Abstract
The small integrin-binding ligand, N-linked glycoprotein (SIBLING) family is closely related to osteogenesis. Until recently, little was known about their existence in articular cartilage. In this study, we systematically evaluated the presence and distribution of four SIBLING family members in rat femoral head cartilage: dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), osteopontin (OPN), and dentin sialophosphoprotein (DSPP). First, non-collagenous proteins were extracted and then separated by ion-exchange chromatography. Next, the protein extracts eluted by chromatography were analyzed by Stains-all staining and Western immunoblotting. IHC was used to assess the distribution of these four SIBLING family members in the femoral head cartilage. Both approaches showed that all the four SIBLING family members are expressed in the femoral head cartilage. IHC showed that SIBLING members are distributed in various locations throughout the articular cartilage. The NH2-terminal fragments of DMP1, BSP, and OPN are present in the cells and in the extracellular matrix, whereas the COOH-terminal fragment of DMP1 and the NH2-terminal fragment of DSPP are primarily intracellularly localized in the chondrocytes. The presence of the SIBLING family members in the rat femoral head cartilage suggests that they may play important roles in chondrogenesis. (J Histochem Cytochem 58:1033–1043, 2010)
Keywords: femoral head, cartilage, dentin matrix protein 1, dentin sialophosphoprotein, bone sialoprotein, osteopontin, chondrogenesis, proteoglycan
The articular cartilage lining the surface of the femoral head contributes to the growth of the cartilage during development, cell proliferation, and extracellular matrix (ECM) production. In addition to type II collagen, the ECM of the cartilage contains many types of non-collagenous proteins (NCPs). The small integrin-binding ligand, N-linked glycoprotein (SIBLING) family is one category of NCPs, which includes dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), osteopontin (OPN), dentin sialophosphoprotein (DSPP), and matrix extracellular phosphoglycoprotein (Fisher et al. 2001). In addition to the functions of signaling, cell development, and cell–matrix interaction, the SIBLING family members play important roles in osteogenesis and dentinogenesis (Huang et al. 2008b). However, no systemic evaluation of the presence and distribution of SIBLING proteins in articular cartilage has been performed.
DMP1 is an acidic phosphoprotein predominantly expressed in bone, dentin, and cementum (George et al. 1993; MacDougall et al. 1998). Lower level of DMP1 expression has also been found in non-mineralized tissue (Terasawa et al. 2004; Qin et al. 2007). In the ECM of bone and dentin, DMP1 mainly occurs as proteolytically processed fragments, including a 37-kDa fragment (designated as DMP1-N) and a 57-kDa fragment (referred to as DMP1-C), originating from the NH2-terminal region and COOH-terminal region of the DMP1 amino acid sequence, respectively (Qin et al. 2003b). In addition to the core protein form (i.e., DMP1-N), NH2-terminal fragment of DMP1 also occurs as a proteoglycan (referred to as DMP1-PG). More recently, the full-length form of DMP1 has been identified in the ECM of rat dentin and bone, the concentration of which is considerably lower than that of its processed fragments (Huang et al. 2008a). The importance of DMP1 for dentin and bone mineralization has been demonstrated by in vitro mineralization studies (Gericke et al. 2010), gene ablation experiments in mice (Ye et al. 2004,2005), and human genetic studies (Feng et al. 2006).
BSP is primarily found in bone, cementum, reactionary dentin, and mineralizing cartilage (Ganss et al. 1999; Moses et al. 2006). In vitro studies suggest that BSP facilitates the nucleation of hydroxyapatite crystals and that, as the crystals grow, BSP acts to inhibit the growth of the crystals (Ganss et al. 1999; Qin et al. 2004). Gene ablation studies showed that BSP-deficient mice have defects in the growth and repair of the long bone, along with a greater mass of trabecular bone and a lower rate of bone turnover (Malaval et al. 2009).
OPN is abundant in non-mineralized and mineralized tissues. In mineralized tissues, OPN is expressed in bone, cementum, and tertiary dentin (Sodek et al. 2000; Qin et al. 2004; Moses et al. 2006). Both in vitro and in vivo studies have shown that OPN is an effective inhibitor of apatite crystal formation and growth (Boskey et al. 1993, 2002; Sodek et al. 2000). In addition, OPN mRNA has been detected in cartilage by RT-PCR (Attur et al. 2001). Gene ablation experiments showed that the articular cartilage of OPN-null mice displays structural changes and loss of proteoglycans (Matsui et al. 2009).
DSPP is expressed at a high level in dentin and at a relatively low level in cementum, bone, and certain non-mineralized tissues (D'souza et al. 1997; MacDougall et al. 1997; Qin et al. 2002; Fisher et al. 2004). Similar to DMP1, DSPP in the ECM of dentin is proteolytically processed into the NH2-terminal fragment termed dentin sialoprotein (DSP) and into the COOH-terminal fragment termed dentin phosphoprotein (DPP) (MacDougall et al. 1997; Prasad et al. in press). Although DSP and DPP are abundant in the ECM of dentin (Qin et al. 2003a; Yamakoshi et al. 2005), only a trace amount of the full-length form of DSPP was found in the mouse/rat dentin–pulp complex (Sun et al. 2010b). Gene ablation experiments in mice demonstrated that DSPP is critical for the mineralization of dentin and bone (Sreenath et al. 2003; Verdelis et al. 2008).
Although a great deal of information regarding the biochemical properties and distribution of the SIBLING family members in mineralized tissue has accumulated, little is known about the distribution and function of these molecules in the articular cartilage.
