Abstract
Background
No study has systematically evaluated the significance of involvement of the superficial specimen margin in skin-sparing mastectomies (SSMs).
Methods
168 SSMs with a small, additional superficial margin (ASM) specimen taken directly over the tumor to the dermis intraoperatively were studied.
Results
64 SSMs (38%) had a positive superficial specimen margin but only 13 (20%) of these had residual breast carcinoma in ASMs. Only 1 of 104 SSMs with a negative superficial specimen margin had residual breast carcinoma in its ASM (P < 0.05). ASM sampling rendered the final true margin directly over the tumor negative in 54 of 58 (93%) SSMs with a focally positive superficial specimen margin, but did not negate the nonfocally positive superficial specimen margin in six other cases. In SSMs with a positive superficial specimen margin, multivariate analysis revealed that the presence of extensive ductal carcinoma in situ (DCIS) in the SSM and a thicker ASM specimen were the only independent factors predictive of residual breast carcinoma in ASM. Eighty-nine (53%) ASMs contained benign breast tissue.
Conclusions
Superficial specimen margins in SSMs are often microscopically positive and approximately half of ASMs contain benign breast tissue, likely reflecting the difficulty in completely removing breast tissue near the skin flaps in SSMs. ASM sampling effectively decreases positive superficial specimen margins directly over the tumor in SSMs, but fails to account for positive superficial specimen margins in other quadrants in patients with multicentric disease, especially extensive DCIS. Patients whose superficial margins remain positive could potentially represent a subset of patients for whom postmastectomy radiation is beneficial.
Keywords: Breast carcinoma, Skin-sparing mastectomy, Additional superficial margin, Residual carcinoma
In spite of the increasing trend towards breast conservation therapy, patients with locally extensive breast carcinoma still require mastectomy. Traditionally, mastectomy entails removal of the entire breast and its overlying skin. A skin-sparing mastectomy (SSM), first introduced in 1991 and usually followed by immediate reconstruction, is a mastectomy, either simple or modified radical, with excision of the nipple-areolar complex, biopsy scar, and a minimal amount of periareolar skin.1 It has received a high level of patient satisfaction because the preservation of the patient’s natural skin envelope (skin flaps) and inframammary fold allows breast reconstruction with a more natural-appearing cosmetic shape and contour in a one-stage procedure.2,3
One major concern with the SSM procedure is that not all of the breast tissue, and hence not all breast carcinoma, is removed at the superficial specimen margin, where breast tissue intermingles with the dermis of the skin flaps that remain in the patient’s body, potentially promoting local recurrence.4 Benign breast tissue left behind may also give rise to a metachronous second breast carcinoma, as patients with one breast carcinoma are at increased risk of developing another carcinoma.5 Nonetheless, the superficial specimen margin of SSM has received little attention in the literature. Some pathologists do not even ink the superficial SSM surface, believing that the surgeon has gone as far as he/she can go in this area.
To assess the frequency and significance of involvement of the superficial specimen margin in SSM, we evaluated a large consecutive series of SSMs performed by a single surgeon. In each case, after the SSM was performed but during the same operation, a small, additional, oriented superficial margin specimen was taken directly over the tumor to the dermis, representing that would normally have been included in the skin flap. These additional superficial margins (ASM) allowed an assessment of what breast tissue or breast disease would otherwise have been left behind in this location. Moreover, they represent the true superficial margin in this location (analogous to a separate cavity margin for a lumpectomy specimen) and could potentially diminish false positive superficial mastectomy specimen margins. However, because of their small size, these samples do not negate the significance of positive superficial margins away from the main tumor (i.e., in other quadrants of the breast). Our study evaluates the efficacy of this technique.
MATERIALS AND METHODS
We obtained approval to perform this study from The Johns Hopkins Hospital institutional review board. The surgical pathology files of the Johns Hopkins Hospital were searched for SSMs with ASM taken at the time of surgery performed by one surgeon (T.N.T.) between January 2004 and June 2006. A total of 168 consecutive SSM from 163 patients (five patients had bilateral SSM) were retrieved. Each SSM was inked yellow at the superficial specimen margin and black at the deep specimen margin (Fig. 1). Representative sections from each SSM were submitted: at least one section per 1 cm tumor size showing relationship to the margins, at least two sections from other quadrants without tumor involvement, two sections (one from the biopsy scar area) from skin, and one section from the nipple. The ASMs were inked at the final true margin surface (designated by a suture by the surgeon), sectioned perpendicular to this surface at 2–3 mm intervals, and then submitted entirely. All slides were re-reviewed for the measurements described below.
