Abstract
Ischemic preconditioning (IPC) is a protective phenomenon in which brief ischemia renders the myocardium resistant to subsequent ischemic insults. Here, we used A2BAR gene knock-out (A2BKO)/β-galactosidase reporter gene knock-in mice and the A2BAR antagonist ATL-801 to investigate the potential involvement of the A2BAR in IPC, focusing on the acute phase of protection. Cardioprotection provided by acute IPC elicited by two 3-min occlusion/3-min reperfusion cycles was readily apparent in an isolated, Langendorff-perfused mouse heart model in studies using hearts from A2BKO mice. IPC equivalently improved the recovery of contractile function following 20 min of global ischemia and 45 min of reperfusion in both WT and A2BKO hearts by ~30–40%, and equivalently decreased the release of cardiac tropinin I during the reperfusion period (from 5,969±925 to 1,595±674 ng/g and 4,376±739 to 2,278±462 ng/g using WT and A2BKO hearts, respectively). Similarly, the infarct size-reducing capacity of acute IPC in an in vivo model of infarction was fully manifest in experiments using A2BKO mice, as well as in experiments using rats pretreated with ATL-801. We did observe, however, a marked reduction in infarct size in rats following administration of the selective A2BAR agonist BAY 60-658 (~25% reduction at a dose of 1.0 mg/kg). While supportive of its concept as a cardioprotective receptor, these experiments indicate that the mechanism of the early phase of IPC is not dependent on signaling by the A2BAR. We present the idea that the A2BAR may contribute to the later stages of IPC dependent on the induction of stress-responsive genes.
Introduction
Ischemic preconditioning is a phenomenon whereby exposure to brief periods of ischemia renders the myocardium resistant to subsequent ischemic insults, manifest as a reduction in myocardial infarct size [1]. IPC appears to consist of two phases, an acute phase (early IPC) that develops immediately but wanes within 1–2 hrs, and a delayed phase (late IPC) that appears 12–24 h later but lasts for several days [2–4]. The time-course and duration of the delayed phase of IPC is consistent with a mechanism involving the synthesis of cardioprotective proteins [5], whereas the early phase is explained by metabolic slowing that preserves stores of high energy phosphates thereby promoting cell survival [6]. The early phase of IPC can be elicited in isolated heart and cardiomyocyte models of ischemic injury, inferring that the mechanism of protection is intrinsic to the cardiac muscle [7, 8].
Current evidence suggests that adenosine and other factors (i.e., opioid peptides and bradykinin) released during preconditioning ischemia serve to initiate the development of the cardioprotected phenotype associated with IPC [9]. Although there is support for involvement of the A3AR, most evidence implicates the A1 in IPC [10–13], which is the predominant AR subtype expressed in cardiac myocytes well-known to regulate heart rate and to suppress responses to β-adrenergic stimulation [14, 15]. Previous studies have identified the importance of the A1AR in IPC using pharmacological strategies and gene knock-out mice [9–13, 16].
It has recently been reported by Eckle and colleagues [16, 17], however, that cardioprotection by what appears to be the early phase of IPC is completely lost in a commercially available line of A2BKO mice, suggesting that the A2BAR also plays an important role in the mechanism of IPC. These studies also reported that IPC protection is absent in gene-ablated mice lacking the extracellular adenosine-generating enzyme ecto-5′-nucleotidase (CD73). Based on these observations, the authors developed the intriguing hypothesis that, as a result of the presence of hypoxia-inducible-1 (HIF-1) elements within their respective gene promoters, the level of expression of the A2BAR and CD73 are increased following IPC. Through a combined increase in adenosine production by CD73 and expression of functional A2BARs in the myocardium, the theory proposes that signaling via the A2BAR is increased during reperfusion providing physiological cardioprotection. While this hypothesis is relevant to the late phase of IPC, it does not account for the finding that the early phase of IPC is not prevented by protein synthesis inhibitors [18].
In this study, we sought to confirm and extend our understanding of the contribution of the A2BAR in IPC, focusing exclusively on the acute phase of protection. Our studies utilized A2BAR gene knock-out/β-galactosidase reporter gene knock-in mice (A2BKO; [19]) and the selective A2BAR antagonist ATL-801 [20].
Materials and Methods
Animals
All experiments were performed with male mice weighing ~25–30 g (12–16 weeks of age) and male rats weighing ~250–350 g (8–12 weeks). C57BL/6J wild-type (WT) mice were purchased from Jackson Laboratories, (Bar Harbor ME) and Sprague-Dawley rats were purchased from Harlan Laboratories (Madison, WI). A2BAR gene KO/β-galactosidase reporter gene “knock-in” mice made congenic on the C57BL/6J genetic background were a kind gift from Dr. Katya Ravid (Boston University; [19]. All animals in the study received humane care in accordance with the guidelines established by the Biomedical Resource Center of the Medical College of Wisconsin, which conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication 85–23, revised 1996) and is accredited by the American Association of Laboratory Animal Care (AALAC).