In this study, protein chemistry and IHC approaches were utilized to analyze these molecules in the rat femoral head cartilage at different ages. These analyses revealed that DMP1, BSP, OPN, and DSPP are expressed in the femoral head cartilage and are distributed in various locations throughout the cartilage. The findings from this investigation provide new information and clues for understanding the biological roles of the SIBLING family members in chondrogenesis and osteogenesis.
Materials and Methods
Tissue Acquisition/Extraction of NCPs
For histological analysis, the femoral head cartilage from 4-, 8-, 16-, and 24-week-old male Sprague–Dawley rats (Harlan; Indianapolis, IN) was used. For protein chemistry analyses, the femoral head cartilage was obtained from rats at 10 weeks after birth because rats at this age provide ample tissue mass while they are still young enough to maintain the momentum of growth with abundant matrix protein secretion. Rats were first anesthetized, then perfused for histological analysis or sacrificed using CO2 to acquire articular cartilage for protein extraction. The animal protocol was approved by the Animal Welfare Committee of Baylor College of Dentistry of the Texas A&M University System Health Science Center (Dallas, TX).
Extraction and Separation of NCPs in the Femoral Head Cartilage
Fifty 10-week-old rats were used for the extraction of NCPs. The femoral head cartilage was carefully separated at the cartilage–bone interface under a dissecting microscope. Because of its unusual histological structure involving an articular cartilage superimposed on a growth plate, there is less chance of protein contamination from the bone matrix when the femoral head cartilage is used to evaluate molecules in the articular cartilage by a protein chemistry approach. To ensure that our method yields only cartilage, we performed histology on five test samples of dissected femoral head cartilage. All the test samples were separated above the cartilage–bone interface. Thus, we were sure that the extracted NCPs were from the cartilaginous tissues. The extracted SIBLING family members were mainly from the articular cartilage portion because the growth plate cartilage contained relatively lower levels of these molecules (see the IHC staining results). For the extraction of NCPs from the femoral head cartilage, the cartilage was placed in 4 M guanidium–HCl (Gdm–HCl; Acros Organics, Fairlawn, NJ) solution (pH 7.2) containing proteinase inhibitors for 48 hr. Because Gdm–HCl without EDTA can only extract proteins from the non-calcified tissue (e.g., cartilage), this protein extraction method also ensured that the protein extracted from the femoral head cartilage was from the unmineralized portion of the cartilage. The Gdm–HCl extracts were subjected to Q-Sepharose (Amersham Biosciences; Uppsala, Sweden) ion-exchange chromatography with a gradient ranging from 0.1 to 0.8 M NaCl in 6 M urea solution (pH 7.2), as described previously (Qin et al. 2001). NCPs eluted into 120 fractions (0.5 ml each), and the sample from each fraction was analyzed by SDS-PAGE and Western immunoblotting to evaluate DMP1, BSP, OPN, and DSPP.
SDS-PAGE and Western Immunoblotting
For SDS-PAGE, 60 μl of sample from each chromatographic fraction was loaded onto 5–15% gradient gels. First, Stains-all staining was used to profile NCPs in all the chromatographic fractions. Then, Western immunoblotting was carried out to confirm and/or identify the individual SIBLING family members. For Western immunoblotting, we used five types of well-validated antibodies against the SIBLING family members (Table 1). For detection of DMP1, two types of anti-DMP1 antibodies were used at a dilution of 1:2000: anti-DMP1-N-9B6.3, a monoclonal antibody (MAb) immunoreactive to the NH2-terminal region of DMP1 (Qin et al. 2001), and anti-DMP1-C-857, an affinity-purified polyclonal antibody which recognizes the COOH-terminal region of DMP1 (Maciejewska et al. 2009). For detection of DSPP/DSP, an anti-DSP MAb (anti-DSP-2G7.3) (Qin et al. 2003a) was used at a dilution of 1:2000. For detection of BSP, an anti-BSP MAb (anti-BSP-10D9.2) (Huang et al. 2008b) was used at a dilution of 1:2000. For detection of OPN, an anti-OPN MAb (Santa Cruz Biotechnology; Santa Cruz, CA) was used at a dilution of 1:2000. Blots were washed three times in PBS containing 0.3% Tween 20, followed by incubation in the alkaline phosphate-conjugated anti-mouse IgG or anti-rabbit IgG (Sigma Aldrich; Louis, MO) at a dilution of 1:5000. Finally, the blots were incubated with the chemiluminescent substrate CDP-Star (Ambion; Austin, TX) for 5 min and exposed to X-ray films.
Table 1.
Antibodies used in this study
| Antibody | Antibody type | Immunizing antigen | Immunoreactivity in Western blotting | Immunoreactivity in IHC |
|---|---|---|---|---|
| Anti-DMP1-N-9B6.3a | Monoclonal | 37 kDa (N-terminal fragment) | Yes | Yes |
| Anti-DMP1-C-8G10.3b | Monoclonal | 57 kDa (C-terminal fragment) | No | Yes |
| Anti-DMP1-C-857c | Polyclonal | Oligopeptide (residues 471–485) | Yes | Yes |
| Anti-BSP-10D9.2d | Monoclonal | Purified rat bone BSP | Yes | Yes |
| Anti-OPNe | Monoclonal | Mouse recombinant OPN | Yes | Yes |
| Anti-DSP-2G7.3f | Monoclonal | Purified rat dentin DSP | Yes | No |
| Anti-DSP-2G12.3g | Monoclonal | Purified rat dentin DSP | No | Yes |
This monoclonal antibody (MAb) (Qin et al. 2006) was used to detect the NH2-terminal fragment of DMP1 by IHC and Western immunoblotting analysis.
This MAb (Baba et al. 2004b) was used to detect the COOH-terminal fragment of DMP1 by IHC analysis in this study.
