Abstract
Vitamin D receptors have been shown to be present in human skeletal muscle using different techniques. We developed a multi-staining immunofluorescent method to detect vitamin D receptor expression and co-localize it with myosin heavy chain isoform expression in skeletal muscle biopsies in older female subjects. Serial sections were cut from frozen samples obtained by needle biopsy of the vastus lateralis. Samples were probed with a primary vitamin D receptor monoclonal antibody and then re-probed with a type IIa myosin heavy chain isoform-specific antibody. Independent unfixed sections followed a similar protocol and were probed with type IIx and type I myosin heavy chain isoform-specific antibodies. Immunohistochemistry and fluorescent microscopy co-localized vitamin D receptor loci and myosin heavy chain isoforms in whole skeletal muscle sections. We quantified intranuclear vitamin D receptor staining patterns and number of individual muscle fiber subtypes within a muscle section. Immunohistochemical staining of the vitamin D receptor was confirmed by Western blot using the same monoclonal antibody. This multi-staining immunofluorescent technique allows for measurement of intranuclear vitamin D receptor expression in the context of the specific muscle fiber type profile in a single section. This method can thus be a useful approach to study potential relationships between muscle fiber subtypes and vitamin D receptor expression.
Keywords: Vitamin D receptor, Immunohistochemistry, Skeletal muscle
Introduction
Recent data suggest that vitamin D supplementation reduces the risk of falls (Bischoff-Ferrari et al. 2009) and improves measures of muscle performance (Pfeifer et al. 2000, 2009; Bischoff et al. 2003) in older adults with low vitamin D status. Muscle biopsies in adults with profound vitamin D deficiency reveal atrophy of type II (fast-twitch) muscle fibers, which are the first fibers to be recruited when preventing a fall (McComas 1996). While the actions of vitamin D on skeletal muscle are not well-understood, current research suggests that the effects of vitamin D may, at least in part, be mediated through the vitamin D receptor (VDR). The VDR is a ligand-activated transcription factor and a member of the superfamily of nuclear receptors for steroid hormones (DeLuca 1988). The active form of vitamin D, 1α,25-dihydroxyvitamin D [1,25(OH)2D], is believed to bind to a nuclear VDR which, in turn, modulates the expression of genes related to the regulation of calcium transport and cell proliferation and differentiation (Boland et al. 1985; Simpson et al. 1985). The nuclear VDR has been identified in human skeletal muscle tissue using different techniques (Costa et al. 1986; Bischoff et al. 2001). Prior published localization techniques, however, have not had the capability of studying potential relationships between degree of intranuclear receptor expression and muscle fiber subtype.
The aim of this study was to co-localize VDR-positive myonuclei and specific skeletal muscle fiber subtypes within a human skeletal muscle section. To accomplish this aim, we developed a multiple immunofluorescent staining technique that identifies the VDR and muscle fiber subtypes by incubating a muscle section with a monoclonal antibody to the VDR and then myosin heavy chain (MHC) isoform-specific antibodies directly labeled with distinct fluorophores. The intranuclear VDR signal detected using a monoclonal antibody was verified by Western blot and compared to two alternative primary VDR antibodies.
Methods
Subjects
Eight muscle biopsy specimens used to develop the method were obtained from four healthy postmenopausal female participants, age 65–85. The Tufts Medical Center-Tufts University Health Sciences Campus Institutional Review Board approved the study, and written informed consent was obtained from each participant.
Muscle biopsy
Eight muscle biopsies were obtained from the vastus lateralis at the level of the mid-thigh under local anesthesia (xylocaine 1%) with a 5-mm Duchenne biopsy needle and suction (Bergström 1975). The specimens were mounted in a vinyl cryomold (Tissue-Tek, USA) and secured using a viscous mounting medium (O.C.T., Tissue-Tek, USA) and then frozen in isopentane/liquid nitrogen slurry.