Langendorff-perfused isolated mouse heart model
Experimental preparation
Mouse hearts were isolated from WT or A2BKO mice and perfused by the Langendorff method, as described previously [21, 22]. Briefly, mice were anesthetized with sodium pentobarbital (100 mg/kg i.p.). Immediately upon achievement of complete anesthesia, the hearts were removed and arrested in ice-cold perfusion solution. The hearts were cannulated via the aorta and perfused retrogradely by the Langendorff method at a continuous pressure of 80 mmHg using Krebs-Henseleit (KH) buffer containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM KH2PO4, 0.5 mM EDTA, 25 mM NaHCO3, and 11 mM glucose. The perfusion buffer was equilibrated with 95% O2/5% CO2 at 37°C and filtered through a 0.22 μm in-line Sterivex filter unit (Millipore, Bedford, MA) to remove particulate matter. A polyethylene drain was inserted in the apex of each heart, and a fluid-filled balloon was inserted through the left atrium and mitral valve to occupy the left ventricular cavity. The balloon was connected to a pressure transducer (ADInstruments, Inc., Colorado Springs, CO) for continuous acquisition of left ventricular pressure. The hearts were immersed in perfusion buffer maintained at 37° C, and the balloons were inflated to achieve end-diastolic pressures of 5 – 10 mmHg. Coronary flow was monitored by an in-line flow probe connected to a flowmeter (model T206; Transonics Systems Inc., Ithaca, NY). The left ventricular (LV) pressure signals were acquired continuously using a PowerLab data acquisition system (ADInstruments) and processed (Chart software) to yield heart rate and LV dP/dt.
Experimental protocol
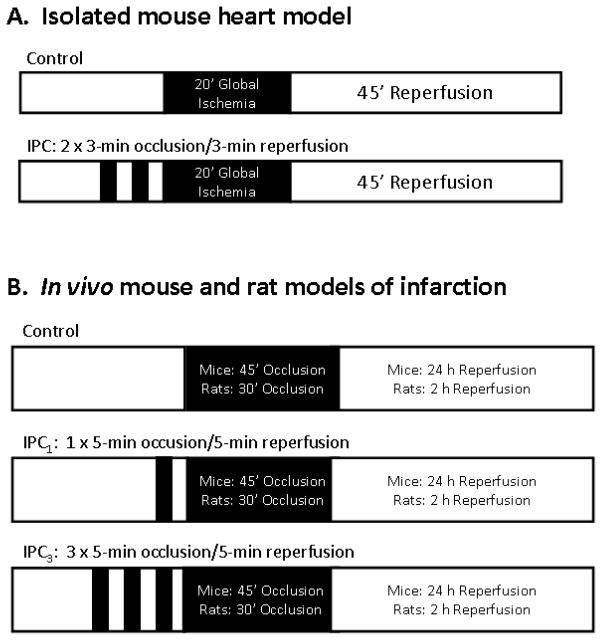
The experimental protocol for the isolated heart experiments is illustrated in Fig. 1. Hearts were perfused for 20 min to allow for stabilization and then perfused for an additional 15 min while pacing at 420 beats/min (ventricular pacing with 2-ms square waves and a voltage set 20% above threshold). Following acquisition of baseline measurements, the hearts were randomly allocated to a control group (Con) or to an ischemic preconditioned group (IPC) in which the hearts were subjected to two cycles of 3-min occlusion/3-min reperfusion achieved by closing and opening an in-line stopcock. Both groups of hearts were subsequently subjected to 20 min of no-flow ischemia and 45 min of reperfusion during which the recovery of contractile function was assessed. During the reperfusion period, the coronary perfusate was collected and stored at −80° C until assayed for cardiac troponin I (cTnI) content by radioimmunoassay (Life Diagnostics, Inc., West Chester, PA). To collect the effluent, the hearts were re-immersed in fresh perfusion buffer immediately before reperfusion and the effluent mixed in bathing buffer was collected for the entire 45-min reperfusion period. cTnI was measured in an aliquot of the effluent/bathing buffer and calculated as the total amount released during the reperfusion period per g of heart weight.
Figure 1.
Schematic diagram of the experimental protocols. Abbreviations: IPC, ischemic preconditioning; O, occlusion; R, reperfusion.
In vivo rat and mouse models of infarction
Experimental preparations
The in vivo rat and mouse models of infarction have been described previously in detail [21–23]. The rat model was an acute model involving 2 h of reperfusion (Fig. 1). The mouse model involved recovery surgery allowing for a longer reperfusion period (24 h; Fig. 1).