This polyclonal antibody (Maciejewska et al. 2009) was used to detect the COOH-terminal fragment of DMP1 by Western immunoblotting analysis.
This MAb (Huang et al. 2008a) was used to detect BSP by IHC analysis and Western immunoblotting in this study.
This MAb (Santa Cruz) was used to detect OPN by IHC analysis and Western immunoblotting in this study.
This MAb (Qin et al. 2003a) was used to detect DSP by Western immunoblotting in this study.
This MAb (Baba et al. 2004a) was used to detect DSP by IHC analysis.
DMP1, dentin matrix protein 1; BSP, bone sialoprotein; OPN, osteopontin; DSP, dentin sialoprotein.
Hematoxylin and Eosin (HE) and IHC Staining
HE staining was performed to examine the histological appearance of the femoral head cartilage from rats at 4, 8, 16, and 24 weeks after birth. IHC was done to analyze the difference in the distribution of these SIBLING family members in the femoral head cartilage from 4-, 8-, and 16-week-old rats. Under anesthesia, male Sprague–Dawley rats (Harlan) were perfused from the ascending aorta with 4% paraformaldehyde in 0.1 M phosphate buffer. The femurs were dissected and further fixed in 4% paraformaldehyde for 48 hr, followed by decalcification in 8% EDTA (pH 7.4) at 4C. The decalcified specimens were processed for paraffin embedding, and serial 5-μm sections were prepared. For the IHC of DMP1, two types of antibodies were used: the anti-DMP1-C-8G10.3, a MAb that recognizes the COOH-terminal region of DMP1 (Baba et al. 2004b) (used at a dilution of 1:800), and the anti-DMP1-N-9B6.3, which is immunoreactive to the NH2-terminal fragment of DMP1 (used at a dilution of 1:300). For the IHC of DSPP/DSP, the anti-DSP MAb, referred to as anti-DSP-2C12.3 (Baba et al. 2004a), was used at a dilution of 1:800. For BSP and OPN, antibodies were the same as those used in the Western immunoblotting analyses and the dilution was 1:400. Mouse IgG at the same concentrations as the corresponding primary antibodies was used to replace the primary antibodies in the negative control experiments. All IHC experiments were carried out using the M.O.M kit and DAB kit (Vector Laboratories; Burlingame, CA) following the manufacturer's instructions. Table 1 is a summary of all well-characterized antibodies used in Western immunoblotting and IHC.
Results
DMP1, BSP, OPN, and DSPP in the Gdm–HCl Extracts of the Femoral Head Cartilage
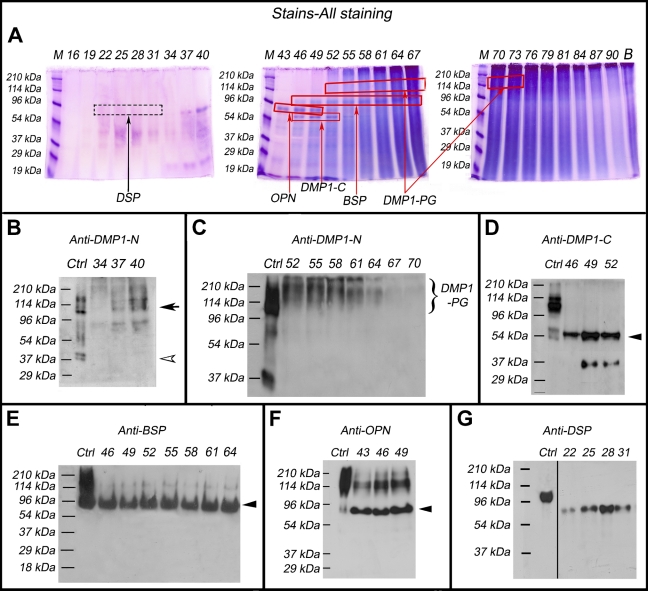
The Gdm–HCl extracts of the articular cartilage were separated into 120 fractions by Q-Sepharose ion-exchange chromatography. Each of the chromatographic fractions that potentially contained any of the four SIBLING family members was assayed by Stains-all staining (Figure 1A) and subsequently by Western immunoblotting (Figures 1B–1G).
Figure 1.
Identification of the small integrin-binding ligand, N-linked glycoprotein (SIBLING) family members in the femoral head cartilage by Stains-all staining and Western immunoblotting. Numbers at the top of each image represent fraction numbers of the Q-Sepharose chromatography. (A) Stains-all staining for every third fraction in the chromatographic region from fractions 16–90 is presented here to illustrate the existence of the four SIBLING family members. The blue protein bands in fractions 43–52 migrating between the 53- and 78-kDa molecular mass markers represent osteopontin (OPN; boxed). The dentin matrix protein 1 (DMP1)-C fragment (∼57 kDa, boxed) co-eluted with OPN in fractions 46–52. Bone sialoprotein (BSP; ∼90 kDa, boxed) mainly eluted in fractions 46–64. The identities of these SIBLING family members were confirmed by Western immunoblotting (B–G). (B) Control (Ctrl): 1 μg of DMP1 (including the full-length DMP1 and its processed fragments) extracted from long bone of rat. Western immunoblotting using the anti-DMP1-N-9B6.3 antibody demonstrated the presence of DMP1-N (37 kDa, white arrowhead) and full-length DMP1 (∼110 kDa, black arrow) in fractions 34–40. (C) Ctrl: 1 μg of DMP1 isolated from long bone of rat. Note the presence of DMP1-proteoglycan (DMP1-PG) recognized by the anti-DMP1-N-9B6.3 antibody in fractions 52–70. (D) Ctrl: 1 μg of DMP1 isolated from long bone of rat. DMP1-C (arrowhead) was detected by the anti-DMP1-C-857 antibody in fractions 46–52. (E) Ctrl: 1 μg of BSP isolated from long bone of rat. BSP (arrowhead) was recognized by the anti-BSP-10D9.2 antibody in fractions 46–64. (F) Ctrl: 1 μg of OPN isolated from long bone of rat. OPN (arrowhead) was detected by the anti-OPN antibody in fractions 43–49. (G) Ctrl: 0.3 μg of dentin sialoprotein (DSP) isolated from incisor dentin of rat. DSP was detected by the anti-DSP-2G7.3 antibody in fractions 22–31. Note that DSP extracted from the cartilage migrated faster than that isolated from rat dentin (i.e., the positive Ctrl). The lane for the positive Ctrl sample in the original Western immunoblotting gel was two lanes away from the lane of fraction 22; we horizontally moved the Ctrl lane to the position next to fraction 22 to better illustrate the results.
