Abstract
Satellite cells are quiescent cells located under the basal lamina of skeletal muscle fibers that contribute to muscle growth, maintenance, repair, and regeneration. Mouse satellite cells have been shown to be muscle stem cells that are able to regenerate muscle fibers and self-renew. As human skeletal muscle is also able to regenerate following injury, we assume that the human satellite cell is, like its murine equivalent, a muscle stem cell. In this review, we compare human and mouse satellite cells and highlight their similarities and differences. We discuss gaps in our knowledge of human satellite cells, compared with that of mouse satellite cells, and suggest ways in which we may advance studies on human satellite cells, particularly by finding new markers and attempting to re-create the human satellite cell niche in vitro. (J Histochem Cytochem 58:941–955, 2010)
Keywords: stem cells, human satellite cells, mouse satellite cells, skeletal muscle regeneration
The satellite cell is defined by its location between the basal lamina and sarcolemma of skeletal muscle fibers. It was identified first in adult frog skeletal muscle (Mauro 1961) and was subsequently found in other vertebrates (Muir et al. 1965), including human (Laguens 1963; Shafiq et al. 1967).
Experiments in rodents demonstrated that satellite cells contribute to the growth and regeneration of skeletal muscle (Moss and Leblond 1971; Bischoff 1975; Cardasis and Cooper 1975a; Konigsberg et al. 1975; Schultz 1976; Snow 1978).
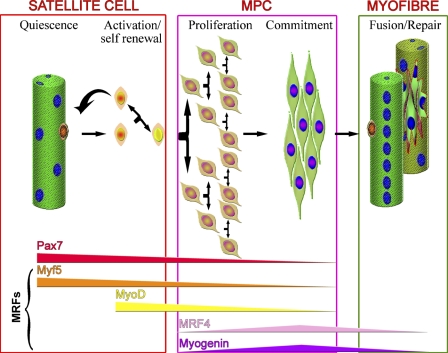
Normally quiescent in adult muscle, satellite cells become activated when muscle is injured and proliferate to generate a pool of muscle precursor cells (mpc) or myoblasts. These can then either repair damaged segments of fibers or fuse together to generate entirely new multinucleated muscle fibers. During the processes of proliferation and differentiation into myofibers, the satellite cell recapitulates the myogenic program that is not an exact recapitulation of muscle development, although the myogenic regulatory factors (MRFs) Myf5, MyoD, MRF4, and myogenin (Figure 1) [reviewed by Weintraub (1993)] are expressed in similar sequence in both processes.
Figure 1.
Model of satellite cell activation and progression through the myogenic program. Quiescent satellite cells, underneath the basal lamina of muscle fibers, express Pax7 and Myf5. Upon activation, they upregulate MyoD and divide to produce a pool of muscle precursor cells (mpc). Satellite cell progeny then follow one of two fates. They may downregulate MyoD and self-renew to give rise to a Pax7+ satellite cell. Alternatively, they may differentiate, downregulating Pax7, Myf5, and MyoD and expressing MRF4 and myogenin, eventually fusing either to form new or to repair damaged myofibers.
Early interest in satellite cells and their progeny mpc was due to their role in muscle repair and regeneration, which is particularly relevant to the treatment of inherited muscle diseases. Recently, satellite cells have emerged as a model of a postnatal stem cell because the availability of markers and genetically modified mice has allowed investigators to follow in vitro and in vivo the processes of activation, proliferation, differentiation, and self-renewal.
Challenges in Studying Satellite Cells
Despite being of such interest, quiescent satellite cells are very difficult to study. Their scarcity and location under the basal lamina of muscle fibers makes them difficult to isolate. Another major problem relates to the difficulty encountered in attempting to separate them from other cells present within skeletal muscle (e.g., fibroblasts, endothelial cells, interstitial cells, and blood vessel–associated cells), in order to obtain a pure population of satellite cells. Because of these factors, performing experiments that require large numbers of satellite cells can be challenging, even in rodents. These difficulties are significantly compounded when studying human muscle, not only because of the practical difficulties in obtaining muscle biopsies to prepare cells but also because of the lack of markers to distinguish satellite cells from myonuclei and other cells present within skeletal muscle. It is because of these reasons that most of the work done so far is focused on rodents, particularly mice.
Satellite cells may be studied either in frozen sections of skeletal muscle, or following their isolation from fresh muscle, by growing muscle explants from which muscle cells, including mpc, migrate onto the culture dish substrate (Harvey et al. 1979; Garrett and Anderson 1995; Conboy and Rando 2002; Conboy et al. 2003; Smith and Merrick 2010), or by mincing and enzymatically disaggregating skeletal muscle (Naffakh et al. 1993; Partridge 1997; Yablonka-Reuveni et al. 1999a; Conboy et al. 2003; Montarras et al. 2005). Both methods give rise to a mixture of cell types, but do not necessarily release all cells from the muscle. Flow cytometry has been used to purify both mouse and human satellite cells on the basis of expression of marker proteins (Baroffio et al. 1996; Conboy et al. 2003,2010; Sherwood et al. 2004; Montarras et al. 2005; Fukada et al. 2007; Pallafacchina et al. 2010), but this method does not give rise to completely pure populations of cells. In addition, enzymatic treatment removes cells from their niche and may strip or alter cell surface markers so that satellite cells prepared in this way may not completely maintain their in vivo phenotype.
An elegant method to prepare rodent satellite cells in their niche on the fiber was developed by Bischoff (1986) and used in many in vitro and in vivo studies (Rosenblatt et al. 1995; Shefer et al. 2004,2006; Zammit et al. 2004; Collins et al. 2005; Yablonka-Reuveni et al. 2008; Boldrin et al. 2009). Isolated viable muscle fibers bearing their satellite cells under the basal lamina may be either fixed immediately or placed in suspension culture so that the activation, proliferation, differentiation, and self-renewal of satellite cells in their niche can be followed (Beauchamp et al. 2000; Zammit et al. 2004; Collins et al. 2007,2009; Day et al. 2007; Gnocchi et al. 2009). Fibers may be cultured on a substrate, usually Matrigel (a commercially available mixture of basement membrane components and growth factors), or other substrates, such as collagen (Shefer et al. 2004); under these conditions, satellite cells migrate from the fiber, proliferate, and differentiate into myotubes (Rosenblatt et al. 1995; Blaveri et al. 1999; De Coppi et al. 2006; Yablonka-Reuveni and Anderson 2006; Boldrin et al. 2007; Malerba et al. 2009). Alternatively, single muscle fibers may be carefully washed to eliminate any extraneous cells, and satellite cells removed from them by either physical (Shefer et al. 2004; Collins et al. 2005; Boldrin et al. 2009) or enzymatic (Ono et al. 2009) methods.
Using this single fiber protocol, it is possible to obtain pure populations of satellite cells, albeit in relatively small number. However, a major limitation of this method is that it requires entire freshly dissected muscles to allow the isolated fibers to remain intact and is therefore not easily applicable to human muscle biopsies. Nevertheless, protocols using particularly short muscles (Bonavaud et al. 2002; De Coppi et al. 2006) or fiber fragments (Cardasis and Cooper 1975b) may be suitable for human muscle preparations.
Studies on quiescent satellite cells need to be performed as soon as possible after fiber or cell preparation as they become activated extremely rapidly (Wozniak et al. 2003; Zammit et al. 2004). On activation, satellite cells promptly enter the myogenic program (Cornelison and Wold 1997; Yablonka-Reuveni et al. 2008) and may not therefore retain the capabilities of quiescent satellite cells.
Identification of Satellite Cells
In early studies, satellite cells were identified by electron microscopy on the basis of their position between the basal lamina and sarcolemma of muscle fibers (Cardasis and Cooper 1975a), but this method is technically demanding and not suitable to study large portions of muscle. There is now a panel of reliable markers of satellite cells in mouse, but not in human. However, it must be borne in mind that some are expressed on activated and quiescent satellite cells, whereas others are also expressed on other cell types (Table 1).
Table 1.