For the mouse model, the mice were anesthetized with sodium pentobarbital (75 mg/kg i.p.) and then respirated (model 845, Harvard Apparatus; tidal volume = 200 μl; rate =125 strokes/min) via an endotracheal tube with room air supplemented with 100% oxygen to maintain blood gases within normal limits. The electrocardiogram (limb lead II configuration) was continuously recorded (Powerlab) using needle electrodes and rectal temperature was controlled at 36.5°C throughout the experiments using a servocontrolled heating pad. Coronary occlusion and reperfusion was achieved by passing an 8–0 nylon suture under the left coronary artery (LCA) at the point of its emergence from under the left atrial appendage. Ischemia and subsequent reperfusion were accomplished respectively by tying and then loosening the suture around a piece of wetted gauze. Upon termination of the occlusion period, the chest wall was closed with 7–0 polypropylene suture with one layer to close the thoracic cavity and one to close the skin and musculature. The mice were then removed from the ventilator and monitored in a warm, oxygen-enriched environment. The endotracheal tube was removed as the mice regained their righting reflex.
For the rat model, rats were anesthetized with thiobarbital sodium (100 mg/kg i.p.) and respirated via a tracheal tube with room air supplemented with 100% oxygen to maintain appropriate blood gas levels. Body temperature was maintained with a heating pad. Following a thoracotomy, a 6–0 prolene suture was passed below the left descending vein and left coronary artery to allow for occlusion and reperfusion. Prior to subjecting the rats to ischemia, the jugular vein and carotid artery were cannulated to allow for drug administration and determination of heart rate and aortic blood pressure, respectively.
Experimental protocols
The experimental protocols for the mouse and rat models of infarction are depicted in Fig. 1. The mouse model consisted of 45 min of coronary occlusion followed by 24 h of reperfusion, whereas the rat model consisted of 30 min of coronary occlusion and 2 h of reperfusion. In both models, IPC was elicited by either one (IPC1) or three (IPC3) 5-min coronary occlusion/5-min reperfusion cycles applied immediately before the sustained occlusion period. Control, non-preconditioned mice or rats (Con) received no intervention prior to the sustained occlusion. For the mouse studies, all three protocols (Con, IPC1, and IPC3) were conducted with either WT mice or A2BKO mice. For the rat studies, experiments were conducted with animals pretreated intravenously 10 min before the first occlusion with the selective A2BAR antagonist ATL-801 (1 mg/kg i.v.; [20]) or equivalent vehicle. Using methods described previously for mouse and human ARs [20], ATL-801 was determined in radioligand binding studies to bind to the rat A2BAR with a Ki value of 47.5 ± 7.0 nM. In comparison, ATL-801 bound to the rat A1AR, A2AAR, and A3AR with Ki values that were >1 μM, >10 μM, and >10 μM, respectively. In preliminary experiments, we determined that pretreating rats with 1 mg/kg of ATL-801 blocked completely the hypotensive effect of bolus administration of the selective A2BAR agonist BAY 60-6583 (1 mg/kg i.v.; Fig 2).
Figure 2.
ATL-801 effectively blocks the hypotensive effect of the A2BAR agonist BAY 60-6583 in rats. Rapid bolus administration of BAY 60,6583 (1 mg/kg i.v.) produced a transient (~5 min) reduction in mean arterial blood pressure in anesthetized rats. This effect of ATL-801 was blocked completely in rats pretreated with ATL 801 (1 mg/kg). Blockade persisted for at least 2 h. Shown is a representative tracing from three different experiments.
We performed an additional experimental protocol in the rat infarction model to determine whether direct activation of the A2BAR with BAY 60-6583 produces a reduction in infarct size. Rats were subjected to 30 min of coronary occlusion followed by 2 h of reperfusion. BAY 60-6583 (0.3 or 1.0 mg/kg i.v.) or equivalent vehicle (PEG-400) was administered as an i.v. bolus 10 min before occlusion in control rats and in rats pretreated with ATL-801 (1 mg/kg i.v. 10 min before administration of BAY 60-6583).
Measurement of ischemic area and infarct size
For the mouse studies, the mice were re-anesthetized with pentobarbital, intubated, and ventilated following the 24-h reperfusion period. To delineate the ischemic area at risk (AAR), the LCA was re-occluded and 100 μl of a 5% phthalo blue dye solution was injected into the LV cavity while the aorta was occluded. After a few seconds of perfusion, the heart was excised and washed in PBS. The pericardium, aorta, atria, and right ventricle were removed and the remaining left ventricle was sliced into 5–6 sections of uniform thickness. The sections were then incubated in a 0.5% triphenyltetrazolium chloride (TTC) solution in phosphate-buffered saline for 20 min at 37°C to stain live tissue red, and then fixed in 10% formaldehyde for 24 hours. The sections were photographed on both sides using a dissecting microscope fitted with a SPOT Insight digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI), blotted dry, and weighed. The infarcted area, AAR (combined infarcted tissue and viable red tissue), and non-ischemic area of the left ventricle (blue stained tissue) were measured by digital planimetry. The AAR size was calculated as a percentage of the entire left ventricle, and infarct size was determined as a percentage of the AAR.