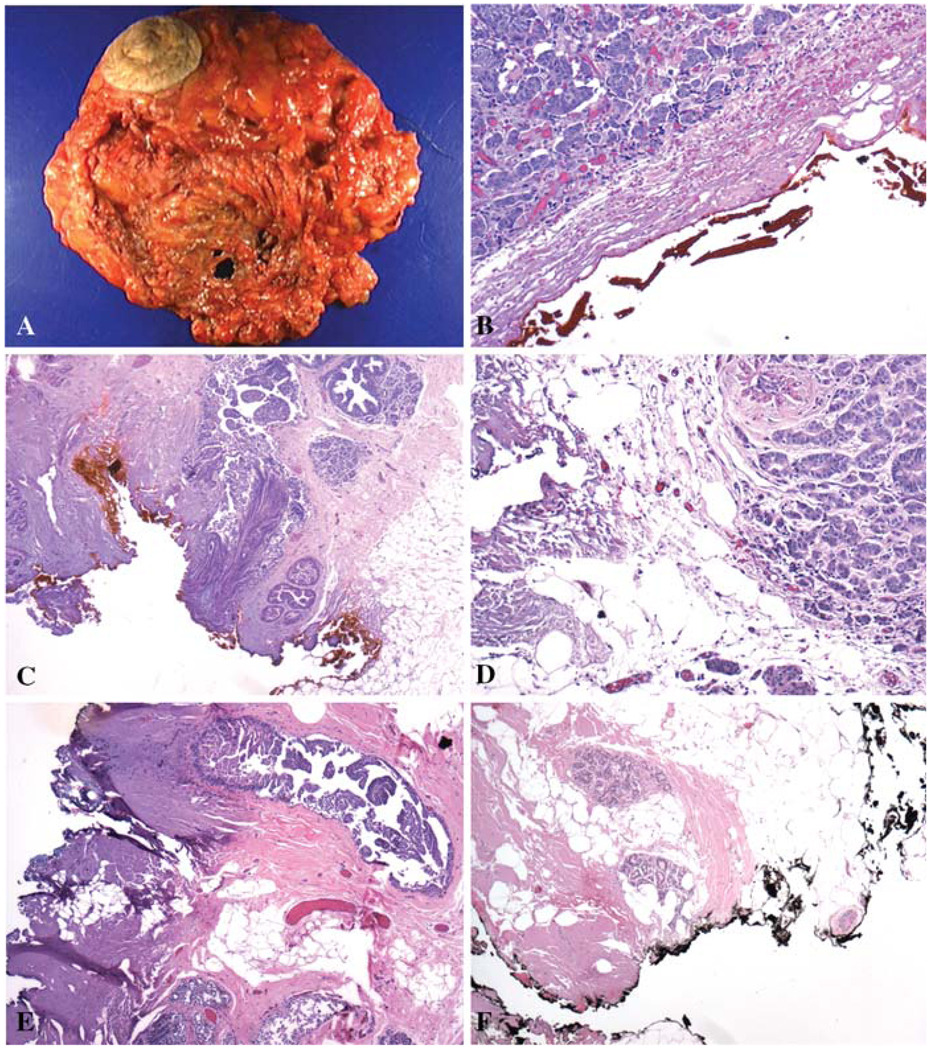
FIG. 1.
Skin-sparing mastectomy (SSM) usually includes the areolar-nipple complex with only a small area of skin (A). The exposed breast tissue represents the superficial specimen margin. Sixty-four of 168 (38%) SSMs had a positive superficial specimen margin, with carcinoma either within 1 mm of (B, ×100) or at (C, ×100) the inked margin. Thirteen of 64 (20%) SSMs with a positive superficial specimen margin had residual carcinoma in the additional superficial margin (ASM), a few as invasive carcinoma (D, ×100) but most as DCIS (E, ×40). Fifty-three percent of ASMs contained benign breast epithelium (F, ×40).
The SSMs were evaluated for their volume (calculated by multiplying the three gross dimensions), skin area (calculated by multiplying the two gross dimensions), skin/SSM surface area ratio, breast carcinoma size, breast carcinoma pathological stage [using the most recent 2003 American Joint Committee on Cancer (AJCC) staging system], Elston grade of the invasive carcinoma, nuclear grade of ductal carcinoma in situ (DCIS), vascular invasion, presence of extensive DCIS (defined for this study as multicentric disease in pure DCIS cases or multiple foci of DCIS away from the invasive carcinoma in invasive carcinoma plus DCIS cases), the number of quadrants involved by breast carcinoma, Paget’s disease, hormone receptor status (estrogen receptor, progesterone receptor), Her-2/neu status, deep margin status (positive or negative), superficial specimen margin status (distance of carcinoma from the inked superficial specimen margin, and extent of involvement, i.e., the aggregate length of carcinoma within 1 mm of the inked superficial specimen margin). ASMs were evaluated for size (surface area, thickness, and volume), the presence and type of breast carcinoma (if any), breast carcinoma distance to their inked true margin, and presence of benign breast tissue. A margin was considered positive if breast carcinoma was at or within 1 mm of the inked surface.
Statistical analyses were performed with SAS software version 9.1 (SAS Institute, Carry, NC, USA). Range and frequency distributions of all continuous and categorized variables were examined. The t-test was used to compare the differences in age, mastectomy weight, mastectomy volume, skin area on the mastectomy, ratio of skin/mastectomy surface area, thickness of additional superficial margin, surface area of additional superficial margin, volume of additional superficial margin, tumor size, number of lymph nodes with metastatic carcinoma, and the extent of involvement by carcinoma at the superficial specimen margin between groups. Fisher’s exact or chi-square test was applied to compare the number of cases with DCIS only, the number of cases with invasive carcinoma with or without DCIS, the number of cases with extensive DCIS, DCIS nuclear grade, Elston grade of the invasive carcinoma, pathological stage of invasive carcinoma, the number of cases with carcinoma in more than one quadrant, lymph node metastasis, vascular invasion, the status of estrogen and progesterone receptors in tumor cells, the status of Her-2/neu, deep margin status, the presence of prior neoadjuvant therapy, carcinoma transected at the superficial mastectomy specimen margin, carcinoma less than 1 mm of the superficial specimen margin, benign breast tissue transected at the superficial specimen margin, frequency of additional superficial margins with benign breast tissue, and residual carcinoma between groups. Multivariate analysis was performed using the Cox proportional-hazard model. For all these analyses, a P-value of less than 0.05 was considered statistically significant.