Stains-all staining demonstrated blue protein bands corresponding to DMP1-C (Figure 1A), whereas Western immunoblotting revealed the presence of full-length DMP1 (black arrow, Figure 1B) and DMP1-N (white arrowhead, Figure 1B) in fractions 37–40, DMP1-PG in fractions 52–70 (Figure 1C), and DMP1-C in fractions 46–52 (black arrowhead, Figure 1D). Among these four forms of DMP1, DMP1-PG was the most abundant. The quantity of full-length DMP1 in the cartilage appeared greater than that of DMP1-N, an observation that is opposite to that seen in the mineralized tissues (i.e., bone and dentin), in which the fragment forms of DMP1 are predominant.
In Stains-all staining, blue protein bands representing BSP were observed in fractions 46–64 (Figure 1A), and its identity as BSP was further confirmed by Western immunoblotting (Figure 1E, black arrowhead). OPN in fractions 43–49 was visualized by Stains-all staining (Figure 1A) and further confirmed by Western immunoblotting (Figure 1F, black arrowhead).
Stains-all staining failed to demonstrate any protein bands corresponding to the migration rates of DSPP or DSP (Figure 1A), whereas Western immunoblotting by the anti-DSP antibody clearly showed the presence of DSP in fractions 22–31, which migrated between the 54- and 96-kDa molecular mass markers (Figure 1G). The migration rate of DSP extracted from the cartilage was faster than that from rat dentin (∼100 kDa). Note that DPP was not detected in any of the chromatographic fractions by Stains-all staining, and an appropriate antibody to detect DPP by Western immunoblotting is not available.
HE and IHC Staining
First, HE staining was carried out to reveal the structure of the rat femoral head cartilage (Figure 2). In the femoral head cartilage, before formation of the secondary ossification center, the articular cartilage is located directly adjacent to the growth plate.
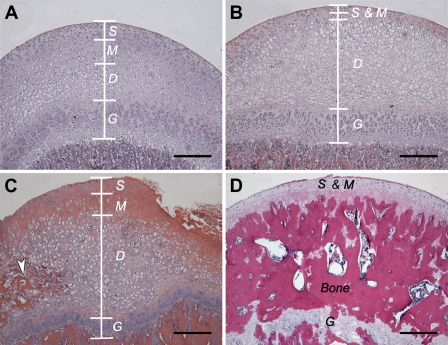
Figure 2.
Hematoxylin and eosin (HE) staining of the femoral head cartilage from 4-, 8-, 16-, and 24-week-old rats. (A) Femoral head cartilage from a 4-week-old rat, (B) femoral head cartilage from an 8-week-old rat, (C) femoral head cartilage from a 16-week-old rat. (D) Femoral head cartilage including the secondary ossification center from a 24-week-old rat. In 4-, 8-, and 16-week-old rats, the femoral head cartilage was divided into three distinct layers: the superficial layer (S), the middle layer (M), and the deep layer (D). The deep layer is continuous to the growth plate (G). The structure of articular cartilage changes with aging. Compared with the younger rats (4-week-old; A), the cartilage of the older rats (16-week-old; C) has more matrix in the middle layer and a greater number of hypertrophic cells in the deep layer. The secondary ossification center (white arrowhead; C) was formed in the deep layer at 16 weeks after birth. At 24 weeks after birth, the secondary ossification center completely separated the articular cartilage from the growth plate (D). Bar = 100 μm.
We divided the rat femoral head cartilage into three layers according to the orientation and the morphology of cells. These three layers are the superficial layer (S), the middle layer (M), and the deep layer (D), which contains enlarged cells at early ages. The major structural changes of the femoral head cartilage from week 4 to week 16 included decreased width of the superficial and middle layers, increased cell number in the deep layer, and the presence of a secondary ossification center interior to the deep layer at 16 weeks. In the 24-week-old rats, the articular cartilage and the growth plate of the femoral head were completely separated by the secondary ossification center.
Immunohistochemical staining of the femoral head cartilage from 4-, 8-, and 16-week-old rats showed that each of the four SIBLING members had its own distribution pattern in specific layers. In addition, the distribution profile of each molecule also changed in relation to postnatal growth (Figures 3–5).