A similar dual staining protocol was used to assess infarct size in the rat model. Upon completion of the experimental protocols, the coronary artery was re-occluded and patent blue dye was injected into the jugular vein to delineate the AAR. The hearts were then cut into 5 thin cross-sections and the AAR tissue samples were placed in a 1% phosphate-buffered TTC solution for 15 min at 37°C. Tissues were stored in 10% formaldehyde overnight. AAR and infarct sizes were calculated following gravimetric analysis. In all of the in vivo rat and mouse experiments, personnel responsible for measuring infarct size were blinded to the experimental protocol (i.e., control or IPC) and the genotype/pharmacological treatment.
Statistical analysis
All data are presented as means ± S.E.M. All parameters were analyzed by two-way ANOVA (genotype/pharmacological treatment and ± IPC) to determine whether there was a main effect of genotype/pharmacological treatment, IPC, or an interaction. A repeated-measures format was used to analyze hemodynamic data from the in vivo experiments. If global tests showed a main effect or interaction, post hoc analysis was performed using an unpaired t test with the Bonferroni correction or a Dunnett’s t test (hemodynamic data).
Results
Isolated mouse heart studies
Baseline functional data from the isolated mouse heart studies are reported in Table 1. During pacing at 420 bpm, there were no differences in any of the parameters measured at baseline including LV developed pressure (DP), maximal LV +/−dP/dt, and coronary flow. Following 20 min of global ischemia and 45 min of reperfusion, recovery of contractile function averaged ~50% of baseline values in the control, non-preconditioned group (Fig. 3). Although not statistically significant, tolerance to ischemia/reperfusion injury tended to be less (~42% recovery) in experiments using hearts from A2BKO mice (Fig. 3).
Table 1.
Baseline data from the isolated mouse heart studies.
| n | Age (weeks) | Heart Weight (g) | Body Weight (g) | DP (mmHg) | Max+dP/dt (mmHg/s) | Max −dP/dt (mmHg/s) | Flow (ml/min/g) | |
|---|---|---|---|---|---|---|---|---|
| WT Ctrl | 11 | 15.1 ± 0.3 | 0.11 ± 0.01 | 26.9 ± 1.0 | 102.8 ± 5.7 | 3979 ± 354 | −2504 ± 161 | 27.5 ± 0.2 |
| WT IPC | 13 | 15.3 ± 0.4 | 0.11 ± 0.01 | 26.8 ± 0.6 | 117.7 ± 7.1 | 4331 ± 276 | −2605 ± 136 | 25.1 ± 0.2 |
| KO Ctrl | 7 | 13.4 ± 0.7 | 0.12 ± 0.01 | 26.7 ± 0.9 | 131.4 ± 10.2 | 4873 ± 408 | −2684 ± 137 | 25.3 ± 0.2 |
| KO IPC | 8 | 14.2 ± 0.6 | 0.12 ± 0.01 | 25.4 ± 0.4 | 125.9 ± 5.9 | 4711 ± 337 | −2823 ± 173 | 21.9 ± 0.3 |
Figure 3.
LV functional recovery data at 45 min of reperfusion (A–D) and cTnI release data (E) from the isolated mouse hearts studies of IPC. Functional data are expressed as a percentage of baseline values. Refer to Fig. 1 for a detailed description of the experimental protocol. IR, non-preconditioned control group; IPC, preconditioned group. *P<0.05 versus the respective non-preconditioned control group (IR). n = 8–10 hearts/group.
Preconditioning elicited by two cycles of ischemia/reperfusion improved recovery of LV function to an equivalent extent in both WT and A2BKO hearts. As shown in Fig. 3, recovery of LV DP increased from 51.3 ± 2.8% to 66.6 ± 2.1% (30% improvement) in WT hearts following IPC, and from 42.1 ± 3.6% to 60.0 ± 3.5% (42% improvement) in A2BKO hearts (Fig. 3A). IPC also improved recovery of maximal +dP/dt (WT: 50.9 ± 3.5% to 75.6 ± 2.1%; KO: 41.2 ± 4.6% to 67.6 ± 4.6%) and −dP/dt (WT: 51.8 ± 4.5% to 70.6 ± 2.8%; KO: 42.9 ± 4.9% to 61.8 ± 4.8%) in both WT and A2BKO mouse hearts (Fig. 3B & C).
Myocardial necrosis was measured indirectly in the isolated mouse hearts by quantifying the amount of cTnI released into the coronary effluent during reperfusion. As shown in Fig. 3E, the amount of cTnI released was found to be decreased significantly by IPC in both WT (5,969 ± 925 ng cTnI/g to 1,595 ± 674 ng cTnI/g) and A2BKO (4,376 ± 739 ng cTnI/g to 2,278 ± 462 ng cTnI/g) mouse hearts.