RESULTS
Cases and Specimens
The mean age of these 163 patients was 51.6 years (26–85 years) (Table 1). Of the 168 SSMs, 35 were performed for pure DCIS, 80 for invasive carcinoma with DCIS, and 53 for invasive carcinoma without evidence of DCIS. The mean SSM superficial surface area was 343.8 cm2, and the mean excised periareolar skin area was 51.3 cm2, so the mean non-skin-covered SSM surface area was 292.5 cm2. The mean ASM surface area was 10.3 cm2. The ratio of the mean ASM size to non-skin-covered SSM surface area was 0.035, or 3.5%. Hence, the ASM covers only the localized area over the breast carcinoma, not the entire superficial specimen margin.
TABLE 1.
Clinicopathological features in skin-sparing mastectomies (SSM) with positive (N = 64) and negative (N = 104) superficial specimen margins
| Clinicopathological factors | Positive (N = 64) | Negative (N = 104) | P-value |
|---|---|---|---|
| Mean age (range)a | 50.0 ± 12.4 (32–80) | 52.7 ± 14.6 (26–85) | 0.1226 |
| Mean mastectomy weight (g) (range) | 436 (103–1842) | 510 (148–2318) | 0.018 |
| Mean mastectomy volume (cm3) (range) | 1084 (202–5967) | 1331 (163–4173) | 0.07 |
| Mean skin area (cm2) (range) | 34.4 (4.0– 247.5) | 61.7 (1.4–359.1) | 0.01 |
| Mean skin/mastectomy surface area (cm2) (range) | 0.11 (0.02–0.79) | 0.17 (0.01–0.84) | 0.01 |
| Mean ASM thickness (cm) (range) | 0.93 (0.1–2.5) | 0.77 (0.1–1.6) | 0.03 |
| Mean ASM surface area (cm2) (range) | 9.36 (0.5 –31.76) | 10.82 (0.49–90.0) | 0.3620 |
| Mean ASM volume (cm3) (range) | 10.41 (0.18–61.88) | 9.29 (0.30–98.8) | 0.6042 |
| DCIS only | 13 | 22 | 0.055 |
| IMC ± DCIS | 51 | 82 | |
| Extensive DCIS | 17 | 13 | 0.032 |
| DCIS nuclear grade | |||
| I | 4 | 2 | 0.6172 |
| II | 20 | 24 | |
| III | 31 | 34 | |
| IMC Elston grade | |||
| I | 5 | 6 | 0.6788 |
| II | 21 | 33 | |
| III | 23 | 38 | |
| No grade given | 2 | 5 (too small to grade) | |
| Stage (invasive carcinoma) | |||
| pT1 | 31 | 51 | 0.9710 |
| pT2 | 14 | 22 | |
| pT3 | 5 | 6 | |
| pT4 | 1 | 2 | |
| Not available | 0 | 1 | |
| Mean tumor size (cm) (range) | 2.2 (0.1–11.7) | 2.5 (0.1–15.7) | 0.4965 |
| Carcinoma in > 1 quadrant | 35 | 32 | 0.02 |
| Paget’s disease | 0 | 1 | 1.00 |
| Lymph node metastasis | 23 | 25 | 0.10 |
| Mean number of lymph node metastases | 4.9 (1–25) | 3.6 (1–14) | 0.3616 |
| Extranodal extension | 6/23 | 5/25 | 0.7362 |
| Vascular invasion | 18/51 | 14/82 | 0.025 |
| Estrogen receptor positive | 52 of 60 | 51 of 81 | 0.01 |
| Progesterone receptor positive | 46 of 60 | 40 of 81 | 0.01 |
| Her-2/neu status (IHC score) | |||
| 0–1 | 25/46 | 44/62 | 0.002 |
| 2–3 | 21/46 | 18/62 | |
| Positive deep margin | 24 | 6 | < 0.001 |
| Prior neoadjuvant chemotherapy | 10 | 28 | 0.04 |
The number of patients in the former group was 61 (three patients had bilateral mastectomies: two patients had bilateral positive superficial margins and one patient had one positive and one negative superficial margin). Accordingly the number of patients in the latter group was 102.
ASM, additional superficial margin; DCIS, ductal carcinoma in situ; IMC: invasive mammary carcinoma, ductal or lobular; IHC, immunohistochemical stain.
Bold values indicates statistically significant differences.
What Predicts a Positive Superficial Specimen Margin in Skin-Sparing Mastectomy Specimens (SSMs)?
Among the 168 SSMs, 64 (38%) had a positive superficial specimen margin and 104 (62%) did not (Fig. 1). On univariate analysis, mastectomies with a positive superficial specimen margin had a smaller weight (P = 0.018), a smaller skin area on the SSM (P = 0.01), and a lower skin/SSM surface ratio (P = 0.01). They tended more often to have extensive DCIS (P = 0.032), vascular invasion (P = 0.025), carcinoma in more than one quadrant (P = 0.02), positive estrogen receptor (P = 0.01), positive progesterone receptor (P = 0.01), 2–3+ score in Her-2/neu status by immunostain (P = 0.002), a thicker ASM (P = 0.03), and a positive deep margin (P < 0.001) (Table 1). However, multivariate analysis showed that only a positive deep margin (P = 0.01) was a strong independent predictive marker for a positive superficial specimen margin after adjusting other variables. A thicker ASM was also an independent marker for predicting a positive superficial margin on multivariate analysis but the significance was borderline (P = 0.05).
Frequency of and Predictive Factors for Residual Carcinoma in Additional Superficial Margins (ASMs)
Among the 64 SSMs with a positive superficial specimen margin, 13 (20%) had residual carcinoma in the ASMs (Fig. 2) whereas only 1 of the 104 (1%) patients with a negative superficial specimen margin did so (P < 0.05). Of note, the latter consisted of carcinoma in lymphatic spaces, reflecting disease not amenable to local excision. Therefore, a positive superficial specimen margin was a significant predictor of residual carcinoma in the ASM.