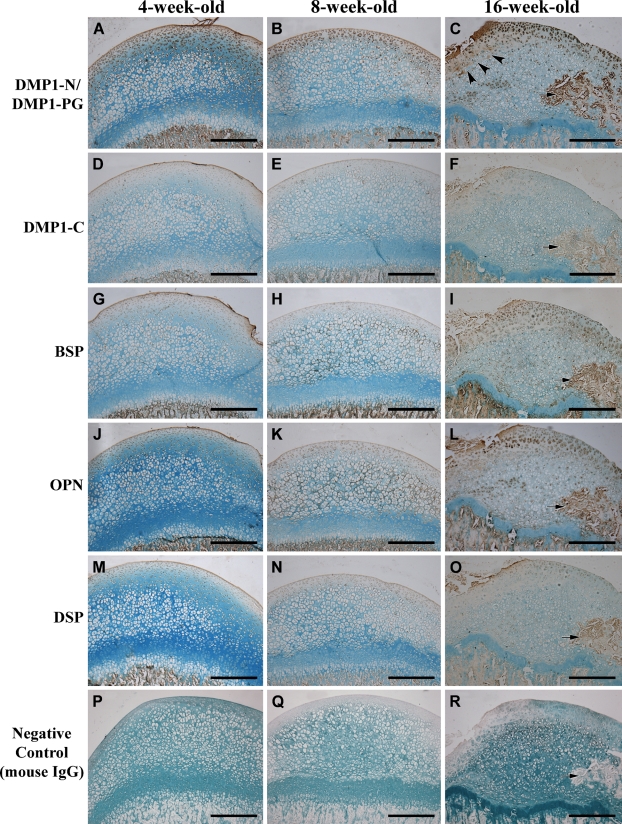
Figure 3.
IHC staining of the femoral head cartilage from 4-, 8-, and 16-week-old rats. Column 1, 4-week-old rat; column 2, 8-week-old rat; column 3, 16-week-old rat. (A–C) IHC for DMP1-N/DMP1-PG. The signal for DMP1-N/DMP1-PG was mainly observed in the chondrocytes and in the extracellular matrix (ECM) of the superficial layer. The intensity of IHC staining in the superficial layer increased with aging. At 16 weeks (C), the signal for DMP1-N/DMP1-PG was very strong in the ECM near the fovea (arrowhead) of the femoral head and in the secondary ossification center (arrow). (D–F) IHC for DMP1-C. The signal for DMP1-C was primarily observed in the cell nuclei in all layers. The staining intensity of DMP1-C is not as strong as DMP1-N/DMP1-PG in the same area. Arrow indicates the secondary ossification center (F). (G–I) IHC for BSP. BSP was observed in the cells of all three layers and in the ECM of the superficial layer at 4 weeks after birth (G). At 8 weeks (H), BSP signal was also observed in the ECM of the middle layer. At 16 weeks (I), the signal for BSP became stronger in the ECM beneath the superficial layer and in the chondrocytes in all layers of the cartilage. The staining was also intense in the secondary ossification center (arrow, I). (J–L) IHC for OPN. At 4 weeks after birth, OPN was observed in the cells of all layers and in the ECM of the superficial layer (J). At 8 weeks (K), the signal for OPN was also observed in the ECM of the middle layer. At 16 weeks (L), the signal for OPN became stronger in the ECM of the superficial and middle layers and in the chondrocytes in all layers of the cartilage. The staining was also strong in the secondary ossification center (arrow, L). (M–O) IHC for DSP. A weak signal for DSP was observed in the cells of all layers and in the ECM of the superficial layer at 4, 8, and 16 weeks. The staining for DSP was observed in the secondary ossification center (arrow, O). (P–R) Negative controls. In these IHC experiments, normal mouse IgG was used to replace the corresponding primary antibodies. Arrow indicates secondary ossification center (R). Bar = 200 μm.
Figure 4.
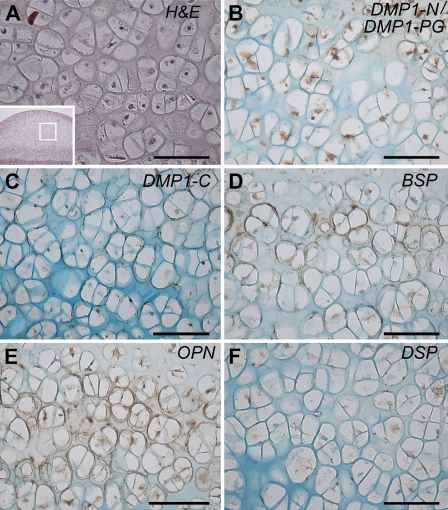
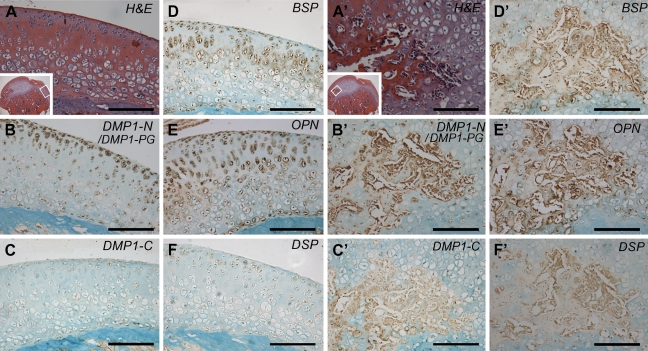
IHC—higher magnification of the deep layer in the femoral head cartilage from an 8-week-old rat. (A) HE staining: the microphotograph is a higher magnification of boxed area in inset; (B) IHC for DMP1-N/DMP1-PG; (C) IHC for DMP1-C; (D) IHC for BSP; (E) IHC for OPN; and (F) IHC for DSP. Note the presence of BSP (D) and OPN (E) in the matrix surrounding chondrocytes in the deep layer. The staining for DMP1-N was weak in the matrix (B). DMP1-C and DSP were observed only within the cells (C,F). Bar = 100 μm.
Figure 5.