Immunohistochemistry
Eight muscle tissue specimens were cut in seven μm sections at −23°C with a cryostat microtome (Leica CM1850, Leica Microsystems, Germany), placed onto microscope slides, and left to air-dry at room temperature for at least 15 min. Samples were washed with phosphate buffered saline (PBS) for 5 min, fixed in 3% neutral buffered formalin for 10 min, and then blocked in PBS/2% goat serum for 20 min. Slides were probed overnight (4°C) with a primary mouse/anti-human VDR/NR1I1 monoclonal antibody (clone H4537, Perseus Proteomics, Inc.; Table 1). Subsequently, sections were probed with goat/anti-mouse IgG2A Alexa Fluor-568® secondary antibody conjugate (Table 1). Samples that were probed with the VDR antibody, were then re-probed with a type IIa (N2.261 IgG1) MHC isoform-specific antibody (Table 1). Unfixed sections followed a similar protocol to identify other muscle fiber subtypes. Mouse antibodies against human type I (A4.951 IgG1; A4.840 IgM) and IIx (212F IgG1) MHC were used (Table 1). A rabbit anti-human antibody (IgG) raised against laminin was used to facilitate identifying individual muscle fibers. Specific goat anti-mouse and anti-rabbit Alexa Fluor® secondary antibodies were used for detection of MHC and laminin primary antibodies, respectively (Table 1). Slides were mounted with 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting medium to stain myonuclei (Vectashield H-1500; Vector Laboratories, Inc). Control sections were processed independently as described above, without primary, secondary or both antibodies (blank control). No staining signal higher than the natural self-fluorescence of the sections was observed (results not shown).
Table 1.
List of primary and secondary antibodies used in immunohistochemistry and immunoblotting
| Antibody | Host | Reactivity | Clone | Dilution | Vendor |
|---|---|---|---|---|---|
| Primaries | |||||
| Anti-VDR (IH/WB) | Mouse | Anti-human IgG2A | H4537 | 1:40 (IH) 1:1,000 (WB) |
R&D systems |
| Anti-VDR (WB) | Mouse | Anti-human IgG2A | D6 | 1:1,000 (WB) | Santa Cruz Biotechnology |
| Anti-VDR (WB) | Mouse | Anti-human IgG1 | 333C6a | 1:1,000 (WB) | Santa Cruz Biotechnology |
| Anti-MHC-I | Mouse | Anti-human IgM | A4.840 | 1:250 | DSHB |
| Anti-MHC-I | Mouse | Anti-human IgG1 | A4.951 | 1:250 | DSHB |
| Anti-MHC-IIa/neonatal I | Mouse | Anti-human IgG1 | N2.261 | 1:333 | DSHB |
| Anti-MHC-IIx | Mouse | Anti-human IgG1 | 212F | Undiluted (cell culture supernatant) | Produced in-house** |
| Anti-Laminin | Rabbit | Anti-human IgG | Polyclonal | 1:250 | Sigma–Aldrich |
| Antibody | Host | Reactivity | Dilution | Emission wavelength | Vendor |
| Secondaries | |||||
| Alexa Fluor® 350 | Goat | Anti-mouse IgG1 | 1:250 | 442 (Blue) | Invitrogen Corp. |
| Alexa Fluor® 430 | Goat | Anti-rabbit IgG | 1:250 | 539 (Light Green) | Invitrogen Corp. |
| Alexa Fluor® 488 | Goat | Anti-mouse IgG1 | 1:250 | 519 (Green) | Invitrogen Corp. |
| Alexa Fluor® 568 | Goat | Anti-mouse IgG1 | 1:250 | 603 (Orange Red) | Invitrogen Corp. |
| Alexa Fluor® 568 | Goat | Anti-mouse IgG2A | 1:250 | 603 (Orange Red) | Invitrogen Corp. |
| HRP-Labeled | Horse | Anti-mouse IgG | 1:2,000 | n/a | Cell Signaling |
| HRP-Labeled | Goat | Anti-mouse IgG1 | 1:2,000 | n/a | Invitrogen Corp. |
VDR Vitamin D receptor, IH immunohistochemistry, WB Western blot, MHC myosin heavy chain, DBHB Developmental Studies Hybridoma Bank
From hybridomas generously donated by Dr. Peter Merrifield, University of Ontario, Canada
Digital imaging was performed through 100× and/or 400× final magnification. Adobe Photoshop® CS3, Nikon NIS-AR (3.01) and NIH Image J software (1.37v) were employed for data acquisition and data analysis.