Markers of satellite cells
| Satellite cell marker | Majority of satellite cells | Subset of satellite cells | Quiescent satellite cells | Activated satellite cells | Detection | Mouse | Human | Marker localization | Transgenic mouse | Expressed by other cells | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pax7 | ✓ | − | ✓ | ✓ | Ab | ✓ | ✓ | Nucleus | Pax7-Zs Green mouse | Neurons | Seale et al. 2000; Bosnakovski et al. 2008 |
| Pax3 | − | ✓ | ✓ | − | TM | ✓ | ? | Nucleus | Pax3GFP/+ mouse | Neural crest lineages | Relaix et al. 2006 |
| Myf5 | ✓ | − | ✓ | ✓ | KI, Ab, mRNA | ✓ | ? | Nucleus | Myf5nlacZ/+ mouse | Neurons, muscle spindle, denervated fibers | Tajbakhsh and Buckingham 1995; Kitzmann et al. 1998; Beauchamp et al. 2000; Friday and Pavlath 2001; Day et al. 2007,2010 |
| MyoD | − | − | − | ✓ | Ab | ✓ | ? | Nucleus | − | Myogenic cells | Yablonka-Reuveni et al. 1999a; Zammit et al. 2004 |
| MNF | − | ✓ | ✓ | ✓ | Aba | ✓ | ? | Nucleus | − | Myonuclei in regenerating fibers, cardiac and skeletal myocytes, brain, and kidney | Garry et al. 1997 |
| c-met | − | ✓ | ✓ | ✓ | ISH | ✓ | ? | Cell membrane | − | Pericytes, neural crest lineage, and other tissues | Cornelison and Wold 1997; Wozniak et al. 2003 |
| Syndecan-3 | ✓ | ✓ | ✓ | ✓ | Aba | ✓ | ? | Cell membrane | − | Macrophages, leukocytes, uterine tissue, ovarian cancer, chondrocytes | Cornelison et al. 2001 |
| Syndecan-4 | ✓ | ✓ | ✓ | ✓ | Aba | ✓ | ? | Cell membrane | − | Macrophages, leukocytes, mammary cells, breast cancer | Cornelison et al. 2001; Tanaka et al. 2009 |
| CD34 | ✓ | − | ✓ | ✓ | Ab | ✓ | ? | Cell membrane | − | Hematopoietic stem and progenitor cells, small-vessel endothelium | Beauchamp et al. 2000 |
| M-cadherin | ✓ | − | ✓ | ✓ | Aba | ✓ | ✓ | Cell membrane | − | Granular cells of the cerebellum and brain | Irintchev et al. 1994; Cornelison and Wold 1997; Beauchamp et al. 2000; Reimann et al. 2004 |
| CD56 | ✓ | ? | ✓ | ✓ | Ab | NA | ✓ | Cell membrane | − | Human peripheral lymphocytes, NK cells, dendritic cells, neurons, glia | Schubert et al. 1989 |
| α7-Integrin | ✓ | − | ✓ | ✓ | Ab | ✓ | ? | Cell membrane | − | Fibers, neurons, vasculature and nervous system | Blanco-Bose et al. 2001; Gnocchi et al. 2009; Rooney et al. 2009 |
| β1-Integrin | ✓ | − | ✓ | ✓ | Ab | ✓ | ? | Cell membrane | − | Widely expressed, isoform β-1D expressed specifically in striated muscle | Sherwood et al. 2004; Cerletti et al. 2008 |
| Caveolin-1 | ✓ | − | ✓ | ✓ | Ab | ✓ | ? | Cell membrane | − | Prostate cancer, adipocytes, neurons, fibroblasts, smooth muscle cells | Volonte et al. 2005; Gnocchi et al. 2009 |
| Calcitonin receptor | ✓ | − | ✓ | ✓ | Ab | ✓ | ? | Cell membrane | − | Kidney, central and peripheral nervous systems, osteoclasts | Gnocchi et al. 2009 |
| Jagged-1 | − | ✓ | − | ✓ | Ab | ✓ | ? | Cell membrane | − | Widely expressed in many tissues, mainly in brain, heart, muscle, and thymus | Gnocchi et al. 2009 |
| Nestin | ✓ | − | ✓ | ✓ | mRNA TM | ✓ | ? | Cell membrane | NES-GFP | Neural progenitors | Shefer et al. 2004; Day et al. 2007 |
| Desmin | ? | ? | ✓ | ✓ | mRNA Ab | ✓ | ? | Cytoplasm | − | Expressed in skeletal, smooth, and cardiac muscle | Yablonka-Reuveni et al. 1999a; Day et al. 2007 |
| Fzd7 | − | ✓ | ✓ | ✓ | Ab | ✓ | ? | Cell membrane | − | Highly expressed in adult skeletal muscle and fetal kidney, fetal lung, adult heart, brain, and placenta. Specifically expressed in squamous cell esophageal carcinomas | Le Grand et al. 2009 |
| Vangl2 | − | ✓ | − | ✓ | Ab | ✓ | ? | Cell membrane | − | Widely expressed, mainly in thymus, brain, spinal cord, heart, lung, prostate | Le Grand et al. 2009 |
| SM/C-2.6 | ✓ | ? | ✓ | ? | Aba | ✓ | ? | Cell membrane | − | ? | Fukada et al. 2004 |
| ABCG2 | − | ✓ | ✓ | ? | Ab | ✓ | ? | Cell membrane | − | Highly expressed in placenta. Low expression in small intestine, liver, and colon | Tanaka et al. 2009 |
| CXCR4 | ✓ | − | ✓ | ? | Ab | ✓ | ? | Cell membrane | − | Expressed in numerous tissues | Sherwood et al. 2004; Cerletti et al. 2008 |
| Robo1 | ? | ? | ? | ✓ | Ab | ✓ | ? | Cell membrane | − | Widely expressed, with exception of kidney | Siegel et al. 2009 |
| Nap1l1 | ? | ? | − | ✓ | Aba | ✓ | ? | Nucleus | − | Ubiquitously expressed | Pallafacchina et al. 2010 |
| Doublecortin | ? | ? | − | ✓ | Aba | ✓ | ? | Cytoplasm | − | Neurons | Pallafacchina et al. 2010 |
| Adam19 | ? | ? | − | ✓ | Aba | ✓ | ? | Cell membrane | − | Widely expressed | Pallafacchina et al. 2010 |
| Sca-1 | − | ✓ | ✓ | ✓ | Ab | ✓ | ? | Cell membrane | − | Hematopoietic stem cells | Mitchell et al. 2005; Kirillova et al. 2007; Tanaka et al. 2009 |
| CD56 | ? | ? | ✓ | ? | Ab | − | ✓ | Cell membrane | − | Myoblast, myotubes, lymphocyte, NK cells, neuronal derived tissues, lung cancer, and other neoplasm | Schubert et al. 1989 |
Non-commercially available.
Ab, antibody; TM, transgenic mouse; KI, knockin mouse; NK, natural killer.
The majority of mouse satellite cells can be defined by their expression of Pax7, CD34, caveolin, calcitonin receptor, β1-integrin, M-cadherin, α7-integrin, and nestin (Table 1), but only for the first five of these markers are there commercially available antibodies. Furthermore, it should be noted that nestin expression in quiescent satellite cells has been revealed only by means of green fluorescent protein (GFP) positivity in the nestin-GFP mouse.
In the Myf5nLacZ/+ mouse, which has nuclear-localizing β-galactosidase targeted to the Myf5 locus, quiescent and activated satellite cells are β-galactosidase positive. However, Myf5 protein expression has not been described in quiescent satellite cells, even though Myf5 transcripts can be detected in sorted mouse satellite cells (Day et al. 2007) (Table 1). Possible explanations may be either the protein level is too low to be detected, or instability of the protein, or simply the lack of a clear signal from the antibody used.
Because of the limitations highlighted previously, it is at present unclear whether all of these markers recognize satellite cells in human muscle (Table 1).
Satellite Cell Heterogeneity
There is clear evidence from mouse studies that satellite cells, both within the same muscle and even on the same fiber, are different in terms of their marker expression (Beauchamp et al. 2000; Montarras et al. 2005; Kuang et al. 2007) and/or function (Collins et al. 2005,2007; Kuang et al. 2007; Sacco et al. 2008; Boldrin et al. 2009). It is also clear that numbers of satellite cells per fiber (Collins et al. 2005; Shefer et al. 2006; Zammit 2008; Ono et al. 2009) and capacity of satellite cells to differentiate in vitro, or contribute to muscle regeneration in vivo (Pavlath et al. 1998; Collins et al. 2005; Montarras et al. 2005; Ono et al. 2009), differ depending on which muscle is used for their isolation. These observations all derive from mouse muscle, and therefore, we do not know whether the human satellite cell pool is, as in the mouse, heterogeneous.
Quantification of Mouse Satellite Cells
Early ultrastructural studies of mouse muscles suggest that 30–35% of fiber nuclei are satellite cells at birth, declining to 5–7% in adults (Allbrook et al. 1971; Cardasis and Cooper 1975a; Schultz 1976). Subsequent studies using either electron microscopy or M-cadherin staining to identify satellite cells in adult mouse soleus muscles give similar numbers of satellite cells (4.6% and 3.4% of nuclei, respectively; Snow 1977; Reimann et al. 2004). Many later studies have relied on counting the number of satellite cells per fiber, based on expression of different marker proteins (Yablonka-Reuveni and Rivera 1994; Beauchamp et al. 2000; Zammit et al. 2004). Nevertheless, even using the same marker, there are differences in the estimated numbers of satellite cells per fiber between laboratories (Collins et al. 2005; Shefer et al. 2006) or even between experiments performed at different times in the same laboratory (Collins et al. 2005,2007). These discrepancies may be due to age, sex, or strain of mouse.