IHC—higher magnification of the femoral head cartilage and the secondary ossification center of the cartilage in the 16-week-old rats. (A) HE staining; the microphotograph is a higher magnification of the boxed area in inset. IHC staining (B–F) was performed on sections that were serial to A. (B) IHC for DMP1-N/DMP1-PG; (C) IHC for DMP1-C; (D) IHC for BSP; (E) IHC for OPN; (F) IHC for DSP. Note that the signals for DMP1-N/DMP1-PG, BSP, and OPN were strong in the chondrocytes and in the ECM (B,D,E), whereas the signals for DMP1-C and DSP were relatively weak in the chondrocytes but not in the ECM (C,F). A′–F′ is a higher magnification of the secondary ossification center in the femoral head cartilage. (A′) HE staining of the boxed area in inset. IHC staining (B′–F′) was performed on sections that were serial to A′. (B′) IHC for DMP1-N/DMP1-PG; (C′) IHC for DMP1-C; (D′) IHC for BSP; (E′) IHC for OPN; (F′) IHC for DSP. All these SIBLING family members were detected at a relatively high level in the newly formed bone matrix of the secondary ossification center. Bar = 200 μm.
In the 4- and 8-week-old rats, the signal recognized by the anti-DMP1-N-9B6.3 antibody was strong in the chondrocytes and in the ECM of the superficial and middle layers, but weak in the deep layer of the cartilage (Figures 3A and 3B). In the 16-week-old rat (Figures 3C and 5B), strong staining was observed in some of the deep layer cells and in the secondary ossification center. In addition, staining by the anti-DMP1-N-9B6.3 antibody in the ECM under the fovea of the femoral head was stronger than that in other areas (Figure 3C). Relatively weak signal was recognized by the anti-DMP1-C-8G10.3 antibody in chondrocytes across all layers of the femoral head cartilage from 4 to 16 weeks of age (Figures 3D–3F); the detection of the DMP1-C fragment by Western immunoblotting (Figure 1D) may be attributed to the signal in the cells.
BSP was observed in all three layers of the femoral head cartilage from all three age groups (Figures 3G–3I). The ECM in the cartilage also showed staining for BSP. In the 4-week-old rat, the signal for BSP was strong in the ECM of the superficial layer (Figure 3G). In the 8-week-old rat, the ECM around deep layer cells was positively stained (Figures 3H and 4D). In the 16-week-old rats, the ECM of the middle layer showed stronger signal than that of the superficial layer (Figures 3I and 5D).
The distribution pattern of OPN (Figures 3J–3L, 4E, and 5E) was very similar to that seen for BSP. In the femoral head cartilage of the 16-week-old rats, staining in the ECM and chondrocytes of the middle layer was stronger than that seen in the superficial layer (Figure 5E).
The signal for DSP was observed in cells across all three layers of the cartilage (Figures 3M–3O, 4F, and 5F). Although DSP was found in the ECM of the superficial and middle layers (Figures 3M–3O), the expression level was very low.
For the convenience of comparison, we summarized the IHC results in Table 2; the staining intensity was graded as strongly positive (+++), moderately positive (++), slightly positive (+), and negative (−). As shown in this table, all the four SIBLING family members were present in the cells of all cartilage layers at all stages of growth, and the signals for these molecules in the matrix were more variable than those in the cells. These observations suggested that the cells in all layers of the cartilage produced these proteins at different levels, and the cells synthesizing greater amounts of an individual member may have secreted more of that molecule in the matrix.
Table 2.
Distribution of SIBLING proteins in the ECM and chondrocytes of the femoral head cartilage
| 4-Week-old rat |
8-Week-old rat |
16–Week-old rat |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| S | M | D | S | M | D | S | M | D | |
| Matrix/cell | M/C | M/C | M/C | M/C | M/C | M/C | M/C | M/C | M/C |
| DMP1-N | ++/++ | +/++ | −/+ | ++/++ | +/++ | −/+ | ++/+++ | ++/++ | +/++ |
| DMP1-C | +/+ | −/+ | −/+ | +/+ | −/+ | −/+ | +/+ | −/+ | −/+ |
| BSP | +/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/+ | ++/+ | +/+ |
| OPN | +/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/+ | ++/++ | +/++ |
| DSP | +/+ | −/+ | −/+ | +/+ | −/+ | −/+ | +/+ | −/+ | −/+++ |
We divided the femoral head cartilage from 4- to 16-week-old rats into three layers: the superficial layer (S), the middle layer (M), and the deep layer (D). M, signal in ECM; C, signal in chondrocytes. SIBLING, small integrin-binding ligand, N-linked glycoprotein; ECM, extracellular matrix; DMP1-N, NH2-terminal region of the DMP1 amino acid sequence; DMP1-C, COOH-terminal region of DMP1 amino acid sequence; ++, moderately positive; +, slightly positive; −, negative; +++, strongly positive.
In summary, the distribution of the four SIBLING members showed the following general tendencies: (1) at 4 weeks after birth, the signals for these SIBLING family members were observed in the ECM of superficial layer; (2) as the animals grow (8 and 16 weeks after birth), the signals for DMP1-N/DMP1-PG, BSP, and OPN in the ECM of the deeper layer became stronger; and (3) in addition to their presence in the ECM, these SIBLING members were also observed within cells.
Discussion
The protein-related macromolecules in articular cartilage are classified into the following categories: (1) collagens (mainly type II collagen), (2) proteoglycans, and (3) NCPs including the SIBLING family. Although numerous studies have been done regarding the SIBLING family members in mineralized tissue, there are few reports citing their presence in the non-mineralized, cartilaginous tissues (Attur et al. 2001; Feng et al. 2003; Gao et al. 2010; Sun et al. 2010a). Thus, the existence and distribution of the SIBLING family members in the articular cartilage are ill-defined. Articular cartilage is a common type of cartilaginous tissue in the body, and investigations on the SIBLING family members in this class of cartilage are needed for a better understanding of chondrogenesis. In this study, the presence and distribution of DMP1, BSP, OPN, and DSPP in the articular cartilage of the rat femoral head were systematically evaluated using protein chemistry and IHC approaches.