Immunoblotting
To confirm VDR expression, three samples were prepared by homogenization in lysis buffer (50 mM Tris HCl pH 7.5, 1 mM EDTA, 1 mM EGTA, 10% glycerol (v/v), 1% Triton-X (v/v), 50 mM NaF, 5 mM Na Pyrophosphate) and then by centrifugation at 10,000 rpm/4°C. Supernatants were stored at −80°C. Total protein concentrations of muscle extracts were measured using the Pierce 660 nm protein assay (Thermo Fisher Scientific). To detect VDR, 30 μg of whole cell muscle lysates were loaded onto and resolved by SDS–PAGE using 10% mini-gels (150 V/60 min with the BioRad Mini-Protean Tetra Cell System) and electrophoretically transferred (350 mA/120 min) to 0.45 μm nitrocellulose membranes. Five μg of 293T lysate (Santa Cruz Biotechnology, Inc.) were used as control. Membranes were blocked in 5% non-fat dry milk (NFM)/Tris-Buffered Saline-0.05% Tween 20 (TBS-T) solution, washed with 0.05% TBS-T, and probed with three different commercially-available primary antibodies to the VDR (VDR/NR1I1 monoclonal antibody, clone H4537 [Perseus Proteomics, Inc.]; VDR D-6 monoclonal antibody [sc-13133 Santa Cruz Biotechnology, Inc.] and VDR 333C6a [sc-81423 Santa Cruz Biotechnology, Inc.] Table 1) in 5% BSA/TBS-T (diluted 1:1,000) overnight. Membranes were then washed with 0.05% TBS-T and incubated with different HRP-conjugated secondary antibodies (Table 1) in 1%NFM/TBS-T (diluted 1:2,000) solution for detection using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
Results
Immunohistochemistry and fluorescent microscopy co-localized VDR and MHC isoforms in whole skeletal muscle sections. Figure 1 illustrates incubation of muscle cryosections with directly labeled antibodies to types I, IIa, and IIx MHC isoforms and laminin. The emission wavelength of each different secondary antibody (Table 1) employed on the MHC isoform staining allows simultaneous identification of the muscle fiber subtypes in two muscle sections overlayed to create a single multi-colored image (Fig. 1). We measured the relative number of individual muscle fiber subtypes within a cryosection, which in the Fig. 1 section revealed a predominance of type II fibers (Table 2).
Fig. 1.
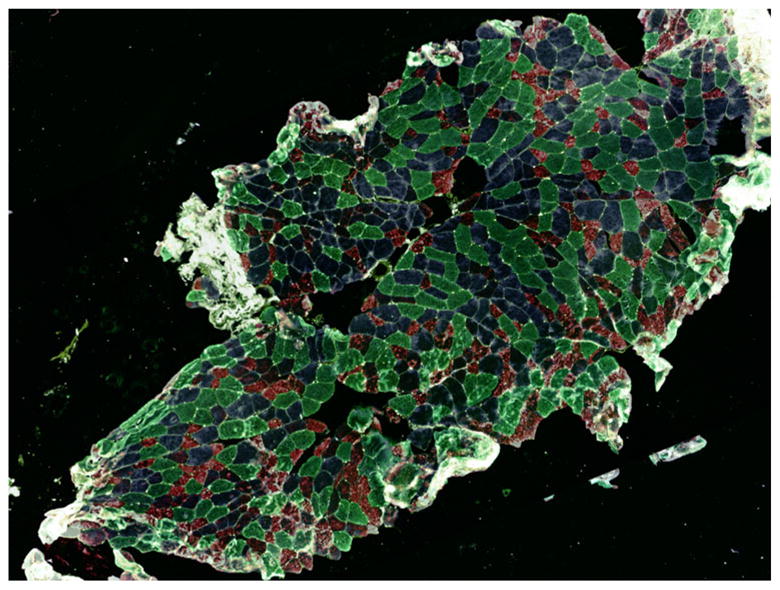
Composite image (100×) of multi-immunofluorescent staining of MHC isoforms in a human skeletal muscle tissue section. Colorization patterns are: dark green = type I, green/blue = hybrid I/IIa, blue = type IIa, red = type IIx, purple = hybrid IIax, and light green = laminin
Table 2.