In vivo mouse infarction studies
We next sought to ascertain the response of A2BKO mice to IPC in an in vivo model of infarction induced by 45 min of ischemia and 24 h of reperfusion (Fig. 1). As expected (Fig. 4A), infarct size was reduced extensively by IPC elicited by either one (IPC1) or three cycles (IPC3) of IPC. Infarct size was reduced from 29.2 ± 3.2% of the AAR in control mice to 10.0 ± 2.0% (66% reduction) in the IPC1 group and to 15.3 ± 2.0% (48% reduction) in the IPC3 group. In comparison, single or multiple-cycle IPC elicited similar reductions in infarct size in A2BKO mice. Infarct size was reduced from 27.9 ± 2.2% of the AAR in non-preconditioned control A2BKO mice to 10.3 ± 2.1% (63% reduction) in the IPC1 group and to 12.9 ± 2.4% (54% reduction) in the IPC3 group (Fig. 4). There were no differences in various determinants of infarct size between the experimental groups including body weight (from 24.2 ± 1.0 to 27.9 ± 0.9 g), AAR size (from 47.5 ± 9.6 % to 61.9 ± 3.0 % of the left ventricle) and heart rate during occlusion and the first 10 min of reperfusion (Table 2).
Figure 4.
Myocardial infarct size data from the in vivo mouse (A) and rat (B) studies of IPC. Refer to Fig. 1 for a detailed description of the experimental protocols. The data are presented as a percentage of the AAR. *P < 0.05 vs. the respective control (Ctrl) group.
Table 2.
Heart rate during in vivo mouse experiments of IPC.
| Mice | Baseline | 15′ Occlusion | 30′ Occlusion | 45′ Occlusion | 5′ Reperfusion | 10′ Reperfusion |
|---|---|---|---|---|---|---|
| Heart Rate (bpm) | ||||||
| WT Ctrl | 421.9 ± 25 | 418.1 ± 15 | 407.0 ± 9 | 398.1 ± 30 | 432.2 ± 21 | 438.9 ± 28 |
| WT IPC1 | 430.1 ± 13 | 419.8 ± 9 | 435.6 ± 10 | 429.3 ± 10 | 437.9 ± 10 | 430.8 ± 12 |
| WT IPC3 | 428.6 ± 17 | 435.3 ± 31 | 437.5 ± 27 | 450.2 ± 37 | 461.9 ± 29 | 449.7 ± 19 |
| KO Ctrl | 422.7 ± 11 | 410.4 ± 13 | 423.3 ± 12 | 393.7 ± 24 | 424.5 ± 20 | 440.9 ± 20 |
| KO IPC1 | 420.7 ± 14 | 413.0 ± 16 | 429.3 ± 14 | 421.3 ± 13 | 431.1 ± 11 | 424.5 ± 12 |
| KO IPC3 | 424.8 ± 15 | 410.0 ± 10 | 416.8 ± 13 | 392.5 ± 19 | 426.4 ± 17 | 438.7 ± 17 |
In vivo rat infarction studies
We finally investigated the potential involvement of the A2BAR in IPC in a rat model of infarction using the selective A2BAR antagonist ATL-801. Rats were subjected to 30 min of coronary artery occlusion and 2 h of reperfusion (Fig. 1). ATL-801 (1 mg/kg i.v.) was administered 10 min before the first coronary occlusion. Compared to vehicle-treated rats, pretreating with ATL-801 had no effect on infarct size in non-preconditioned control rats (Fig. 4). Similar to the mouse model, infarct size was greatly reduced by IPC elicited by one (IPC1) or three (IPC3) cycles of ischemia/reperfusion in vehicle-treated rats. Infarct size was reduced from 64.1 ± 0.9% of the AAR to 24.0 ± 2.4% (63% reduction) in the IPC1 group and to 4.5 ± 1.3% (93% reduction) in the multiple cycle IPC3 group. In rats pretreated with ATL-801, single and multiple cycle IPC produced similar reductions in infarct size. In ATL-801-treated rats, infarct size was reduced from 60.5 ± 1.7% in vehicle-treated rats to 21.8 ± 4.4% (64% reduction) in the IPC1 group and to 4.0 ± 1.1% (93% reduction) in the IPC3 group.
As shown in Fig. 5, administering the selective A2BAR agonist BAY 60-6583 produced a dose-dependent reduction in infarct size in the in vivo rat model. Infarct size was reduced from 61.9 ± 1.1% of the AAR in the vehicle-treated control group to 52.2 ± 1.7% (0.3 mg/kg) and 46.4 ± 2.0% (1 mg/kg) in the BAY 60-6583-treated groups. BAY 60-6583 (1 mg/kg) did not reduce infarct size in rats pretreated with ATL-801 (61.0 ±1.0%). Although ATL-801 tended to increase mean arterial blood pressure (~10–15 mmHg; not significant) immediately prior to coronary occlusion, there were no differences between any of the experimental groups in body weight (from 259 ± 7 to 294 ± 6 g), AAR size (from 35.8 ± 3.0 to 42.1 ± 0.9% of the left ventricle), as well as heart rate or mean arterial blood pressure during the ischemic period and throughout reperfusion (Tables 3 and 4).