In bone and dentin, DMP1 is primarily present as the processed fragments and only a trace amount of the full-length form of DMP1 is found in these tissues (Huang et al. 2008a). In this study, protein chemistry analysis showed that the major form of DMP1 in the extract from the articular cartilage is DMP1-PG. IHC revealed that the signal detected by the anti-DMP1-N-9B6.3 antibody in the rat femoral head cartilage increased as these animals age. On the basis of findings from protein chemistry analyses, which showed strong and broad band matching the migration pattern of DMP1-PG and remarkably weaker band for the 37-kDa fragment or the full-length DMP1 (Figures 1B and 1C), we believe that the IHC signal detected by the anti-DMP1-N-9B6.3 antibody must be primarily from DMP1-PG (the proteoglycan form of the NH-terminal fragment of DMP1). The proteoglycans (e.g., aggrecan, decorin, and biglycan) are known to play important roles in chondrogenesis. The observations that the DMP1-PG is abundant in the extract of the femoral head cartilage along with its increase with aging suggest that this proteoglycan may play a role in chondrogenesis during the development of the cartilage. This belief is consistent with the findings from the Dmp1 knockout studies, which showed that mice devoid of Dmp1 gene have severe phenotypic changes in the growth plate of their long bones (Ye et al. 2005). Recently, an in vitro study showed that DMP1-PG dose dependently inhibited mineral accumulation (Gericke et al. 2010). An in vivo study showed that the level of DMP1-PG was remarkably elevated in the accumulated osteoid in the poorly mineralized long bones of hypophosphatemic mice (Zhang et al. 2010). Taken together, these findings indicate that DMP1-PG may function as an inhibitor of mineralization during the formation of cartilage and bone. Another interesting finding is that the ratio of full-length DMP1 to its processed fragments was greater in the cartilage than that seen in dentin or long bone, indicating that there were more full-length forms of DMP1 that were not cleaved in the cartilage.
BSP and OPN were mainly found in the cells and in the ECM of the fast-growing superficial layer at 4 weeks after birth. At later time points (8 and 16 weeks), the location of BSP and OPN expanded into the deeper layers of the femoral head cartilage. It is also worth noting that BSP and OPN appeared in the ECM of deep layer earlier (∼8 weeks) than the formation of secondary ossification center in this layer (Figure 4). The expansion of BSP and OPN from the superficial layer to the deeper layer may be related to the requirements for cartilage development or the formation of the secondary ossification center in the cartilage (Copray et al. 1988; Sasaguri et al. 1998; Hinton and Carlson 2005; Shen and Darendeliler 2005). Previous observations that OPN deficiency led to structural changes in cartilage (Matsui et al. 2009) and that the level of OPN was elevated in the cartilage of osteoarthritis patients (Gao et al. 2010) support our belief that OPN may play an important role in the formation of healthy cartilage.
This investigation is the first to report the expression of DSP/DSPP in articular cartilage. It appeared that the concentration of DSP in the femoral head cartilage was greater than that in the long bone (excluding the epiphysis/metaphysis regions) because 60 μl of sample from a fraction of the cartilage extract gave rise to a clear DSP band in Western immunoblotting analysis, whereas we had to concentrate the corresponding fraction of the long-bone extract from the same number of rats by 10 times for a clear detection of DSP (data not shown). Additionally, the migration rate of DSP extracted from the cartilage was faster than that from rat incisor dentin. The migration rate of DSP isolated from the cartilage was similar to rat dentin DSP sequentially treated with sialidase, O-glycosidase N-glycosidase F, indicating that DSP in the cartilage may be devoid of any carbohydrate substituents (Qin et al. 2003a). Similar to DMP1-PG, DSP also has a proteoglycan form referred to as DSP-PG (Zhu et al. 2010). In this investigation, we failed to detect DSP-PG in the protein extracts and this failure to detect DSP-PG may be due to the low expression level of DSPP in the cartilage.
In summary, protein chemistry and IHC analyses demonstrated the presence of the four SIBLING family members (DMP1, BSP, OPN, and DSPP) in the rat femoral head cartilage. The localization of these four SIBLING family members varied in the different zones of the cartilage and changed with age. The localization of these SIBLING family members and/or their fragments provides clues about the potential roles of these molecules in the development of the articular cartilage.