Muscle fiber type profile of Fig. 1
| Muscle fiber type | Number of fibers | Percent (%) |
|---|---|---|
| Type I | 200 | 28.6 |
| Hybrid I–IIa | 9 | 1.3 |
| Type IIa | 196 | 28.0 |
| Type IIx | 198 | 28.3 |
| Hybrid IIa–IIx | 96 | 13.7 |
| All type Ia | 209 | 29.9 |
| All type IIb | 490 | 70.1 |
| Total | 699 | 100 |
Type I fibers consist of type I and hybrid I–IIa
Type II fibers consist of type IIa, type IIx and hybrid IIa–IIx
In each sample, we randomly selected ten higher magnification (400×) fields, which were representative of the whole section. In Fig. 1, the ten fields comprised 46% of the muscle fibers in the whole section and had a similar type I to type II muscle fiber type ratio (32% to 68%) to that of the whole section (30% to 70%). The 400× images were captured using fluorescent (Fig. 2a/b) and bright field microscopy (Fig. 2c). Figure 2a shows DAPI staining. Figure 2b illustrates co-localization of VDR-positive nuclei with DAPI. Bright field images (Fig. 2c) were used to assign the myonuclei to a particular fiber. We measured the relative number of VDR-positive myonuclei within each muscle fiber subtype in all 10 fields. Table 3 lists the ratio of VDR-positive myonuclei to total myonuclei by muscle fiber subtype from 10 fields from Fig. 1. Of note, VDR immunohistochemical staining was not solely associated with clearly identified intranuclear staining as shown in Fig. 2b (arrows).
Fig. 2.
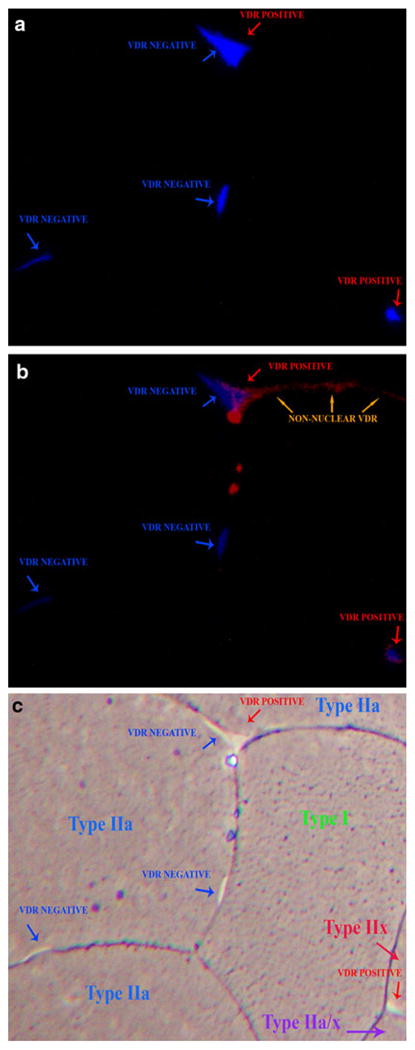
VDR-positive myonuclei in skeletal muscle section at 400× magnification showing: a DAPI staining myonuclei; b co-localization of VDR-positive myonuclei with DAPI; c bright field microscopy
Table 3.