Figure 5.

Pretreating with the A2BAR agonist BAY 60-6583 reduced myocardial infarct size in rats. Anesthetized rats were subjected to 30 min of coronary occlusion and 2 h of reperfusion. Vehicle (control) or BAY 60-6583 (0.3 or 1.0 mg/kg i.v.) were administered 10 min before occlusion. In the combination group (BAY+ATL), ATL-801 was administered (1.0 mg/kg i.v.) 10 min before administering BAY 60-6583. The data are presented as a percentage of the AAR. *P<0.05 vs the vehicle-treated control group.
Table 3.
Heart rate (HR; beats/nun) and mean arterial pressure (MAP; mmHg) data during in vivo rat experiments of IPC.
| HR | MAP | HR | MAP | HR | MAP | HR | MAP | |
|---|---|---|---|---|---|---|---|---|
| Pre-Drug Baseline | 1′ Pre-Ischemia | 15′ Ischemia | 2h Reperfusion | |||||
| Veh Ctrl | 378 ± 3 | 115 ± 4 | 377 ± 3 | 114 ± 4 | 388 ± 4 | 107 ± 3 | 366 ± 5 | 85 ± 3* |
| Veh IPC1 | 377 ± 12 | 116 ± 7 | 378 ± 11 | 117 ± 6 | 397 ± 13 | 104 ± 14 | 373 ± 7 | 83 ± 5* |
| Veh IPC3 | 387 ± 18 | 110 ± 6 | 383 ± 15 | 109 ± 6 | 380 ± 10 | 95 ± 2 | 380 ± 12 | 78 ± 5* |
| ATL Ctrl | 370 ± 6 | 110 ± 3 | 363 ± 7 | 124 ± 4 | 365 ± 6 | 100 ± 4 | 355 ± 8 | 72 ± 4* |
| ATL IPC1 | 368 ± 5 | 103 ± 4 | 385 ± 9 | 121 ± 6 | 373 ± 9 | 87 ± 7 | 365 ± 14 | 78 ± 4* |
| ATL IPC3 | 390 ± 12 | 111 ± 6 | 390 ± 12 | 120 ± 5 | 377 ± 12 | 89 ± 7 | 380 ± 10 | 76 ± 6* |
P < 0.5 vs. respective pre-drug baseline value by repeated-measures ANOVA and Dunnett’s t test
Table 4.
Heart rate (HR; beats/min) and mean arterial pressure (MAP; mmHg) data during in vivo rat experiments of with BAY 60-6583.
| HR | MAP | HR | MAP | HR | MAP | HR | MAP | |
|---|---|---|---|---|---|---|---|---|
| Pre-Drug Baseline | 1′ Pre-Ischemia | 15′ Ischemia | 2h Reperfusion | |||||
| Vehicle | 358 ± 12 | 117 ± 6 | 362 ± 13 | 116 ± 6 | 372 ± 12 | 97 ± 7 | 350 ± 14 | 80 ± 10* |
| BAY 0.3 mg/kg | 350 ± 15 | 111 ± 4 | 367 ± 9 | 112 ± 4 | 383 ± 12 | 95 ± 10 | 343 ± 9 | 70 ± 8* |
| BAY 1.0 mg/kg | 384 ± 7 | 115 ± 7 | 378 ± 15 | 111 ± 6 | 388 ± 9 | 108 ± 8 | 352 ± 8 | 80 ± 7* |
| BAY 1 mg/kg +ATL 1 mg/kg | 385 ± 13 | 110 ± 3 | 383 ± 8 | 125 ± 4 | 365 ± 8 | 96 ± 7 | 350 ± 13 | 74 ± 3* |
P < 0.5 vs. respective pre-drug baseline value by repeated-measures ANOVA and Dunnett’s t test
Discussion
The possibility has arisen in recent years that the A2BAR subtype may play an important role in the mechanism of the early phase of IPC [9, 16, 17, 24]. To further test this theory, we examined the potential involvement of the A2BAR in acute IPC using a comprehensive experimental approach. We first examined whether IPC can be demonstrated in A2BKO mice. For our studies we used a well-characterized line of A2BKO/β-galactosidase reporter gene “knock-in” mice developed by Yang and colleagues [19]. These mice were initially studied in an isolated, buffer-perfused mouse heart model of IPC that provides very precise measurement of ischemic injury. Subsequently, the A2BKO mice were examined in an in vivo model of infarction in which IPC was elicited by either a single cycle or multiple cycles of coronary occlusion and reperfusion. We used two different protocols to induce IPC, since the participation of adenosine in IPC-induced cardioprotection is very much dependent on the intensity (duration and number of cycles) of preconditioning ischemia [9]. In a final series of experiments, we examined the role of the A2BAR in IPC in an additional species using a pharmacological approach. We examined whether cardioprotection provided by IPC induced by single or multiple ischemia/reperfusion cycles is blocked by the selective A2BAR antagonist ATL-801 [20] in an in vivo rat model of infarction. We used a dose of ATL-801 that blocked the hypotensive and cardioprotective actions of the A2BAR agonist BAY 60-6583 (Figs. 2 and 5). In both the isolated mouse heart model and in the in vivo mouse model of infarction comprising an extended reperfusion period, we observed that IPC was fully manifest in experiments using A2BKO mice (Figs. 3 and 4). Similarly, we found that the full benefit of IPC to reduce infarct size was readily apparent in the rat model of infarction during pharmacological blockade of the A2BAR with ATL-801. Our data using two different animal species and two different model systems indicate that the early phase of IPC is not exclusively dependent on signaling by the A2BAR.