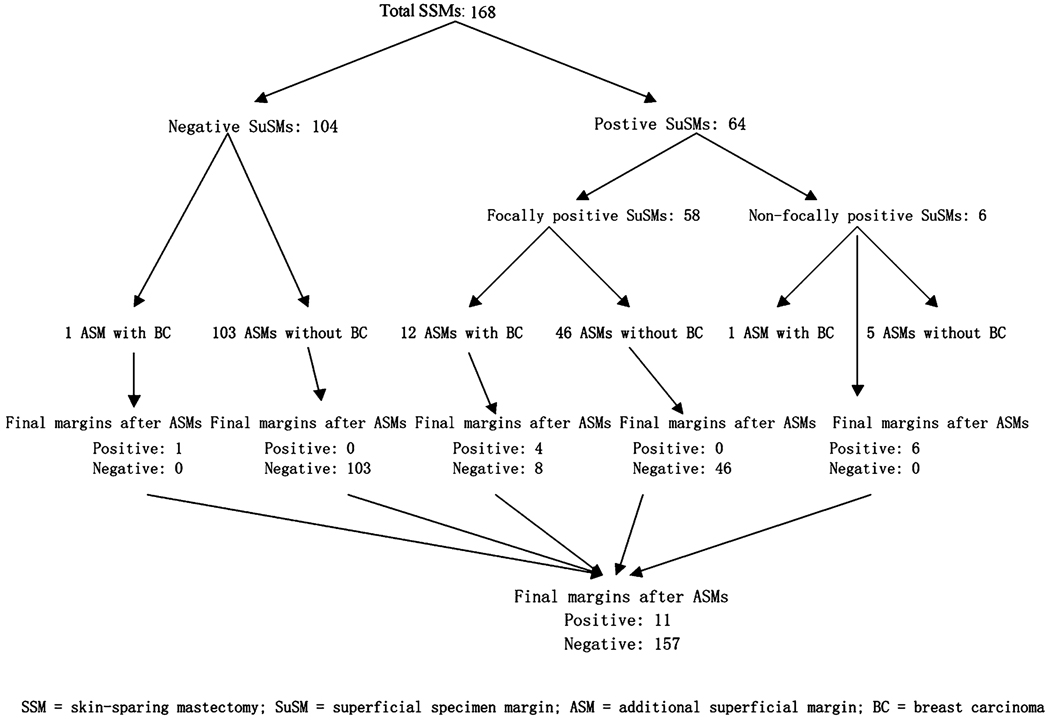
FIG. 2.
Efficacy of additional superficial margin (ASM) sampling in skin-sparing mastectomy (SSM).
Among the 64 cases with a positive superficial specimen margin (13 with residual carcinoma in ASMs and 51 without), the only factors predictive of residual carcinoma in the ASMs were the presence of extensive DCIS and thicker ASM specimens (Table 2), both on univariate analysis and multivariate analysis. Eight of 13 cases (52%) with residual carcinoma in ASMs had extensive DCIS in contrast to only 8 of the 51 patients (16%) without residual carcinoma in ASMs (P = 0.002 on univariate analysis, P = 0.01 on multivariate analysis). The mean ASM thickness was 1.20 cm in the former 13 cases in contrast to 0.86 cm in the latter 51 cases (P = 0.019 on univariate analysis, P = 0.026 on multivariate analysis). Whether the carcinoma was transected at the superficial specimen margin or only close to the superficial specimen margin was not associated with residual carcinoma in the ASM (P = 0.7520) (Table 3).
TABLE 2.
Mastectomies with positive superficial specimen margins: clinicopathological features of cases with residual carcinoma in additional superficial margin (ASM) (N = 13) versus those without (N = 51)
| Clinicopathological factors | Additional superficial margin with carcinoma (N = 13) |
Additional superficial margin without carcinoma (N = 51) |
P-value |
|---|---|---|---|
| Mean age (range) | 50.3 (42–64) | 49.4 (32–80) | 0.7715 |
| Mean mastectomy weight (g) (range) | 425 (210–823) | 447 (103–1842) | 0.8113 |
| Mean mastectomy volume (cm3) (range) | 1010 (390–2035) | 1102 (163–4173) | 0.7525 |
| Mean mastectomy skin area (cm2) (range) | 37.2 (8.32–153.4) | 33.7 (3.96–247.5) | 0.8221 |
| Mean skin/specimen area (cm2) (range) | 0.13 (0.03–0.55) | 0.11 (0.02–0.79) | 0.6255 |
| Mean ASM thickness (cm) (range) | 1.20 (0.5–2.5) | 0.86 (0.1–2.5) | 0.019 |
| Mean ASM surface area (cm2) (range) | 11.11 (1.98–19.9) | 8.91 (0.5–31.76) | 0.2722 |
| Mean ASM volume (cm3) (range) | 14.88 (1.19–28.13) | 9.27 (0.18–61.88) | 0.1290 |
| DCIS only | 4 | 9 | > 0.05 |
| IMC ± DCIS | 9 | 42 | |
| Extensive DCIS | 8 | 8 | 0.002 |
| DCIS nuclear grade | |||
| I | 1 | 3 | 0.3296 |
| II | 5 | 15 | |
| III | 7 | 24 | |
| IMC Elston grade | |||
| I | 0 | 5 | 0.3089 |
| II | 4 | 17 | |
| III | 5 | 18 | |
| Not available | 0 | 2 | |
| Mean tumor size (cm) (range) | 2.49 (0.1–11.7) | 2.00 (0.2–6.2) | 0.6092 |
| Carcinoma in > 1 quadrant | 11 | 29 | 0.3381 |
| Pathological stage pT | |||
| pT1 | 7 | 23 | 0.6474 |
| pT2 | 1 | 13 | |
| pT3 | 1 | 4 | |
| pT4 | 0 | 2 | |
| Lymph node metastasis | 4/9 (44%) | 18/43 (43%) | 1.00 |
| Vascular invasion | 2/9 (22%) | 16/43 (38%) | 0.6994 |
| Estrogen receptor positive | 13/13 (100%) | 40/45 (89%) | 0.5785 |
| Progesterone receptor positive | 12/13 (92%) | 34/45 (78%) | 0.0967 |
| Her-2/neu status (IHC score) | |||
| 0–1 | 2 | 20 | > 0.05 |
| 2–3+ | 3 | 18 | |
| Not available | 4 | 14 | |
| Positive deep margin | 7 | 17 | 0.5191 |
| Prior chemotherapy | 1 | 9 | 0.6721 |
ASM, additional superficial margin; DCIS, ductal carcinoma in situ; IMC, invasive mammary carcinoma; IHC, immunohistochemical stain.