Relative number of VDR-positive myonuclei in 10 randomly selected 400 × fields
| Muscle fiber type | Number of VDR- positive nuclei | Total number of nuclei | Ratio (VDR/Total) |
|---|---|---|---|
| Type I | 165 | 178 | 0.93 |
| Hybrid I–IIa | 14 | 14 | 1.00 |
| Type IIa | 155 | 180 | 0.86 |
| Type IIx | 145 | 170 | 0.85 |
| Hybrid IIa–IIx | 70 | 83 | 0.84 |
| All type Ia | 179 | 192 | 0.93 |
| All type IIb | 370 | 433 | 0.86 |
| Total | 549 | 625 | 0.88 |
Type I fibers consist of type I and hybrid I–IIa
Type II fibers consist of type IIa, type IIx and hybrid IIa–IIx
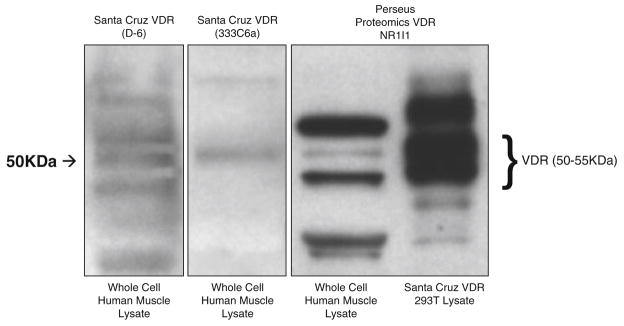
To confirm identification of VDR by immunohistochemical staining in these cryosections, we performed Western blot analysis using the same VDR monoclonal antibody (VDR/NR1I1). The immunoblot was run alongside two other commercially-available primary antibodies to the VDR (D-6 and 333C6a) to confirm the accuracy of the VDR antibody used in the immunohistochemical stain (Fig. 3). The molecular weight band at approximately 50 kDa is consistent across all three lanes incubated with a VDR antibody (Fig. 3).
Fig. 3.
VDR expression in whole cell lysate of human skeletal muscle is shown using three commercially available primary antibodies. From left to right are VDR (D-6), VDR (333C6a) and VDR (NR1I1). VDR expression was verified by use of human VDR transfected 293T cell lysate from Santa Cruz Biotechnology, Inc
Discussion
This study describes a multiple immunofluorescent staining technique which co-localizes the VDR and muscle fiber subtypes in a single human skeletal muscle section. Similar to a prior study in human skeletal muscle specimens (Bischoff et al. 2001), we identified VDR myonuclear staining using a human monoclonal antibody to the VDR. Unlike prior studies, however, our technique provides information on VDR expression in the context of muscle fiber subtype and its distribution. Using type I, IIa and IIx MHC isoform-specific antibodies directly labeled with distinct fluorophores, we were able to identify hybrid fibers which are of particular interest when studying factors such as vitamin D supplementation that may have effects on muscle morphology (Sorensen et al. 1979; Boland 2005; Sato et al. 2005). Our technique could be utilized in future studies to provide information on whether the VDR has differential expression based on muscle fiber subtype. Although we cannot generalize on a pattern of VDR expression by fiber subtype in this study, a more extensive sampling of muscle specimens in a larger study would allow for such an analysis. If VDR has a direct role on muscle as proposed, this knowledge may help to explain the morphological changes noted in vitamin D deficiency (Boland 2005) and in vitamin D repletion (Sorensen et al. 1979; Sato et al. 2005) demonstrating a potentially selective effect on type II muscle fibers.
Notably, our technique stained peripheral areas of the muscle fiber that did not appear connected with a myonucleus within these 7 μm sections. This preliminary finding will need to be explored further to determine whether this signal may be representative of the putative membrane-associated VDR believed to activate rapid, non-genomic, second messenger intracellular signaling cascades that influence muscle intracellular calcium regulation, muscle contractility and myogenesis (Boland 2005). Recent animal studies indentified VDR in isolated membrane fractions of both chick intestinal cells and chick embryonic skeletal muscle cells (Capiati et al. 2002; Huhtakangas et al. 2004).