Quantification of Human Satellite Cells
Comparison of ultrastructural data suggests that there are similar percentages of satellite cells in adult mouse and human muscles—4% ± 2% of all nuclei within the fiber basal lamina of human muscles (Schmalbruch and Hellhammer 1976), which is similar to the 5–7% for mouse satellite cells. More direct comparison is however difficult, as in the mouse, satellite cell number depends on the muscle in which they reside (Collins et al. 2005; Zammit 2008), whereas we lack details in the human.
Lack of specific satellite cell markers in the human has led to equivocal and sometimes contradictory reports on their presence and number in human muscle sections. The first antibodies used to identify satellite cells in sections of human skeletal muscle were Leu19 and NKH-1, which recognize CD56, or neural cell adhesion molecule (NCAM; Schubert et al. 1989; Illa et al. 1992; Belles-Isles et al. 1993). NCAM is expressed by quiescent human (Fidzianska and Kaminska 1995) and rat (Irintchev et al. 1994), but not mouse, satellite cells; mouse satellite cells only express NCAM when they become committed to differentiation (Capkovic et al. 2008). Despite NCAM expression not being satellite cell specific (Cashman et al. 1987; Schubert et al. 1989; Mechtersheimer et al. 1992) (Table 1), it has been extensively used for identification of satellite cells on sections of human muscle (Illa et al. 1992; Charifi et al. 2003; Kadi et al. 2006; Doppler et al. 2008; Mackey et al. 2009).
M-cadherin, a reliable marker for mouse satellitecells (Irintchev et al. 1994; Beauchamp et al. 2000), has also been used to identify human satellite cells (Sajko et al. 2004), but this particular antibody is not commercially available and has therefore not been widely used.
Although Pax7 is a reliable mouse satellite cell marker (Seale et al. 2000), in human muscle it appears not to identify all satellite cells; in addition, it may also stain myonuclei (Reimann et al. 2004). In an attempt to distinguish Pax7+ satellite cells from myonuclei, some authors combined Pax7 and NCAM antibodies for satellite cell quantification (Verdijk et al. 2007) or counted a satellite cell as being a NCAM- and/or Pax7-positive cell in a sublaminar position (identified by laminin immunostaining) (Lindstrom and Thornell 2009). The latter study showed that the majority of human satellite cells expressed both Pax7 and NCAM, but there were also small numbers of NCAM+/Pax7− and NCAM−/Pax7+ satellite cells, which may have been either activated or differentiating (Lindstrom and Thornell 2009).
To further complicate comparison between studies, the following parameters have been used by different authors for normalization of the satellite cell count: number of myonuclei (Crameri et al. 2004; Eriksson et al. 2005; Sinha-Hikim et al. 2006), length of muscle fiber (Sinha-Hikim et al. 2003; Sajko et al. 2004), and fiber cross-sectional area (Charifi et al. 2003; Dreyer et al. 2006; Kadi et al. 2006; Verdijk et al. 2007; Lindstrom and Thornell 2009). The correlation with the cross-sectional fiber area allows processes like muscle growth, pathological events, and ageing to be taken into account (Sajko et al. 2004), but it has been suggested that ideally both satellite cells per fiber and satellite cells per number of myonuclei should be used together for a more accurate measurement (Lindstrom and Thornell 2009).
In conclusion, it is still not clear if different antibodies detect all satellite cells and if satellite cell number determined by antibody staining and electron microscopy concur. In addition, it remains to be determined if differences between immunostaining of human and mouse satellite cells are an indication of species-specific differences, or merely reflect that the antibody itself is species specific. There is therefore a pressing need to standardize the identification and quantification of satellite cells in transverse sections of skeletal muscle so that comparisons of satellite cell numbers in different muscles, or the same muscles in individuals of different age and sex or individuals suffering from different pathological conditions, can be made.
Control of Satellite Cell Activation and Proliferation
Nearly all the work done on the processes of quiescence, activation, and self-renewal has been done on mouse, not human, satellite cells [reviewed by Dhawan and Rando (2005), Collins (2006), and Zammit (2008)]. We therefore summarize briefly findings on rodent satellite cells, but whether the same mechanisms apply to the human remains to be demonstrated.
Satellite cells are awakened from quiescence in response to normal physiological stimuli, e.g., exercise (Darr and Schultz 1987), and mechanical stretch (Tatsumi et al. 2002; Wozniak et al. 2003) and by pathological degeneration of muscle fibers, as seen for example in muscular dystrophy. These events lead to the activation of specific signaling pathways. Stretch-induced satellite cell activation is mediated by nitric oxide (NO) (Wozniak et al. 2003) that activates hepatocyte growth factor (HGF) (Tatsumi et al. 1998,2002; Anderson and Pilipowicz 2002). Notch receptors on the satellite cell membrane play a crucial role in regulating self-renewal of satellite cells (Conboy et al. 2003; Kuang et al. 2007), and the wnt pathway is involved in both activation and self-renewal (Fuchs et al. 2004; Brack and Rando 2007; Le Grand et al. 2009) of mouse satellite cells. Sphingosine 1 phosphate induces mouse satellite cells to enter the cell cycle (Nagata et al. 2006). Growth factors such as fibroblast growth factor (FGF) (DiMario and Strohman 1988; DiMario et al. 1989) and insulin-like growth factor (IGF)-1 (Hill and Goldspink 2003) play a part in satellite cell proliferation and muscle regeneration (Charge and Rudnicki 2004). Other signals that are involved in controlling satellite cell function include stromal cell–derived factor (SDF)-1 that binds to CXCR4 and CXCR7 receptors on myogenic cells (Melchionna et al. 2010) and M-cadherin (Irintchev et al. 1994) that is involved in both satellite cell quiesence (Irintchev et al. 1994) and fusion into muscle fibers (Charrasse et al. 2007). Other cells within skeletal muscle may influence satellite cells, e.g., smooth muscle cells and fibroblasts secrete angiopoietin 1 that is involved in satellite cell quiescence and self-renewal (Abou-Khalil et al. 2009). However, the control of satellite cell quiescence, activation, proliferation, differentiation, and self-renewal within adult skeletal muscle in vivo has yet to be fully elucidated.
Satellite Cell Contribution to Skeletal Muscle Regeneration
Evidence that there are some satellite cells that fulfill the definition of a stem cell—a cell that is able to give rise to differentiated progeny and to self-renew (Ramalho-Santos and Willenbring 2007)—came from work in which quiescent satellite cells prepared from genetically modified mice were grafted into dystrophic mouse muscles and contributed both to regenerated muscle fibers and to functional satellite cells (Collins et al. 2005).
In mouse, the regenerative capacity of satellite cells does not appear to depend on the niche, as quiescent satellite cells removed from their fiber retain their ability to regenerate skeletal muscle and functionally reconstitute the satellite cell pool when grafted into dystrophin-deficient host mice (Collins et al. 2005; Boldrin et al. 2009; Ono et al. 2009). Similar work on human satellite cells has not been performed because of the difficulties in obtaining sufficient human satellite cells for grafting and particularly in determining if the donor cells had given rise to satellite cells, which requires the availability of a reliable human-specific satellite cell antibody.
However, as it is impractical to obtain satellite cells directly from donor muscle for treatment of patients, expansion in vitro would be necessary. A major limitation of the use of mpc to treat muscular dystrophies is that they lose their regenerative capacity following tissue culture. In vitro expansion of donor mouse (Montarras et al. 2005) and chicken satellite cells (O'Neill and Stockdale 1972) for only a short time significantly reduces the number of muscle fibers they form in vivo, probably because they commence myogenic differentiation. Similar to mouse, the regenerative capacity of human mpc is reduced after they have been expanded in vitro (Cooper et al. 2003; Brimah et al. 2004), which may be as a result of senescence during the culture period (Decary et al. 1996). This suggests that expansion of both mouse and human mpc in vitro may cause stem-like properties to be outweighed and therefore lost. Stem cell potential may also be affected by tissue culture conditions, e.g., signals from the substrate, medium components, growth factors, the cells themselves, or prior events in the life history of that particular population of cells. Whether there are ways to maintain the stem cell subpopulation in vitro, e.g., by using low-oxygen conditions as in the case of pluripotent stem cells (Millman et al. 2009), or using growth factors and substrates to re-create the niche (Cosgrove et al. 2009), remains to be investigated.
Nevertheless, there are some mouse mpc that retain their ability to regenerate skeletal muscle following a limited period of tissue culture. It is known that only a minority of cultured mouse mpc survive in vivo following intramuscular grafting, and these were the cells that were non-proliferating in vitro (Beauchamp et al. 1999). Within irradiated (but not in non-irradiated) dystrophic mdx hosts, the surviving donor cells proliferated, but nevertheless their contribution to regenerated muscle fibers was inefficient (Beauchamp et al. 1999) compared with freshly isolated satellite cells (Collins et al. 2005). For technical reasons, it has not been possible to follow the kinetics of human mpc grafted into mouse muscles, but similar to mouse mpc (Morgan et al. 1989,1990,1993; Watt et al. 1991; Gross and Morgan 1999), human mpc contribute to regenerated muscle fibers in immunodeficient mouse hosts (Huard et al. 1994; Brimah et al. 2004; Silva-Barbosa et al. 2005; Ehrhardt et al. 2007). However, human mpc repopulate host mouse muscle even less effectively than mouse mpc; fewer fibers of donor origin are found when the same number of human (Brimah et al. 2004) and mouse mpc (Morgan et al. 2002; Cousins et al. 2004) are grafted. This suggests that human myoblasts do not regenerate as effectively as mouse myoblasts, unless the difference in efficiency is related to the xenografts. Furthermore, neither mouse (El Fahime et al. 2000) nor human (Skuk et al. 2006) mpc are able to migrate far from the injection site, which is another major limitation of their use for therapeutic applications.