TABLE 3.
Histological assessment of carcinoma and benign breast tissue at the skin-sparing mastectomy (SSM) superficial specimen margin does not predict involvement of additional superficial margin (ASM)
| ASMs with carcinoma (N = 13) |
ASMs without carcinoma (N = 51) |
P-value | |
|---|---|---|---|
| Carcinoma transected at the mastectomy superficial margin | 6 | 19 | 0.7520 |
| Carcinoma less than 1 mm of the Superficial specimen margin | 7 | 32 | |
| Extent of involvement by carcinoma at the Superficial specimen margin (mm) (range) | 12.5 ± 15.8 (2–58.5) | 11.0 ± 17.4 (0.5–85) | 0.7927 |
| Benign breast tissue transected at the Superficial specimen margin | 9 | 25 | 0.2219 |
As might be expected, the extent of involvement by carcinoma of the superficial specimen margin was higher in SSMs in which the carcinoma was transected at the inked superficial specimen margin (mean 20.8 mm, range 2–85 mm) than in those SSMs in which the carcinoma was within 1 mm of the superficial specimen margin (mean 5.0 mm, range 0.5–16.1 mm) (P < 0.001). However, the extent of involvement at the superficial specimen margin by carcinoma (defined as the aggregate length of carcinoma within 1 mm of the inked superficial specimen margin) did not predict residual carcinoma in the ASMs (Table 3).
Types of Residual Carcinoma in Additional Superficial Margins (ASMs)
DCIS was present in all 13 SSMs that had positive superficial specimen margins and residual carcinoma in ASMs (Table 4). Of the 13 ASMs, 12 contained DCIS (9 had DCIS only, while 3 had both IC and DCIS) and 1 had only IC. Therefore DCIS was responsible for the majority of positive superficial margins in this study.
TABLE 4.
Skin-sparing mastectomies (SSM) with residual carcinoma in the additional superficial margins (ASM)
| Case no. | SSM diagnosis | Extensive DCIS present |
Status of the superficial specimen margin |
Additional superficial margin (ASM) |
|
|---|---|---|---|---|---|
| Type of carcinoma |
Status of final true margin |
||||
| 1 | DCIS and IDC | Yes | DCIS within 1 mm | DCIS | Positive (within 1 mm) |
| 2 | IDC and DCIS | No | DCIS transected | IDC | Positive (at the ink) |
| 3 | DCIS | No | DCIS transected | DCIS | Negative |
| 4 | IDC and DCIS | No | DCIS within 1 mm IDC transected | IDC + DCIS | Negative |
| 5 | DCIS | Yes | DCIS within 1 mm | DCIS | Positive (at the ink) |
| 6 | IDC and DCIS | Yes | Both DCIS and IDC transected | DCIS | Negative |
| 7 | IDC and DCIS | No | IDC transected | DCIS + IDC | Negative |
| 8 | IDC and DCIS | No | DCIS within 1 mm | DCIS | Negative |
| 9 | DCIS | Yes | DCIS within 1 mm | DCIS | Negative |
| 10 | IDC and DCIS | Yes | DCIS within 1 mm | DCIS | Positive (within 1 mm) |
| 11 | IDC and DCIS | Yes | IDC transected | IDC + DCIS | Negative |
| 12 | IDC and DCIS | Yes | Both DCIS and IDC within 1 mm | DCIS | Negative |
| 13 | DCIS | Yes | DCIS within 1 mm | DCIS | Negative |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma.
Efficacy of Additional Superficial Margin (ASM) Sampling
Of the 64 cases with a positive superficial specimen margin, 58 were localized over the tumor, while in the remaining 6 the superficial specimen margin was positive in other quadrants not covered by the ASM. The ASM therefore does not change the positive superficial margin status of these 6 cases (Fig. 2). Of the 58 cases with localized positive superficial specimen margins, 46 ASMs did not contain residual carcinoma while 12 did. Of these 12 cases, the final true margins of the ASM specimens were negative (carcinoma > 1 mm away from the inked true margin of the ASM) in 8 (67%). Therefore, ASM sampling rendered the final margin status histologically negative in 54 (46 + 8) of 58 SSMs (93%) with a focally positive superficial specimen margin, and 54 of 64 overall cases with a positive superficial specimen margin (Fig. 2).
Frequency of Finding Benign Breast Epithelium in Additional Superficial Margins (ASMs)
Eighty-nine of the 168 (53%) ASMs contained benign breast ducts (Fig. 1). While a thicker ASM specimen correlated with having a positive superficial specimen margin, the presence of benign breast ducts in ASM specimens did not correlate with having a positive superficial specimen margin (Table 5). Similarly, while the presence of residual carcinoma in the ASM correlated with the thickness of the ASM specimen, it was not associated with the presence of benign breast ducts in ASM (Table 5).