The strength of this method is that, by incubating a single muscle section with different specific primary antibodies to different MHC isoforms, it successfully reduces the amount of muscle tissue, reagents, and time needed to perform these analyses. A limitation in this study was that we did not verify the VDR signal in muscle using an inhibitor or knockout model; however, we confirmed VDR expression in muscle sections by Western blot analysis using the same antibody and compared it to other antibodies to further corroborate our findings.
In summary, this technique co-localizes VDR using a human monoclonal antibody and type I, IIa, and IIx muscle fibers using specific antibodies to MHC isoforms in a sample obtained from a human skeletal muscle biopsy. The method identifies and quantifies VDR-positive myonuclei in skeletal muscle, identifies specific MHC isoforms and VDR positive myonuclei within individual muscle fiber subtypes, and identifies peripheral VDR staining patterns that need further investigation. Use of this technique in a large sample of muscle specimens would provide patterns of VDR expression and its associations with MHC iso-forms in human muscle.
Acknowledgments
This material is based upon work supported by the U.S. Department of Agriculture, Agricultural Research Service, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
The A4.951, A4.840, and N2.261 monoclonal antibodies developed by H. M. Blau were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. The 212F mAb was produced in-house from hybridomas generously donated by Dr. Peter Merrifield, University of Ontario, Canada. We are grateful to the staff of the Metabolic Research Unit at the Jean Mayer USDA HNRCA at Tufts University for assistance in carrying out on this study. LC is supported by NIH KL2 RR025751. The study was funded by the Jean Mayer USDA HNRCA at Tufts University and P30 AG031679.
Footnotes
None of the authors had any conflicts of interest.
Contributor Information
Lisa Ceglia, Email: lisa.ceglia@tufts.edu, Division of Endocrinology, Diabetes and Metabolism, Tufts Medical Center, Boston, MA, USA. Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111, USA.
Mauricio da Silva Morais, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Lara K. Park, Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111, USA
Evan Morris, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Susan S. Harris, Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111, USA
Heike A. Bischoff-Ferrari, Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111, USA. Centre on Aging and Mobility, University of Zurich, Zurich, Switzerland. Department of Rheumatology and Institute of Physical Medicine, University Hospital Zurich, Zurich, Switzerland
Roger A. Fielding, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA
Bess Dawson-Hughes, Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111, USA.
References
- Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–616. [PubMed] [Google Scholar]
- Bischoff HA, Borchers M, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- Bischoff HA, Stahelin HB, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dawson-Hughes B, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland R. Vitamin D and muscle. In: Feldman D, Pike JW, Glorieux GH, editors. Vitamin D. Elsevier; Amsterdam: 2005. pp. 883–897. [Google Scholar]
- Boland R, Norman A, et al. Presence of a 1,25-dihydroxy-vitamin D3 receptor in chick skeletal muscle myoblasts. Biochem Biophys Res Commun. 1985;128(1):305–311. doi: 10.1016/0006-291x(85)91679-1. [DOI] [PubMed] [Google Scholar]
- Capiati D, Benassati S, et al. 1, 25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002;86(1):128–135. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- Costa EM, Blau HM, et al. 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology. 1986;119(5):2214–2220. doi: 10.1210/endo-119-5-2214. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. Faseb J. 1988;2(3):224–236. [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, et al. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18(11):2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- McComas A. Skeletal muscle: form and function. Human Kinetics Publishers, Inc; Champaign, IL: 1996. [Google Scholar]
- Pfeifer M, Begerow B, et al. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15(6):1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, et al. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20(2):315–322. doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]
- Sato Y, Iwamoto J, et al. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20(3):187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- Simpson RU, Thomas GA, et al. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260(15):8882–8891. [PubMed] [Google Scholar]
- Sorensen OH, Lund B, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 1979;56(2):157–161. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]