Acknowledgments
This work was supported by the National Institutes of Health Grant DE-005092 (to CQ) and by the Department of Science and Technology of Heilongjiang Province of China—Gongguan Project Grant GC09C412-1.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Attur MG, Dave MN, Stuchin S, Kowalski AJ, Steiner G, Abramson SB, Denhardt DT, et al. (2001) Osteopontin: an intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum 44:578–584 [DOI] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, Butler WT (2004a) Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci 112:163–170 [DOI] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, Wygant JN, Mcintyre BW, Butler WT (2004b) Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol 23:371–379 [DOI] [PubMed] [Google Scholar]
- Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW (1993) Osteopontin–hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin gel. Bone Miner 22:147–159 [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, Mckee MD (2002) Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int 71:145–154 [DOI] [PubMed] [Google Scholar]
- Copray JC, Dibbets JM, Kantomaa T (1988) The role of condylar cartilage in the development of the temporomandibular joint. Angle Orthod 58:369–380 [DOI] [PubMed] [Google Scholar]
- D'souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res 12:2040–2049 [DOI] [PubMed] [Google Scholar]
- Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, et al. (2003) The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res 82:776–780 [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, et al. (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1230–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Jain A, Tayback M, Fedarko NS (2004) Small integrin-binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res 10:8501–8511 [DOI] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS (2001) Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun 280:460–465 [DOI] [PubMed] [Google Scholar]
- Ganss B, Kim RH, Sodek J (1999) Bone sialoprotein. Crit Rev Oral Biol Med 10:79–98 [DOI] [PubMed] [Google Scholar]
- Gao SG, Li KH, Zeng KB, Tu M, Xu M, Lei GH (2010) Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthr Cartil 18:82–87 [DOI] [PubMed] [Google Scholar]
- George A, Sabsay B, Simonian PA, Veis A (1993) Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem 268:12624–12630 [PubMed] [Google Scholar]
- Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, et al. (2010) Different forms of DMP1 play distinct roles in mineralization. J Dent Res 89:355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RJ, Carlson DS (2005) Regulation of mandibular cartilage growth. Sem Orthod 11:209–218 [Google Scholar]
- Huang B, Maciejewska I, Sun Y, Peng T, Qin D, Lu Y, Bonewald L, et al. (2008a) Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int 82:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Sun Y, Maciejewska I, Qin D, Peng T, McIntyre B, Wygant J, et al. (2008b) Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur J Oral Sci 116:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Gu T, Luan X, Simmons D, Chen J (1998) Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res 13:422–431 [DOI] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem 272:835–842 [DOI] [PubMed] [Google Scholar]
- Maciejewska I, Qin D, Huang B, Sun Y, Mues G, Svoboda K, Bonewald L, et al. (2009) Distinct compartmentalization of dentin matrix protein 1 fragments in mineralized tissues and cells. Cells Tissues Organs 189:186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaval L, Monfoulet L, Fabre T, Pothuaud L, Bareille R, Miraux S, Thiaudiere E, et al. (2009) Absence of bone sialoprotein (BSP) impairs cortical defect repair in mouse long bone. Bone 45:853–861 [DOI] [PubMed] [Google Scholar]
- Matsui Y, Iwasaki N, Kon S, Takahashi D, Morimoto J, Matsui Y, Denhardt DT, et al. (2009) Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum 60:2362–2367 [DOI] [PubMed] [Google Scholar]
- Moses K, Butler WT, Qin C (2006) Immunohistochemical study of small integrin-binding ligand, N-linked glycoproteins in reactionary dentin of rat molars at different ages. Eur J Oral Sci 114:216–222 [DOI] [PubMed] [Google Scholar]
- Prasad M, Butler WT, Qin C (In Press) Dentin sialophosphoprotein in biomineralization. Connect Tissue Res. Published online April 2, 2010 (DOI: 10.3109/03008200903329789) [DOI] [PMC free article] [PubMed]
- Qin C, Baba O, Butler WT (2004) Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126–136 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Baba O, Wygant JN, Mcintyre BW, Butler WT (2003a) Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci 111:235–242 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H (2002) The expression of dentin sialoprotein gene in bone. J Dent Res 81:392–394 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT (2003b) Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem 278:34700–34708 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT (2001) A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci 109:133–141 [DOI] [PubMed] [Google Scholar]
- Qin C, D'Souza R, Feng JQ (2007) Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res 86:1134–1141 [DOI] [PubMed] [Google Scholar]
- Qin C, Huang B, Wygant JN, McIntyre BW, McDonald CH, Cook RG, Butler WT (2006) A chondroitin sulfate chain attached to the bone dentin matrix protein 1 NH2-terminal fragment. J Biol Chem 281:8034–8040 [DOI] [PubMed] [Google Scholar]
- Sasaguri K, Jiang H, Chen J (1998) The effect of altered functional forces on the expression of bone-matrix proteins in developing mouse mandibular condyle. Arch Oral Biol 43:83–92 [DOI] [PubMed] [Google Scholar]
- Shen G, Darendeliler MA (2005) The adaptive remodeling of femoral head cartilage—a transition from chondrogenesis to osteogenesis. J Dent Res 84:691–699 [DOI] [PubMed] [Google Scholar]
- Sodek J, Ganss B, Mckee MD (2000) Osteopontin. Crit Rev Oral Biol Med 11:279–303 [DOI] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, et al. (2003) Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem 278:24874–24880 [DOI] [PubMed] [Google Scholar]
- Sun Y, Gandhi V, Prasad M, Yu W, Wang X, Zhu Q, Feng JQ, et al. (2010a) Distribution of small integrin-binding ligand, N-linked glycoproteins (SIBLING) in the condylar cartilage of rat mandible. Int J Oral Maxillofac Surg 39:272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Lu Y, Chen S, Prasad M, Wang X, Zhu Q, Zhang J, et al. (2010b) Key proteolytic cleavage site and full-length form of DSPP. J Dent Res 89:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H (2004) Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab 22:430–438 [DOI] [PubMed] [Google Scholar]
- Verdelis K, Ling Y, Sreenath T, Haruyama N, MacDougall M, van der Meulen MC, Lukashova L, et al. (2008) DSPP effects on in vivo bone mineralization. Bone 43:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, Simmer JP (2005) Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem 280:1552–1560 [DOI] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, et al. (2004) Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem 279:19141–19148 [DOI] [PubMed] [Google Scholar]
- Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, Sivakumar P, et al. (2005) Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem 280:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Sun Y, Chen L, Guan C, Guo L, Qin C (2010) Expression and distribution of SIBLING proteins in the predentin/dentin and mandible of hyp mice. Oral Dis 16:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Sun Y, Prasad M, Wang X, Yamoah AK, Li Y, Feng J, et al. (2010) Glycosaminoglycan chain of dentin sialoprotein proteoglycan. J Dent Res 89:808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]