TABLE 5.
Frequency of benign breast tissue in additional superficial margins (ASM)
| Frequency of finding benign breast tissue in additional superficial margins (ASM) |
P-value | |
|---|---|---|
| SSM with a positive superficial specimen margin (N = 64) | 36 (56%) | 1.00 |
| SSM with a negative superficial specimen margin (N = 104) | 53 (51%) | |
| ASMs with residual carcinoma (N = 13) | 8 (62%) | 0.4290 |
| ASMs without residual carcinoma (N = 51) | 28 (55%) |
DISCUSSION
One major concern with skin-sparing mastectomy is that not all of the breast carcinoma is removed at the superficial margin where breast tissue intermingles with the dermis of the skin flaps left behind in the patient, potentially predisposing to local recurrence.4 For these reasons, we assessed the frequency of superficial mastectomy specimen margin involvement in our experience, and investigated the efficacy of taking a limited ASM sample directly over the tumor to the dermis at the time of skin-sparing mastectomy to minimize these risks.
In our study, 64 of 168 (38%) skin-sparing mastectomies (SSM) had a positive superficial specimen margin (25 with carcinoma transected at and 39 with carcinoma within 1 mm of the superficial specimen margin). This high frequency has two probable causes. First, some positive superficial specimen margins are likely false positives. Supporting this assertion is the fact that only 13 of 64 (20%) SSMs with a positive superficial specimen margin had residual carcinoma in their corresponding, completely embedded ASM specimens. We and others have noted a similar phenomenon when assessing intraoperatively obtained additional cavity margins overlying positive lumpectomy specimen margins. In our prior study,6 only 29% of cavity margins overlying positive lumpectomy specimen margins contained carcinoma. Possible factors accounting for false-positive specimen margins which apply to both mastectomies and lumpectomies include tissue retraction after removal from the patient, ink seepage into the specimen, and specimen compression during radiological studies or gross examination in the pathology laboratory.6–9 However, the second and more important and likely cause of the high frequency of positive superficial specimen margin in SSMs is the difficulty of removing all breast tissue superficially. Indeed, in our study, thicker ASMs (likely reflecting greater amounts of breast tissue left behind since a greater amount of tissue was able to be removed before reaching the dermis after the SSM was completed) correlated with positive superficial specimen margins, and 53% of ASMs contained benign breast epithelium. These results are very similar to those reported by Torresan et al.10 These authors first performed an SSM, and then removed the skin flap that would have remained in the patient, essentially converting the procedure to a conventional mastectomy. They found benign breast tissue in 59.5% of their skin flaps, and the presence of residual benign breast tissue was significantly associated with a skin flap thickness of > 5 mm. However, our result and that of Torresan et al.10 are different from that of the study of Slavin et al.,11 who performed 144 biopsies (consisting of strips of skin) of native skin flaps after SSM in 32 consecutive patients and found no breast ducts in the dermis of any biopsy. However, since biopsies are incomplete and limited samples of skin flaps and ducts are scattered focally within breast tissue, it should not be surprising that residual breast ducts would not be detected using this method.
In a skin-sparing mastectomy, the ideal situation is to create a skin flap that is thin enough to allow removal of almost all breast tissue but at the same time thick enough to preserve flap circulation and therefore avoid flap necrosis.12 In order to achieve this goal, one of the common recommendations is to dissect just superficial to the superficial layer of the superficial fascia of the breast, because the superficial fascia encloses the mammary gland ventrally.12 However, as many as 44% of breasts do not have a superficial fascial layer. Even when the superficial layer is present, 42% of them are irregular and contain islands of breast tissue within.12 The minimal distance between the superficial layer and the dermis varied from only 0.2 to 4.0 mm, which means that the superficial fascial layer is too superficial to use as a landmark for dissection since the resulting flap would be too thin. These findings provide an anatomic basis for the observed difficulty in removing all the breast tissue in SSM.
We found that skin-sparing mastectomies with a positive superficial specimen margin generally have a smaller weight, smaller skin area, and a lower skin/mastectomy surface area, but these factors did not independently predict a positive superficial specimen margin after adjusting other variables on multivariate analysis. On multivariate analysis, a positive deep margin (P = 0.01) was a strong independent prognostic marker for a positive superficial specimen margin. A thicker ASM was also an independent marker for predicting a positive superficial specimen margin but statistically it was a borderline factor (P = 0.05) in this study. A thicker ASM sample corresponds to a thicker skin flap in the patient’s body and the presence of a positive deep margin likely reflects a more extensive disease process.