Interestingly, the pretreatment of host muscle (Brimah et al. 2004) and the host mouse strain (Cooper et al. 2001; Morgan et al. 2002; Silva-Barbosa et al. 2005) affects the number of donor-derived muscle fibers formed, and this effect seems to differ for human and mouse mpc. For example, human mpc contribute to more fibers of donor origin in cryoinjured rather than irradiated host muscles (Brimah et al. 2004), whereas the opposite is true for mouse satellite cells (Boldrin et al., unpublished data). Mouse mpc contribute to significantly more muscle fibers of donor origin in mdx nu/nu than in C5-/Rag2-/ γ chain-host mice (Morgan et al. 2002). This implies that mouse and human mpc respond differently to an in vivo environment; human mpc may not undergo an expansion phase within the host mouse muscle and therefore neither regenerate skeletal muscle nor reconstitute the satellite cell niche efficiently (Ehrhardt et al. 2007).
The environmental factors that modulate donor mouse or human myoblast or satellite cell–derived muscle regeneration and self-renewal have yet to be fully determined. Certainly, other cells present within skeletal muscle, e.g., macrophages (Gordon 1995; Tidball 1995; Chazaud et al. 2003; Malerba et al. 2009), microvascular components (Rhoads et al. 2009), nerves (Tatsumi et al. 2009), smooth muscle cells, fibroblasts (Abou-Khalil et al. 2009), and the fiber itself, together with growth factors, gases (e.g., NO) (Anderson and Pilipowicz 2002; Tatsumi et al. 2002), or connective tissue components produced by them (Silva-Barbosa et al. 2008), as well as systemic factors (Conboy et al. 2005; Brack et al. 2007; Brack and Rando 2007), influence the capacity of satellite cells to survive, proliferate, migrate, regenerate muscle fibers, and self-renew.
There is evidence that growth factors such as IGF-1 (Mourkioti and Rosenthal 2005), leukemia inhibitory factor (Kurek et al. 1998), HGF (Miller et al. 2000), and FGF promote endogenous regeneration (Kurek et al. 1998; Yablonka-Reuveni et al. 1999b; Miller et al. 2000) or donor human myoblast–derived regeneration in the mouse (Brimah et al. 2004). However, not all growth factor isoforms have the same effect—for example, in some reports, IGF-6 has no effect on (Fiore et al. 2000) or even impairs (Floss et al. 1997) muscle regeneration; similarly, different FGF isoforms may achieve different effects (Neuhaus et al. 2003). Even the concentration of a particular growth factor and its interaction with other factors, e.g. HGF and myostatin, may be crucial for its effect on satellite cells (Yamada et al. 2010).
Function of Aged Satellite Cells
Satellite cells are lost with age in both mouse and man (Renault et al. 2002; Kadi et al. 2004; Sajko et al. 2004; Brack et al. 2005; Shefer et al. 2006; Collins et al. 2007; Verdijk et al. 2007). Although satellite cells from aged mouse muscles have a reduced capacity to self-renew (Day et al. 2010), there is a satellite cell fraction that retains muscle stem cell characteristics in aged mouse muscle and, if grafted into a young muscle environment, is still capable of muscle regeneration and self-renewal to the same extent as young satellite cells (Collins et al. 2007). However, the equivalent experiments on human satellite cells have not been performed.
Skeletal muscle mass is lost, and there is a decline in the ability of muscle to regenerate with increasing age in both mouse and humans (Alnaqeeb and Goldspink 1987; Cartee 1995; Grounds 1998; Bross et al. 1999). Whether this is due to defects in the environment (either local or systemic), or in muscle satellite cells, or both has been the subject of much recent debate.
In the mouse, satellite cells from aged muscle show impaired activation (Betters et al. 2008; Leiter and Anderson 2010) and increased apoptosis (Collins et al. 2007); similarly, aged human satellite cells are less capable of activation in vitro compared with young satellite cells (Renault et al. 2002). It has been suggested that accumulation of lipofuscin with age on human myofibers and satellite cells could result in a delay in satellite cell activation (Renault et al. 2002; Nakae et al. 2004; Leiter and Anderson 2010). The different expression of muscle actin isoforms in cultures of human mpc from individuals of different ages supports the view that old human satellite cells indeed differ from young human satellite cells (Lancioni et al. 2007).
The aged muscle environment impedes muscle regeneration (Conboy et al. 2005; Solomon and Bouloux 2006; Brack and Rando 2007; Carlson et al. 2009), possibly because of systemic or local levels of wnt or TGF-β1 (Carlson et al. 2009), but it may be modified by preirradiation to allow efficient donor-derived satellite cell regeneration and self-renewal (Boldrin et al. 2009).
Satellite Cell Response to Exercise and Contribution to Skeletal Muscle Hypertrophy
In response to exercise, satellite cells become activated and increase in number (Armand et al. 2003; Parise et al. 2008). Human satellite cells of both young and old individuals respond similarly to exercise, increasing in number and activation status (Crameri et al. 2004; Kadi et al. 2005; Mackey et al. 2009; Verdijk et al. 2009) and contributing to muscle hypertrophy (Kadi et al. 1999; Kadi and Thornell 2000). Interestingly, resistance exercise seems to have a different hypertrophic effect on men and women (Kosek et al. 2006), with satellite cell number only increasing in young men (Petrella et al. 2006).
In the mouse and rat, it was demonstrated that satellite cells are required for hypertrophy of overloaded skeletal muscles (Rosenblatt and Parry 1992; Rosenblatt et al. 1994; Snijders et al. 2009). Growth factors that cause muscle hypertrophy include particular isoforms of IGF-1 (Barton et al. 2002,2010; Goldspink 2003), but only when muscle is growing or regenerating (Shavlakadze et al. 2010).
Muscle atrophy is mediated by interacting signaling pathways (Glass 2003,2005), including FoxO (Southgate et al. 2007) and nuclear factor kB (Li et al. 2009). Myostatin induces muscle atrophy (Lee and McPherron 2001), and its inhibition results in muscle hypertrophy (McPherron and Lee 1997; Lee and McPherron 2001; Bogdanovich et al. 2002). However, satellite cells do not seem to be involved in muscle hypertrophy mediated by this pathway (Amthor et al. 2009). In rodent models of unloading-induced muscle atrophy, satellite cells initially become activated (Ferreira et al. 2006), but eventually decrease in number (Schultz et al. 1994; Mozdziak et al. 2000; Hawke and Garry 2001; Jejurikar et al. 2002; Jejurikar and Kuzon 2003), and those that remain are dysfunctional (Mitchell and Pavlath 2004). These studies have implications not only for repair and maintenance of skeletal muscle during periods of immobilization but also for using satellite cells to repair muscle that is already affected by disuse atrophy as, for example, in muscular dystrophies.
Satellite Cells in Pathological Conditions
Satellite cell dysfunction has been implicated in the muscular dystrophies, and in cases where there is a mouse model of the human dystrophy, comparisons may be made between satellite cells in mouse and human muscles with the same genetic defect. In Duchenne muscular dystrophy (DMD), lack of functional dystrophin leads to sarcolemma fragility and to continuous cycles of muscle degeneration and regeneration, resulting in regeneration failure, loss of muscle mass and function, and progressive substitution of muscle tissue with fibrotic and adipose tissue (Rando 2001; Emery 2002). In contrast, skeletal muscle regenerates at first effectively in the dystrophin-deficient mouse model of DMD, the mdx mouse (Coulton et al. 1988a,b; Stedman et al. 1991), although older mdx mice show a degree of defective regeneration (Zacharias and Anderson 1991) and their muscle eventually deteriorates with age (Pastoret and Sebille 1995). It is not therefore clear whether there is really a difference in dystrophic mouse/human satellite cell regenerative capacity, and if so, whether it is caused by differences in the satellite cells themselves, or the local muscle (e.g., extent of fibrosis), or systemic environment, or even by the genetic background of the mouse model (Fukada et al. 2010).