In addition to the results of the present investigation, there is other supporting evidence to suggest that the A2BAR may not participate in the early phase of IPC. It is well established that protection afforded by early IPC appears immediately, but is short-lived, lasting only 1–2 h and is not blocked by chemical inhibitors of protein synthesis [9, 18]. Thus, the general biology of the early phase of IPC with its rapid onset and short duration is not consistent with a mechanism involving increased synthesis of the A2BAR. Early mechanistic studies have also demonstrated that the formation of adenosine is decreased in preconditioned myocardium [8, 25, 26], explained by the well-known effect of IPC to slow ischemic metabolism and decrease necrotic cell injury [6]. An alternative theory has been forwarded by Downey’s group addressing this discrepancy. Theseinvestigators have proposed that the “sensitivity” of the A2BAR to adenosine is increased following IPC due to a PKC-mediated mechanism [9, 24, 27]. According to this theory, the A2BAR is activated to a greater extent at the time of reperfusion in preconditioned myocardium despite lower levels of extracellular adenosine production. While intriguing, little is know about the regulation of the A2BAR and this theory has yet to be tested directly.
There are several explanations for the different results obtained in the present investigation using A2BKO mice compared to those previously reported by Eckle and colleagues [16, 17]. For instance, the two studies used different lines of A2BKO mice, thus the opposing findings might be due to unknown differences between the two mouse strains. The A2BKO/β-galactosidase reporter gene knock-in mouse line used in the present investigation [19] displays an underlying pro-inflammatory phenotype characterized by increased circulating levels of cytokines and endothelial cell activation. It is unknown at this time whether the Deltagen stock of A2BKO mice displays a similar phenotype. There are also differences in the methodology. Eckle et al [16, 17] used a hanging weight system to produce coronary occlusion, whereas we used the standard method of tightening a ligature around the coronary artery. Another possibility relates to differences in the experimental protocols to induce IPC and to produce infarction. In the experiments by Eckle and colleagues [16, 17], IPC was elicited by four 5-min occlusion/5-min reperfusion cycles and the protocol to induce infarction consisted of 60 min of coronary occlusion followed by 2 h of reperfusion. In our studies, we used fewer preconditioning cycles (1 or 3) and subjected the mice to a slightly shorter period of ischemia (45 min), but a much longer reperfusion period (24 h). We chose a long reperfusion time to allow for the full extent of infarction to develop. One very possible explanation is that the overall experimental approach used by Eckle and colleagues [16, 17] corresponds more closely to the delayed, late phase of IPC dependent on the induction of stress-responsive genes described by others [5], whereas our studies correlate more with the acute phase of IPC. It should be noted that the time-course of the early and late phases of IPC have yet to be clearly delineated in the murine species.
Similar to reports by others [17, 27, 28], we found that administering the selective A2BAR agonist BAY 60-6583 reduced infarct size in our rat model (Fig. 5). Thus, although we found no evidence for its participation in acute IPC, our data support the concept that A2BAR activation elicits protection during ischemia/reperfusion injury. Downey and colleagues [9] have proposed that A2BAR activation reduces myocardial infarct size following prolonged ischemia and reperfusion by stimulating pro-survival signaling pathways in the myocardium, particularly within the first few minutes of reperfusion. This hypothesis is consistent with recent studies implicating the A2BAR in protection provided by post-conditioning [29]. In our studies, infarct size was similar in A2BKO mice compared to wild-type mice and was not influenced in rats by administration of ATL-801. These findings indicate that A2BAR signaling also did not modulate tolerance to ischemia/reperfusion injury in the absence of IPC.
In summary, we were able to observe in this study a robust cardioprotective effect of IPC in various model systems despite the absence of expression of the A2BAR in gene knock-out mice or pharmacological blockade of the A2BAR in rats. Our studies therefore indicate that the cardioprotective mechanism of the early phase of IPC is not universally dependent on signaling by the A2BAR, and must be explained by additional or alternative mechanisms. Based on the exciting finding that the A2BAR is induced in response to hypoxic stress [16, 17], however, we anticipate that the A2BAR and other adenosine regulatory proteins may emerge as important participants in other adaptive responses in the heart, such as later phases of IPC.