In our study, residual carcinoma was seen in 14 of 168 (8.3%) ASMs. The percentage of ASMs containing residual carcinoma in our study is concordant with two previous studies that used different methods of evaluation.10,13 As mentioned previously, Torresan et al.10 initially performed SSM, and then removed the skin flap that would have remained in the patient’s body (essentially converting to a conventional mastectomy) for histological examination. They found a 9.5% incidence of residual carcinoma in the skin flaps, which was strongly associated with the thickness of the skin flap (similar to our results) and the amount of residual glandular tissue (dissimilar to our results). Using a step-serial sectioning technique, Ho et al.13 examined the skin and subcutaneous tissue of mastectomies up to 5 cm from the center of cancers at 5-mm intervals and found carcinoma in 20% (6 of 30 cases). Skin tethering, tumor size, and perineural invasion were strongly associated with skin involvement outside the nipple-areolar complex by carcinoma.13
Our study is the first to correlate superficial specimen margins with ASM sampling. We showed that a positive superficial specimen margin was a strong predictor of residual carcinoma in ASM. Among the SSMs with a positive superficial specimen margin, multivariate analysis revealed that the only clinicopathological factors independently predictive of residual carcinoma in ASMs were the presence of extensive DCIS and thicker ASM samples. Our results should alert surgical oncologists that patients with extensive DCIS (as detected preoperatively by mammography as extensive branching calcifications or possibly by magnetic resonance imaging) are at increased risk for incomplete excision of the DCIS superficially. The prototypical case is that of the young woman with a large, multicentric, high-grade comedo DCIS who requires mastectomy but seeks the best cosmetic result. Unfortunately, such patients may be not the ideal candidates for the SSM procedure. Incomplete excision of high-grade DCIS could predispose to recurrence as an aggressive high-grade invasive ductal carcinoma.14 Such patients require meticulous dissection between the skin and breast tissue to minimize this possibility, and possibly additional therapy (see below). Review of the surgical literature supports this viewpoint. Salas et al.15 reported four women who manifested invasive local recurrences in transverse rectus abdominis musculocutaneous (TRAM) flaps after undergoing SSM and immediate reconstruction for DCIS (two cases) or DCIS with microinvasion (two cases). Importantly, all four of these patients were relatively young (ages 37–48 years), had extensive, high-grade, multifocal DCIS in their initial SSM, and had DCIS along with IDC in their post-SSM recurrence, suggesting that the recurrence arose from incompletely excised breast tissue bearing DCIS. Indeed, margins were reported to be close (< 1 mm) in three of the four SSM. Furthermore, Carlson et al.16 recently reported a retrospective analysis of local recurrence of DCIS after SSM. In their study, high nuclear grade was a significant risk factor for local recurrence; there was a trend towards recurrence in young patients (< 50 years old) with large (> 4 cm) DCIS having a close margin, but these latter factors failed to reach statistical significance.
Our study should be put in the context of the existing literature that emphasizes (perhaps overemphasizes) the oncological safety of SSM. Indeed, most studies of SSM have shown a local recurrence rate similar to that of the traditional mastectomy.11,17–22 Several authors have concluded that the risk factors for local recurrence parallel those for systemic relapse, and that local recurrence represents a component of systemic relapse (tumor biology) and not inadequate surgical excision (surgical modality). 20,23–27 Hence, these studies de-emphasize the importance of complete surgical excision. However, both of these conclusions are easily challenged. First, essentially all studies of SSM safety have been single institution, retrospective studies with limited follow-up and variable adjuvant chemotherapy and postmastectomy radiation therapy. Biases inherent in these studies would seem to be likely to favor SSM; for example, patients with locally advanced cancers that are more likely to recur locally are less likely to be offered SSM. Large, randomized, multicenter studies of SSM safety are conspicuously absent from the literature. More importantly, few studies have evaluated SSM margin status in relation to relapse. Second, more recent studies, including a large metaanalysis, have concluded that isolated local recurrences do impact upon survival.28,29 Moreover, not all local recurrences after SSM have the same clinical implications. Langstein et al.30 showed that patients with subcutaneous recurrences (likely resulting from incomplete excision) have a better prognosis that those with chest wall recurrences (likely reflecting the presence of incurable metastatic disease).
We suspect that superficial margin status may predict local recurrence in low stage patients (such as those with extensive DCIS described above) who are less likely to receive postmastectomy chemotherapy and radiation therapy based upon published data delineating the high frequency of local recurrences following SSM that occur in the skin flap, not in the chest wall. For example, Newman et al.21 reported that 22 of the 23 patients with local recurrence presented as skin-flap masses. Langstein et al.30 reported that 72% of local recurrences in their patient group were in the skin and subcutaneous tissue. Therefore, we suggest careful attention to the superficial specimen margin in all SSM specimens. It should be pointed out that unless a surgeon feels he/she has cut across gross cancer, the general practice is not to treat clinically with either re-excision or radiation therapy. However, more patients overall are receiving postmastectomy radiation today than before. ASM sampling may help clear the superficial margin in the area of the tumor in some cases. The patients whose margins remain positive may be the ones for whom postmastectomy radiation therapy is useful, potentially allowing this treatment and its potential adverse side-effects to be limited to the subset of patients who will benefit from it.
At present, we suggest particularly close follow-up for the subset of patients with positive superficial margins, to determine the frequency of recurrence in this carefully defined group. While our results are preliminary at this time due to limited follow-up, we have noted that a higher rate of local recurrence in those with a positive superficial specimen margin (10%) than those with a negative superficial specimen margin (4%). If the increased frequency proves to be significant, a prospective randomized trial of postmastectomy radiation therapy or extended skin excision at the time of SSM in this subgroup of patients should be considered.
In summary, we have investigated the efficacy of ASM sampling in SSM. Negative superficial specimen margins are reassuring. The presence of extensive DCIS and thick ASMs were strong predictors of residual carcinoma in ASMs in cases with a positive superficial specimen margin. ASM sampling is effective in decreasing positive superficial margins locally in SSM specimens but not in patients with multicentric disease. Patients with extensive high-grade DCIS may not be the ideal candidates for SSMs.
