cancer Res Treat. 2001;69:13–19. doi: 10.1023/a:1012281221647. [DOI] [PubMed] [Google Scholar]
- 4.Vance GH, Barry TS, Bloom KJ, et al. Genetic heterogeneity in HER2 testing in breast cancer. Panel summary and guidelines. Arch Pathol Lab Med. 2009;133:611–612. doi: 10.5858/133.4.611. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JT, Ketterling RP, Halling KC, et al. Analysis of intratumoral heterogeneity and amplification status of breast carcinoma with equivocal (2+) her-2 immunostaining. Am J Clin Pathol. 2005;124:273–281. doi: 10.1309/J9VX-ABUG-KC4Y-07DL. [DOI] [PubMed] [Google Scholar]
- 6.Brunelli M, Manfrin E, Martignoni G, et al. Genotypic intratumoral heterogeneity in breast carcinomas with Her2/neu amplification. Am J Clin Pathol. 2009;131:678–682. doi: 10.1309/AJCP09VUTZWZXBMJ. [DOI] [PubMed] [Google Scholar]
- 7.Chivukula M, Bhargava R, Brufsky A, et al. Clinical importance of HER2 immunohistologic heterogeneous expression in core-needle biopsies vs resection specimens for equivocal (immunohistochemical score 2+) cases. Mod Pathol. 2008;21:363–368. doi: 10.1038/modpathol.3801021. [DOI] [PubMed] [Google Scholar]
- 8.Regitnig P, Schippinger W, Lindbauer M, et al. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J Pathol. 2004;203:918–926. doi: 10.1002/path.1592. [DOI] [PubMed] [Google Scholar]
- 9.Tanner M, Jarvinen P, Isola J. Amplification of HER-2/neu and Topoisomerase IIα in primary and metastatic breast cancer. Cancer Res. 2001;61:5345–5348. [PubMed] [Google Scholar]
- 10.Gancberg D, Di Leo A, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–1043. doi: 10.1093/annonc/mdf252. [DOI] [PubMed] [Google Scholar]
- 11.Tapia C, Savic S, Wagner U, et al. Her2 status in primary breast cancers and matched distant metastases. Breast Cancer Res. 2007;9:R31. doi: 10.1186/bcr1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunimoto K, Inoue S, Ichihara F, et al. A case of metastatic breast cancer with outgrowth of Her2-negative cells after eradication of Her-2 positive cells by humanized anti-Her2 monoclonal antibody (trastuzumab) combined with docetaxel. Hum Pathol. 2004;35:379–391. doi: 10.1016/j.humpath.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Cottu PH, Asselah J, Lae M, et al. Intratumoral heterogeneity of Her2/neu expression and its consequences for the management of advanced breast cancer. Ann Oncol. 2008;19:595–597. doi: 10.1093/annonc/mdn021. [DOI] [PubMed] [Google Scholar]
- 14.Pectasides D, Gaglia A, Arapantoni-Dadioti P, et al. HER-2/neu status of primary breast cancer and corresponding metastatic sites in patients with advanced breast cancer treated with trastuzumab-based therapy. Anticancer Res. 2006;26(1B):647–653. [PubMed] [Google Scholar]
- 15.Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and pactaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for Her2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol. 2003;21:46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]