Whether satellite cell numbers are altered in dystrophic muscle is difficult to determine, as accurate satellite cell quantification in dystrophic muscle is complicated by substitution of muscle fibers by fibrotic and adipose tissue (Desguerre et al. 2009). In addition, “branching” of regenerated myofibers (Bradley 1979; Blaveri et al. 1999) makes quantification of satellite cell numbers per fiber difficult. Nevertheless, based on observations on skeletal muscle sections, it has been reported that satellite cell number is greater in muscles of patients with DMD (Wakayama et al. 1979; Ishimoto et al. 1983; Watkins and Cullen 1988; Maier and Bornemann 1999) and neurogenic atrophy, but not in other muscular dystrophies (Becker muscular dystrophy, limb-girdle dystrophy) or inflammatory myopathies (Maier and Bornemann 1999). Numbers of M-cadherin+ satellite cells (calculated as the ratio of M-cadherin+ cells/total cell nuclei) were greater in mdx than in C57Bl/10 mouse muscles (Yamane et al. 2005), but their activation state was not determined. There is evidence that satellite cells in muscles of DMD patients may be in a more activated state (Wakayama et al. 1979; Watkins and Cullen 1988; Maier and Bornemann 1999), as they seem to be in mdx muscles (Bhagavati et al. 1996; Ikemoto et al. 2007) and be detrimentally influenced by the pathological environment (Watkins and Cullen 1986). This state of constant activation and contribution to the repair of necrotic muscle fibers may deplete the stem cells from the satellite cell pool (Heslop et al. 2000).
Although human DMD mpc have reduced proliferative capacity (Blau et al. 1983; Melone et al. 1999), they can differentiate in vitro into myotubes and normal mpc (Blau et al. 1983), but only when contaminating fibroblasts have been removed from the preparation (Delaporte et al. 1984). The mpc from mdx EDL muscles have been reported to differentiate to the same extent as mpc from age-matched control animals, but mpc from some mdx mice displayed poor differentiation (Schuierer et al. 2005). Although these mice are of the same genetic background, there may be a difference in the muscle pathology between both muscles in the same mouse and the same muscle of different mice. However, the kinetics of differentiation of mdx mpc seems to be accelerated—normal mouse mpc proliferated faster and differentiated earlier than mdx mpc (Cheng et al. 1996), in accordance with work showing that mpc derived from mdx flexor digitorum brevis and diaphragm muscles had accelerated differentiation (Yablonka-Reuveni and Anderson 2006). However, there is no evidence that satellite cell–derived mpc fail to proliferate at later stages of the mdx pathological process (Bockhold et al. 1998). Different dystrophies may affect satellite cells in different ways, as suggested in mpc derived from biopsies of patients with myotonic dystrophy type II, which proliferate, but fail to differentiate (Pelletier et al. 2009).
Finally, in vitro experiments have suggested that satellite cells may actually be contributing to muscle pathology by transdifferentiating into adipocytes or fibroblasts (Asakura et al. 2001; Brack et al. 2007). However, the satellite cell population may be contaminated with other cell types, and recent in vivo experiments have provided evidence that adipocytes and fibroblasts in skeletal muscle derive from interstitial cell progenitors rather than from satellite cells (Joe et al. 2010; Uezumi et al. 2010). There is therefore a need to study human satellite cell number, activation, and differentiative and self-renewal ability in muscles of patients with muscular dystrophies and in particular to address the contribution of satellite cells to fat and fibrotic tissue in different pathological conditions. These studies would be invaluable for our understanding of why skeletal muscle fibers are lost in muscular dystrophies.
Conclusions
Although human and mouse satellite cells express similar markers, it has been difficult to determine whether their phenotype and functions are equivalent. This is mostly due to the difficulty in isolating quiescent human satellite cells and the lack of specific antibodies for their unequivocal identification. In particular, it is not clear whether human satellite cells are indeed effective muscle stem cells. Both mouse and human skeletal muscles are capable of regeneration, but whether human satellite cells can self-renew following injury to give rise to functional satellite cells has not been demonstrated. In order to progress work on human satellite cells, one first needs to ascertain whether newly discovered markers of mouse satellite cells (Fukada et al. 2007; Gnocchi et al. 2009; Pallafacchina et al. 2010) are expressed by human satellite cells. Then, if there was a method to induce cultured mpc to give rise to quiescent satellite cells (e.g., by re-creating the niche in vivo), these markers could be verified and used to either subfractionate human satellite cells or study their activation and differentiative potential in vitro.
Isolation of human satellite cells and investigation of their in vitro and in vivo properties, similar to the extensive work that has been performed on mouse satellite cells, would pave the way to either using donor satellite cells as a therapy for muscular dystrophies or enhancing the function of endogenous satellite cells in dystrophic or aged muscles.
Acknowledgments
We thank Dr. Francesco Conti for his critical reading of the manuscript.
This article is a JHC article of the month. This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication with the exception of the JHC articles of the month which are immediately released for public access.
L.B. is funded by the Muscular Dystrophy Campaign and J.E.M. holds a Wellcome Trust University award. J.E.M. and F.M. are principal investigators of the Medical Research Council Centre for Neuromuscular Diseases.
References
- Abou-Khalil R, Le Grand F, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, et al. (2009) Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell 5:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbrook DB, Han MF, Hellmuth AE (1971) Population of muscle satellite cells in relation to age and mitotic activity. Pathology 3:223–243 [DOI] [PubMed] [Google Scholar]
- Alnaqeeb MA, Goldspink G (1987) Changes in fibre type, number and diameter in developing and ageing skeletal muscle. J Anat 153:31–45 [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, et al. (2009) Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci USA 106:7479–7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Pilipowicz O (2002) Activation of muscle satellite cells in single-fiber cultures. Nitric Oxide 7:36–41 [DOI] [PubMed] [Google Scholar]
- Armand AS, Launay T, Gaspera BD, Charbonnier F, Gallien CL, Chanoine C (2003) Effects of eccentric treadmill running on mouse soleus: degeneration/regeneration studied with Myf-5 and MyoD probes. Acta Physiol Scand 179:75–84 [DOI] [PubMed] [Google Scholar]
- Asakura A, Komaki M, Rudnicki M (2001) Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68:245–253 [DOI] [PubMed] [Google Scholar]
- Baroffio A, Hamann M, Bernheim L, Bochaton-Piallat ML, Gabbiani G, Bader CR (1996) Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation 60:47–57 [DOI] [PubMed] [Google Scholar]
- Barton ER, Demeo J, Lei H (2010) The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol 108:1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL (2002) Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 157:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, et al. (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA (1999) Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol 144:1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles-Isles M, Roy R, Dansereau G, Goulet M, Roy B, Bouchard JP, Tremblay JP (1993) Rapid selection of donor myoblast clones for muscular dystrophy therapy using cell surface expression of NCAM. Eur J Histochem 37:375–380 [PubMed] [Google Scholar]
- Betters JL, Lira VA, Soltow QA, Drenning JA, Criswell DS (2008) Supplemental nitric oxide augments satellite cell activity on cultured myofibers from aged mice. Exp Gerontol 43:1094–1101 [DOI] [PubMed] [Google Scholar]
- Bhagavati S, Ghatpande A, Shafiq SA, Leung B (1996) In situ hybridization analysis for expression of myogenic regulatory factors in regenerating muscle of mdx mouse. J Neuropathol Exp Neurol 55:509–514 [DOI] [PubMed] [Google Scholar]
- Bischoff R (1975) Regeneration of single skeletal muscle fibers in vitro. Anat Rec 182:215–235 [DOI] [PubMed] [Google Scholar]
- Bischoff R (1986) Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 115:129–139 [DOI] [PubMed] [Google Scholar]
- Blanco-Bose WE, Yao CC, Kramer RH, Blau HM (2001) Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res 265:212–220 [DOI] [PubMed] [Google Scholar]
- Blau HM, Webster C, Pavlath GK (1983) Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 80:4856–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaveri K, Heslop L, Yu DS, Rosenblatt JD, Gross JG, Partridge TA, Morgan JE (1999) Patterns of repair of dystrophic mouse muscle: studies on isolated fibers. Dev Dyn 216:244–256 [DOI] [PubMed] [Google Scholar]
- Bockhold KJ, Rosenblatt JD, Partridge TA (1998) Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve 21:173–183 [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS (2002) Functional improvement of dystrophic muscle by myostatin blockade. Nature 420:418–421 [DOI] [PubMed] [Google Scholar]
- Boldrin L, Elvassore N, Malerba A, Flaibani M, Cimetta E, Piccoli M, Baroni MD, et al. (2007) Satellite cells delivered by micro-patterned scaffolds: a new strategy for cell transplantation in muscle diseases. Tissue Eng 13:253–262 [DOI] [PubMed] [Google Scholar]
- Boldrin L, Zammit P, Muntoni F, Morgan J (2009) The mature adult dystrophic mouse muscle environment does not impede efficient engrafted satellite cell regeneration and self-renewal. Stem Cells 27:2478–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavaud S, Agbulut O, D'Honneur G, Nizard R, Mouly V, Butler-Browne G (2002) Preparation of isolated human muscle fibers: a technical report. In Vitro Cell Dev Biol Anim 38:66–72 [DOI] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M (2008) Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells 26:3194–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Bildsoe H, Hughes SM (2005) Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118:4813–4821 [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317:807–810 [DOI] [PubMed] [Google Scholar]