REFERENCES
- 1.Toth BA, Lapport P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87:1048–1053. [PubMed] [Google Scholar]
- 2.Cunnick GH, Mokbel K. Skin-sparing mastectomy. Am J Surg. 2004;188:78–84. doi: 10.1016/j.amjsurg.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Salhab M, al Sarakbi W, Joseph A, et al. Skin-sparing mastectomy and immediate breast reconstruction: patient satisfaction and clinical outcome. Int J Clin Oncol. 2006;11:51–54. doi: 10.1007/s10147-005-0538-1. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel AJ, Butler CE. Recurrence following treatment of ductal carcinoma in situ with skin-sparing mastectomy and immediate breast reconstruction. Plast Reconstr Surg. 2003;111:706–711. doi: 10.1097/01.PRS.0000041440.12442.05. [DOI] [PubMed] [Google Scholar]
- 5.Torresan RZ, Cabello Dos Santos C, Brenelli H, et al. Residual glandular tissue after skin-sparing mastectomies. Breast J. 2005;11:374–375. doi: 10.1111/j.1075-122X.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 6.Cao D, Lin C, Woo SH, et al. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005;29:1625–1632. doi: 10.1097/01.pas.0000180448.08203.70. [DOI] [PubMed] [Google Scholar]
- 7.Dooley WC, Parker J. Understanding the mechanisms creating false positive lumpectomy margins. Am J Surg. 2005;190:606–608. doi: 10.1016/j.amjsurg.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Schnitt SJ, Connolly JL. Processing and evaluation of breast excision specimens. A clinically oriented approach. Am J Clin Pathol. 1992;98:125–137. doi: 10.1093/ajcp/98.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Schnitt SJ, Abner A, Gelman R, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer. 1994;74:1746–1751. doi: 10.1002/1097-0142(19940915)74:6<1746::aid-cncr2820740617>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Torresan RZ, Cabello Dos Santos C, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol. 2005;12:1037–1044. doi: 10.1245/ASO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Slavin SA, Schnitt SJ, Duda RB, et al. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg. 1998;102:49–62. doi: 10.1097/00006534-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer. 2002;94:1619–1625. doi: 10.1002/cncr.10429. [DOI] [PubMed] [Google Scholar]
- 13.Ho CM, Mak CK, Lau Y, et al. Skin involvement in invasive breast carcinoma: safety of skin-sparing mastectomy. Ann Surg Oncol. 2003;10:102–107. doi: 10.1245/aso.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SK, Douglas-Jones AG, Fenn N, et al. The clinical behavior of breast carcinoma is probably determined at the preinvasive stage (ductal carcinoma in situ) Cancer. 1997;80:1740–1745. [PubMed] [Google Scholar]
- 15.Salas AP, Helvie MA, Wilkins EG, et al. Is mammography useful in screening for local recurrences in patients with TRAM flap breast reconstruction after mastectomy for multifocal DCIS? Ann Surg Oncol. 1998;5:456–463. doi: 10.1007/BF02303866. [DOI] [PubMed] [Google Scholar]
- 16.Carlson GW, Page A, Johnson E, et al. Local recurrence of ductal carcinoma in situ after skin-sparing mastectomy. J Am Coll Surg. 2007;204:1074–1078. doi: 10.1016/j.jamcollsurg.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 17.Carlson GW, Styblo TM, Lyles RH, et al. Local recurrence after skin-sparing mastectomy: tumor biology or surgical conservatism? Ann Surg Oncol. 2003;10:108–112. doi: 10.1245/aso.2003.03.053. [DOI] [PubMed] [Google Scholar]
- 18.Fersis N, Hoenig A, Relakis K, et al. Skin-sparing mastectomy and immediate breast reconstruction: incidence of recurrence in patients with invasive breast cancer. Breast. 2004;13:488–493. doi: 10.1016/j.breast.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Kroll SS, Khoo A, Singletary SE, et al. Local recurrence risk after skin-sparing and conventional mastectomy: a 6-year follow-up. Plast Reconstr Surg. 1999;104:421–425. doi: 10.1097/00006534-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Medina-Franco H, Vasconez LO, Fix RJ, et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg. 2002;235:814–819. doi: 10.1097/00000658-200206000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence after skin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol. 1998;5:620–626. doi: 10.1007/BF02303832. [DOI] [PubMed] [Google Scholar]
- 22.Rivadeneira DE, Simmons RM, Fish SK, et al. Skin-sparing mastectomy with immediate breast reconstruction: a critical analysis of local recurrence. Cancer J. 2000;6:331–335. [PubMed] [Google Scholar]
- 23.Barton FE, English JM, Kingsley WB, et al. Glandular excision in total glandular mastectomy and modified radical mastectomy: a comparison. Plast Reconstr Surg. 1991;88:389–392. doi: 10.1097/00006534-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Carlson GW, Bostwick J, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225:570–575. doi: 10.1097/00000658-199705000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donegan WL, Perez-Mesa CM, Watson FR. A biostatistical study of locally recurrent breast carcinoma. Surg Gynecol Obstet. 1966;122:529–540. [PubMed] [Google Scholar]
- 26.Gilliland MD, Barton RM, Copeland EM. The implications of local recurrence of breast cancer as the first site of therapeutic failure. Ann Surg. 1983;197:284–287. doi: 10.1097/00000658-198303000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singletary SE. Skin-sparing mastectomy with immediate breast reconstruction: the M. D. Anderson Cancer Center experience. Ann Surg Oncol. 1996;3:411–416. doi: 10.1007/BF02305673. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan A, Dietz JR, Aft R, et al. Patterns of local breast cancer recurrence after skin-sparing mastectomy and immediate breast reconstruction. Am J Surg. 2007;194:438–443. doi: 10.1016/j.amjsurg.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;17(366):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 30.Langstein HN, Cheng MH, Singletary SE, et al. Breast cancer recurrence after immediate reconstruction: patterns and significance. Plast Reconstr Surg. 2003;111:712–720. doi: 10.1097/01.PRS.0000041441.42563.95. [DOI] [PubMed] [Google Scholar]