Research Highlights.
Protection by early preconditioning can be elicited in A2B receptor knock-out mice
Preconditioning in rats is also present during A2B receptor blockade with ATL-801
Giving the A2B receptor agonist BAY 60-6583, however, reduces infarct size in rats
Acknowledgments
We thank Ms. Anna Hsu for technical assistance and Dr. Thomas Krahn (Bayer HealthCare) for supplying BAY 60-6583. We also thank Drs. Holger Eltzschig (University of Colorado) and Katya Ravid (Boston University) for critical evaluation of this work. This study was supported by grants from the National Institutes of Health (R01 HL077707 to J.A.A., R37 HL074314 to G.J.G., and R01 HL 008311 to G.J.G.) and by a pre-doctoral fellowship from the American Heart Association (0810035Z to J.E.M.).
Footnotes
Disclosures: Dr. Figler is an employee and shareholder of PGxHealth.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Miura T, Goto M, Urabe K, Endoh A, Shimamoto K, Iimura O. Does myocardial stunning contribute to infarct size limitation by ischemic preconditioning? Circulation. 1991;84:2504–12. doi: 10.1161/01.cir.84.6.2504. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Richard VJ, Jennings RB, Reimer KA. Myocardial protection is lost before contractile function recovers from ischemic preconditioning. Am J Physiol. 1991;260:H796–804. doi: 10.1152/ajpheart.1991.260.3.H796. [DOI] [PubMed] [Google Scholar]
- 4.Van Winkle DM, Thornton JD, Downey DM, Downey JM. The natural history of preconditioning: cardioprotection depends on the duration of transient ischemia and time to subsequent ischemia. Cor Art Dis. 1991;2:613–9. [Google Scholar]
- 5.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–83. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 6.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–31. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong SC, Hoover DB, Shivell LC, Ganote CE. Preconditioning of isolated rabbit cardiomyocytes: no evident separation of induction, memory and protection. J Mol Cell Cardiol. 1997;29:2285–98. doi: 10.1006/jmcc.1997.0467. [DOI] [PubMed] [Google Scholar]
- 8.Harrison GJ, Willis RJ, Headrick JP. Extracellular adenosine levels and cellular energy metabolism in ischemically preconditioned rat heart. Cardiovasc Res. 1998;40:74–87. doi: 10.1016/s0008-6363(98)00123-0. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103:203–15. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 10.Auchampach JA, Gross GJ. Adenosine A1 receptors, KATP channels, and ischemic preconditioning in dogs. Am J Physiol. 1993;264:H1327–36. doi: 10.1152/ajpheart.1993.264.5.H1327. [DOI] [PubMed] [Google Scholar]
- 11.Lankford AR, Yang JN, Rose’Meyer R, French BA, Matherne GP, Fredholm BB, et al. Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H1469–73. doi: 10.1152/ajpheart.00181.2005. [DOI] [PubMed] [Google Scholar]
- 12.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–6. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchida A, Miura T, Miki T, Shimamoto K, Iimura O. Role of adenosine receptor activation in myocardial infarct size limitation by ischaemic preconditioning. Cardiovasc Res. 1992;26:456–61. doi: 10.1093/cvr/26.5.456. [DOI] [PubMed] [Google Scholar]
- 14.Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995;9:359–65. doi: 10.1096/fasebj.9.5.7896004. [DOI] [PubMed] [Google Scholar]
- 15.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 16.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 17.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 18.Thornton J, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, et al. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol. 1990;259:H1822–5. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- 19.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–23. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155:127–37. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, et al. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234–43. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, et al. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319:1200–10. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- 23.Gross GJ, Gauthier KM, Moore J, Campbell WB, Falck JR, Nithipatikom K. Evidence for role of epoxyeicosatrienoic acids in mediating ischemic preconditioning and postconditioning in dog. Am J Physiol Heart Circ Physiol. 2009;297:H47–52. doi: 10.1152/ajpheart.01084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Cohen MV, Downey JM. Mechanism of Cardioprotection by Early Ischemic Preconditioning. Cardiovasc Drugs Ther. doi: 10.1007/s10557-010-6236-x. in poress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goto M, Cohen MV, van Wylen DG, Downey JM. Attenuated purine production during subsequent ischemia in preconditioned rabbit myocardium is unrelated to the mechanism of protection. J Mol Cell Cardiol. 1996;28:447–54. doi: 10.1006/jmcc.1996.0041. [DOI] [PubMed] [Google Scholar]
- 26.Schulz R, Post H, Vahlhaus C, Heusch G. Ischemic preconditioning in pigs: a graded phenomenon: its relation to adenosine and bradykinin. Circulation. 1998;98:1022–9. doi: 10.1161/01.cir.98.10.1022. [DOI] [PubMed] [Google Scholar]
- 27.Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, et al. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–71. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, et al. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009;47:684–90. doi: 10.1016/j.yjmcc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–14. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]