- Brack AS, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3:226–237 [DOI] [PubMed] [Google Scholar]
- Bradley W (1979) Muscle fibre splitting. In Mauro A, Bischoff R, Carlson B, Shafiq S, Konisberg I, Lipton B, eds. Muscle Regeneration. New York, Raven Press, 215–232
- Brimah K, Ehrhardt J, Mouly V, Butler-Browne GS, Partridge TA, Morgan JE (2004) Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum Gene Ther 15:1109–1124 [DOI] [PubMed] [Google Scholar]
- Bross R, Storer T, Bhasin S (1999) Aging and muscle loss. Trends Endocrinol Metab 10:194–198 [DOI] [PubMed] [Google Scholar]
- Capkovic KL, Stevenson S, Johnson MC, Thelen JJ, Cornelison DD (2008) Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp Cell Res 314:1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardasis CA, Cooper GW (1975a) An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: a satellite cell-muscle fiber growth unit. J Exp Zool 191:347–358 [DOI] [PubMed] [Google Scholar]
- Cardasis CA, Cooper GW (1975b) A method for the chemical isolation of individual muscle fibers and its application to a study of the effect of denervation on the number of nuclei per muscle fiber. J Exp Zool 191:333–346 [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, et al. (2009) Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell 8:676–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee GD (1995) What insights into age-related changes in skeletal muscle are provided by animal models? J Gerontol A Biol Sci Med Sci 50 Spec No:137–141 [DOI] [PubMed] [Google Scholar]
- Cashman NR, Covault J, Wollman RL, Sanes JR (1987) Neural cell adhesion molecule in normal, denervated, and myopathic human muscle. Ann Neurol 21:481–489 [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ (2008) Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell 134:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238 [DOI] [PubMed] [Google Scholar]
- Charifi N, Kadi F, Feasson L, Denis C (2003) Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 28:87–92 [DOI] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouviere C (2007) M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell 18:1734–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, et al. (2003) Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163:1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KF, Chuang YH, Her WY, Chen SC, Liu KM (1996) Growth characteristics of normal and dystrophic myoblasts in primary myoblast cultures. Proc Natl Sci Counc Repub China B 20:31–38 [PubMed] [Google Scholar]
- Collins CA (2006) Satellite cell self-renewal. Curr Opin Pharmacol 6:301–306 [DOI] [PubMed] [Google Scholar]
- Collins CA, Gnocchi VF, White RB, Boldrin L, Perez-Ruiz A, Relaix F, Morgan JE, et al. (2009) Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS One 4:e4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122:289–301 [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA (2007) A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25:885–894 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302:1575–1577 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA (2002) The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3:397–409 [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Cerletti M, Wagers AJ, Conboy IM (2010) Immuno-analysis and FACS sorting of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol 621:165–173 [DOI] [PubMed] [Google Scholar]
- Cooper RN, Irintchev A, Di Santo JP, Zweyer M, Morgan JE, Partridge TA, Butler-Browne GS, et al. (2001) A new immunodeficient mouse model for human myoblast transplantation. Hum Gene Ther 12:823–831 [DOI] [PubMed] [Google Scholar]
- Cooper RN, Thiesson D, Furling D, Di Santo JP, Butler-Browne GS, Mouly V (2003) Extended amplification in vitro and replicative senescence: key factors implicated in the success of human myoblast transplantation. Hum Gene Ther 14:1169–1179 [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB (2001) Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol 239:79–94 [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ (1997) Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191:270–283 [DOI] [PubMed] [Google Scholar]
- Cosgrove BD, Sacco A, Gilbert PM, Blau HM (2009) A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 78:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton GR, Curtin NA, Morgan JE, Partridge TA (1988a) The mdx mouse skeletal muscle myopathy. II. Contractile properties. Neuropathol Appl Neurobiol 14:299–314 [DOI] [PubMed] [Google Scholar]
- Coulton GR, Morgan JE, Partridge TA, Sloper JC (1988b) The mdx mouse skeletal muscle myopathy. I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol 14:53–70 [DOI] [PubMed] [Google Scholar]
- Cousins JC, Woodward KJ, Gross JG, Partridge TA, Morgan JE (2004) Regeneration of skeletal muscle from transplanted immortalised myoblasts is oligoclonal. J Cell Sci 117:3259–3269 [DOI] [PubMed] [Google Scholar]
- Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, et al. (2004) Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr KC, Schultz E (1987) Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol 63:1816–1821 [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z (2007) Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol 304:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Shearer A, Yablonka-Reuveni Z (2010) The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol 340:330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary S, Mouly V, Butler-Browne GS (1996) Telomere length as a tool to monitor satellite cell amplification for cell-mediated gene therapy. Hum Gene Ther 7:1347–1350 [DOI] [PubMed] [Google Scholar]
- De Coppi P, Milan G, Scarda A, Boldrin L, Centobene C, Piccoli M, Pozzobon M, et al. (2006) Rosiglitazone modifies the adipogenic potential of human muscle satellite cells. Diabetologia 49:1962–1973 [DOI] [PubMed] [Google Scholar]
- Delaporte C, Dehaupas M, Fardeau M (1984) Comparison between the growth pattern of cell cultures from normal and Duchenne dystrophy muscle. J Neurol Sci 64:149–160 [DOI] [PubMed] [Google Scholar]
- Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C (2009) Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol 68:762–773 [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA (2005) Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15:666–673 [DOI] [PubMed] [Google Scholar]
- DiMario J, Buffinger N, Yamada S, Strohman RC (1989) Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science 244:688–690 [DOI] [PubMed] [Google Scholar]
- DiMario J, Strohman RC (1988) Satellite cells from dystrophic (mdx) mouse muscle are stimulated by fibroblast growth factor in vitro. Differentiation 39:42–49 [DOI] [PubMed] [Google Scholar]
- Doppler K, Mittelbronn M, Bornemann A (2008) Myogenesis in human denervated muscle biopsies. Muscle Nerve 37:79–83 [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA (2006) Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33:242–253 [DOI] [PubMed] [Google Scholar]
- Ehrhardt J, Brimah K, Adkin C, Partridge T, Morgan J (2007) Human muscle precursor cells give rise to functional satellite cells in vivo. Neuromuscul Disord 17:631–638 [DOI] [PubMed] [Google Scholar]
- El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP (2000) In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res 258:279–287 [DOI] [PubMed] [Google Scholar]
- Emery AE (2002) The muscular dystrophies. Lancet 359:687–695 [DOI] [PubMed] [Google Scholar]
- Eriksson A, Kadi F, Malm C, Thornell LE (2005) Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem Cell Biol 124:167–175 [DOI] [PubMed] [Google Scholar]
- Ferreira R, Neuparth MJ, Ascensao A, Magalhaes J, Vitorino R, Duarte JA, Amado F (2006) Skeletal muscle atrophy increases cell proliferation in mice gastrocnemius during the first week of hindlimb suspension. Eur J Appl Physiol 97:340–346 [DOI] [PubMed] [Google Scholar]
- Fidzianska A, Kaminska A (1995) Neural cell adhesion molecule (N-CAM) as a marker of muscle tissue alternations. Review of the literature and own observations. Folia Neuropathol 33:125–128 [PubMed] [Google Scholar]
- Fiore F, Vallone P, Ricchi P, Tambaro R, Daniele B, Sandomenico F, De Vivo R, et al. (2000) Levovist-enhanced Doppler sonography versus spiral computed tomography to evaluate response to percutaneous ethanol injection in hepatocellular carcinoma. J Clin Gastroenterol 31:164–168 [DOI] [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T (1997) A role for FGF-6 in skeletal muscle regeneration. Genes Dev 11:2040–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday BB, Pavlath GK (2001) A calcineurin- and NFAT-dependent pathway regulates Myf5 gene expression in skeletal muscle reserve cells. J Cell Sci 114:303–310 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116:769–778 [DOI] [PubMed] [Google Scholar]
- Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, et al. (2004) Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res 296:245–255 [DOI] [PubMed] [Google Scholar]
- Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, et al. (2007) Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25:2448–2459 [DOI] [PubMed] [Google Scholar]
- Fukada SI, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yamaguchi M, Ito T, et al. (2010) Genetic background affects properties of satellite cells and mdx phenotypes. Am J Pathol 176:2414–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett KL, Anderson JE (1995) Colocalization of bFGF and the myogenic regulatory gene myogenin in dystrophic mdx muscle precursors and young myotubes in vivo. Dev Biol 169:596–608 [DOI] [PubMed] [Google Scholar]
- Garry DJ, Yang Q, Bassel-Duby R, Williams RS (1997) Persistent expression of MNF identifies myogenic stem cells in postnatal muscles. Dev Biol 188:280–294 [DOI] [PubMed] [Google Scholar]
- Glass DJ (2003) Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5:87–90 [DOI] [PubMed] [Google Scholar]
- Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984 [DOI] [PubMed] [Google Scholar]
- Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS (2009) Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One 4:e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G (2003) Gene expression in muscle in response to exercise. J Muscle Res Cell Motil 24:121–126 [DOI] [PubMed] [Google Scholar]
- Gordon S (1995) The macrophage. Bioessays 17:977–986 [DOI] [PubMed] [Google Scholar]
- Gross JG, Morgan JE (1999) Muscle precursor cells injected into irradiated mdx mouse muscle persist after serial injury. Muscle Nerve 22:174–185 [DOI] [PubMed] [Google Scholar]
- Grounds MD (1998) Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci 854:78–91 [DOI] [PubMed] [Google Scholar]
- Harvey AL, Robertson JG, Witkowski JA (1979) Maturation of human skeletal muscle fibres in explant tissue culture. J Neurol Sci 41:115–122 [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551 [DOI] [PubMed] [Google Scholar]
- Heslop L, Morgan JE, Partridge TA (2000) Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci 113(Pt 12):2299–2308 [DOI] [PubMed] [Google Scholar]
- Hill M, Goldspink G (2003) Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J, Verreault S, Roy R, Tremblay M, Tremblay JP (1994) High efficiency of muscle regeneration after human myoblast clone transplantation in SCID mice. J Clin Invest 93:586–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto M, Fukada S, Uezumi A, Masuda S, Miyoshi H, Yamamoto H, Wada MR, et al. (2007) Autologous transplantation of SM/C-2.6(+) satellite cells transduced with micro-dystrophin CS1 cDNA by lentiviral vector into mdx mice. Mol Ther 15:2178–2185 [DOI] [PubMed] [Google Scholar]
- Illa I, Leon-Monzon M, Dalakas MC (1992) Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol 31:46–52 [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A (1994) Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn 199:326–337 [DOI] [PubMed] [Google Scholar]
- Ishimoto S, Goto I, Ohta M, Kuroiwa Y (1983) A quantitative study of the muscle satellite cells in various neuromuscular disorders. J Neurol Sci 62:303–314 [DOI] [PubMed] [Google Scholar]
- Jejurikar SS, Kuzon WM Jr (2003) Satellite cell depletion in degenerative skeletal muscle. Apoptosis 8:573–578 [DOI] [PubMed] [Google Scholar]
- Jejurikar SS, Marcelo CL, Kuzon WM Jr (2002) Skeletal muscle denervation increases satellite cell susceptibility to apoptosis. Plast Reconstr Surg 110:160–168 [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, et al. (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J (2004) Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29:120–127 [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, et al. (2005) The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch 451:319–327 [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Henriksson J (2006) The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochem Cell Biol 126:83–87 [DOI] [PubMed] [Google Scholar]
- Kadi F, Eriksson A, Holmner S, Thornell LE (1999) Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc 31:1528–1534 [DOI] [PubMed] [Google Scholar]
- Kadi F, Thornell LE (2000) Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113:99–103 [DOI] [PubMed] [Google Scholar]
- Kirillova I, Gussoni E, Goldhamer DJ, Yablonka-Reuveni Z (2007) Myogenic reprogramming of retina-derived cells following their spontaneous fusion with myotubes. Dev Biol 311:449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A (1998) The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol 142:1447–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg UR, Lipton BH, Konigsberg IR (1975) The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol 45:260–275 [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM (2006) Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101:531–544 [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129:999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek JB, Radford AJ, Crump DE, Bower JJ, Feeney SJ, Austin L, Byrne E (1998) LIF (AM424), a promising growth factor for the treatment of ALS. J Neurol Sci 160(suppl 1):S106–113 [DOI] [PubMed] [Google Scholar]
- Laguens R (1963) Satellite cells of skeletal muscle fibers in human progressive muscular dystrophy. Virchows Arch Pathol Anat Physiol Klin Med 336:564–569 [DOI] [PubMed] [Google Scholar]
- Lancioni H, Lucentini L, Palomba A, Fulle S, Micheli MR, Panara F (2007) Muscle actin isoforms are differentially expressed in human satellite cells isolated from donors of different ages. Cell Biol Int 31:180–185 [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA (2009) Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4:535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter JR, Anderson JE (2010) Satellite cells are increasingly refractory to activation by nitric oxide and stretch in aged mouse-muscle cultures. Int J Biochem Cell Biol 42:132–136 [DOI] [PubMed] [Google Scholar]
- Li H, Mittal A, Makonchuk DY, Bhatnagar S, Kumar A (2009) Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum Mol Genet 18:2584–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom M, Thornell LE (2009) New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-lifters and sedentary men. Histochem Cell Biol 132:141–157 [DOI] [PubMed] [Google Scholar]
- Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F (2009) Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 40:455–465 [DOI] [PubMed] [Google Scholar]
- Maier F, Bornemann A (1999) Comparison of the muscle fiber diameter and satellite cell frequency in human muscle biopsies. Muscle Nerve 22:578–583 [DOI] [PubMed] [Google Scholar]
- Malerba A, Vitiello L, Segat D, Dazzo E, Frigo M, Scambi I, De Coppi P, et al. (2009) Selection of multipotent cells and enhanced muscle reconstruction by myogenic macrophage-secreted factors. Exp Cell Res 315:915–927 [DOI] [PubMed] [Google Scholar]
- Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtersheimer G, Staudter M, Moller P (1992) Expression of the natural killer (NK) cell-associated antigen CD56(Leu-19), which is identical to the 140-kDa isoform of N-CAM, in neural and skeletal muscle cells and tumors derived therefrom. Ann N Y Acad Sci 650:311–316 [DOI] [PubMed] [Google Scholar]
- Melchionna R, Di Carlo A, De Mori R, Cappuzzello C, Barberi L, Musaro A, Cencioni C, et al. (2010) Induction of myogenic differentiation by SDF-1 via CXCR4 and CXCR7 receptors. Muscle Nerve 41:828–835 [DOI] [PubMed] [Google Scholar]
- Melone MA, Peluso G, Petillo O, Galderisi U, Cotrufo R (1999) Defective growth in vitro of Duchenne Muscular Dystrophy myoblasts: the molecular and biochemical basis. J Cell Biochem 76:118–132 [DOI] [PubMed] [Google Scholar]
- Miller KJ, Thaloor D, Matteson S, Pavlath GK (2000) Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol 278:C174–181 [DOI] [PubMed] [Google Scholar]
- Millman JR, Tan JH, Colton CK (2009) The effects of low oxygen on self-renewal and differentiation of embryonic stem cells. Curr Opin Organ Transplant 14:694–700 [DOI] [PubMed] [Google Scholar]
- Mitchell PO, Mills T, O'Connor RS, Kline ER, Graubert T, Dzierzak E, Pavlath GK (2005) Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev Biol 283:240–252 [DOI] [PubMed] [Google Scholar]
- Mitchell PO, Pavlath GK (2004) Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol 287:C1753–1762 [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, et al. (2005) Direct isolation of satellite cells for skeletal muscle regeneration. Science 309:2064–2067 [DOI] [PubMed] [Google Scholar]
- Morgan JE, Coulton GR, Partridge TA (1989) Mdx muscle grafts retain the mdx phenotype in normal hosts. Muscle Nerve 12:401–409 [DOI] [PubMed] [Google Scholar]
- Morgan JE, Gross JG, Pagel CN, Beauchamp JR, Fassati A, Thrasher AJ, Di Santo JP, et al. (2002) Myogenic cell proliferation and generation of a reversible tumorigenic phenotype are triggered by preirradiation of the recipient site. J Cell Biol 157:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JE, Hoffman EP, Partridge TA (1990) Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol 111:2437–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JE, Pagel CN, Sherratt T, Partridge TA (1993) Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. J Neurol Sci 115:191–200 [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP (1971) Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170:421–435 [DOI] [PubMed] [Google Scholar]
- Mourkioti F, Rosenthal N (2005) IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol 26:535–542 [DOI] [PubMed] [Google Scholar]
- Mozdziak PE, Pulvermacher PM, Schultz E (2000) Unloading of juvenile muscle results in a reduced muscle size 9 wk after reloading. J Appl Physiol 88:158–164 [DOI] [PubMed] [Google Scholar]
- Muir AR, Kanji AH, Allbrook D (1965) The structure of the satellite cells in skeletal muscle. J Anat 99:435–444 [PMC free article] [PubMed] [Google Scholar]
- Naffakh N, Pinset C, Montarras D, Pastoret C, Danos O, Heard JM (1993) Transplantation of adult-derived myoblasts in mice following gene transfer. Neuromuscul Disord 3:413–417 [DOI] [PubMed] [Google Scholar]
- Nagata Y, Partridge TA, Matsuda R, Zammit PS (2006) Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol 174:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae Y, Stoward PJ, Kashiyama T, Shono M, Akagi A, Matsuzaki T, Nonaka I (2004) Early onset of lipofuscin accumulation in dystrophin-deficient skeletal muscles of DMD patients and mdx mice. J Mol Histol 35:489–499 [DOI] [PubMed] [Google Scholar]
- Neuhaus P, Oustanina S, Loch T, Kruger M, Bober E, Dono R, Zeller R, et al. (2003) Reduced mobility of fibroblast growth factor (FGF)-deficient myoblasts might contribute to dystrophic changes in the musculature of FGF2/FGF6/mdx triple-mutant mice. Mol Cell Biol 23:6037–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MC, Stockdale FE (1972) A kinetic analysis of myogenesis in vitro. J Cell Biol 52:52–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PS (2009) Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev Biol 337:29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, Montarras D, et al. (2010) An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res 4:77–91 [DOI] [PubMed] [Google Scholar]
- Parise G, McKinnell IW, Rudnicki MA (2008) Muscle satellite cell and atypical myogenic progenitor response following exercise. Muscle Nerve 37:611–619 [DOI] [PubMed] [Google Scholar]
- Partridge TA (1997) Tissue culture of skeletal muscle. Methods Mol Biol 75:131–144 [DOI] [PubMed] [Google Scholar]
- Pastoret C, Sebille A (1995) mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci 129:97–105 [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Thaloor D, Rando TA, Cheong M, English AW, Zheng B (1998) Heterogeneity among muscle precursor cells in adult skeletal muscles with differing regenerative capacities. Dev Dyn 212:495–508 [DOI] [PubMed] [Google Scholar]
- Pelletier R, Hamel F, Beaulieu D, Patry L, Haineault C, Tarnopolsky M, Schoser B, et al. (2009) Absence of a differentiation defect in muscle satellite cells from DM2 patients. Neurobiol Dis 36:181–190 [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM (2006) Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291:E937–946 [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Willenbring H (2007) On the origin of the term “stem cell”. Cell Stem Cell 1:35–38 [DOI] [PubMed] [Google Scholar]
- Rando TA (2001) The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve 24:1575–1594 [DOI] [PubMed] [Google Scholar]
- Reimann J, Brimah K, Schroder R, Wernig A, Beauchamp JR, Partridge TA (2004) Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res 315:233–242 [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, et al. (2006) Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault V, Rolland E, Thornell LE, Mouly V, Butler-Browne G (2002) Distribution of satellite cells in the human vastus lateralis muscle during aging. Exp Gerontol 37:1513–1514 [DOI] [PubMed] [Google Scholar]
- Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, et al. (2009) Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol 296:C1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JE, Gurpur PB, Yablonka-Reuveni Z, Burkin DJ (2009) Laminin-111 restores regenerative capacity in a mouse model for alpha7 integrin congenital myopathy. Am J Pathol 174:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA (1995) Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 31:773–779 [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ (1992) Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol 73:2538–2543 [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Yong D, Parry DJ (1994) Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 17:608–613 [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM (2008) Self-renewal and expansion of single transplanted muscle stem cells. Nature 456:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajko S, Kubinova L, Cvetko E, Kreft M, Wernig A, Erzen I (2004) Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J Histochem Cytochem 52:179–185 [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Hellhammer U (1976) The number of satellite cells in normal human muscle. Anat Rec 185:279–287 [DOI] [PubMed] [Google Scholar]
- Schubert W, Zimmermann K, Cramer M, Starzinski-Powitz A (1989) Lymphocyte antigen Leu-19 as a molecular marker of regeneration in human skeletal muscle. Proc Natl Acad Sci USA 86:307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuierer MM, Mann CJ, Bildsoe H, Huxley C, Hughes SM (2005) Analyses of the differentiation potential of satellite cells from myoD−/−, mdx, and PMP22 C22 mice. BMC Musculoskelet Disord 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E (1976) Fine structure of satellite cells in growing skeletal muscle. Am J Anat 147:49–70 [DOI] [PubMed] [Google Scholar]
- Schultz E, Darr KC, Macius A (1994) Acute effects of hindlimb unweighting on satellite cells of growing skeletal muscle. J Appl Physiol 76:266–270 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786 [DOI] [PubMed] [Google Scholar]
- Shafiq SA, Gorycki MA, Milhorat AT (1967) An electron microscopic study of regeneration and satellite cells in human muscle. Neurology 17:567–574 [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Chai J, Maley K, Cozens G, Grounds G, Winn N, Rosenthal N, et al. (2010) A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J Cell Sci 123:960–971 [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z (2006) Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294:50–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z (2004) Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci 117:5393–5404 [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ (2004) Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell 119:543–554 [DOI] [PubMed] [Google Scholar]
- Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DD (2009) 3D timelapse analysis of muscle satellite cell motility. Stem Cells 27:2527–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Barbosa SD, Butler-Browne GS, de Mello W, Riederer I, Di Santo JP, Savino W, Mouly V (2008) Human myoblast engraftment is improved in laminin-enriched microenvironment. Transplantation 85:566–575 [DOI] [PubMed] [Google Scholar]
- Silva-Barbosa SD, Butler-Browne GS, Di Santo JP, Mouly V (2005) Comparative analysis of genetically engineered immunodeficient mouse strains as recipients for human myoblast transplantation. Cell Transplant 14:457–467 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S (2006) Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab 91:3024–3033 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Roth SM, Lee MI, Bhasin S (2003) Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285:E197–205 [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, Dugre FJ, et al. (2006) Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol 65:371–386 [DOI] [PubMed] [Google Scholar]
- Smith J, Merrick D (2010) Embryonic skeletal muscle microexplant culture and isolation of skeletal muscle stem cells. Methods Mol Biol 633:29–56 [DOI] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, van Loon LJ (2009) The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev 8:328–338 [DOI] [PubMed] [Google Scholar]
- Snow MH (1977) The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res 185:399–408 [DOI] [PubMed] [Google Scholar]
- Snow MH (1978) An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res 186:535–540 [DOI] [PubMed] [Google Scholar]
- Solomon AM, Bouloux PM (2006) Modifying muscle mass: the endocrine perspective. J Endocrinol 191:349–360 [DOI] [PubMed] [Google Scholar]
- Southgate RJ, Neill B, Prelovsek O, El-Osta A, Kamei Y, Miura S, Ezaki O, et al. (2007) FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem 282:21176–21186 [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, et al. (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352:536–539 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Buckingham ME (1995) Lineage restriction of the myogenic conversion factor myf-5 in the brain. Development 121:4077–4083 [DOI] [PubMed] [Google Scholar]
- Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB (2009) Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell 4:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE (1998) HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194:114–128 [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE (2002) Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 13:2909–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, Suzuki T, et al. (2009) Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol 297:C238–252 [DOI] [PubMed] [Google Scholar]
- Tidball JG (1995) Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 27:1022–1032 [DOI] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12:143–152 [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, et al. (2009) Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 89:608–616 [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ (2007) Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292:E151–157 [DOI] [PubMed] [Google Scholar]
- Volonte D, Liu Y, Galbiati F (2005) The modulation of caveolin-1 expression controls satellite cell activation during muscle repair. FASEB J 19:237–239 [DOI] [PubMed] [Google Scholar]
- Wakayama Y, Schotland DL, Bonilla E, Orecchio E (1979) Quantitative ultrastructural study of muscle satellite cells in Duchenne dystrophy. Neurology 29:401–407 [DOI] [PubMed] [Google Scholar]
- Watkins SC, Cullen MJ (1986) A quantitative comparison of satellite cell ultrastructure in Duchenne muscular dystrophy, polymyositis, and normal controls. Muscle Nerve 9:724–730 [DOI] [PubMed] [Google Scholar]
- Watkins SC, Cullen MJ (1988) A quantitative study of myonuclear and satellite cell nuclear size in Duchenne's muscular dystrophy, polymyositis and normal human skeletal muscle. Anat Rec 222:6–11 [DOI] [PubMed] [Google Scholar]
- Watt DJ, Morgan JE, Partridge TA (1991) Allografts of muscle precursor cells persist in the non-tolerized host. Neuromuscul Disord 1:345–355 [DOI] [PubMed] [Google Scholar]
- Weintraub H (1993) The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75:1241–1244 [DOI] [PubMed] [Google Scholar]
- Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, Greenway S, Craven S, Scott E, Anderson JE (2003) C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem 51:1437–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Anderson JE (2006) Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn 235:203–212 [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Day K, Vine A, Shefer G (2008) Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci 86:E207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ (1994) Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol 164:588–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P (1999a) The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol 210:440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seger R, Rivera AJ (1999b) Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem 47:23–42 [DOI] [PubMed] [Google Scholar]
- Yamada M, Tatsumi R, Yamanouchi K, Hosoyama T, Shiratsuchi S, Sato A, Mizunoya W, et al. (2010) High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol 298:C465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane A, Akutsu S, Diekwisch TG, Matsuda R (2005) Satellite cells and utrophin are not directly correlated with the degree of skeletal muscle damage in mdx mice. Am J Physiol Cell Physiol 289:C42–48 [DOI] [PubMed] [Google Scholar]
- Zacharias JM, Anderson JE (1991) Muscle regeneration after imposed injury is better in younger than older mdx dystrophic mice. J Neurol Sci 104:190–196 [DOI] [PubMed] [Google Scholar]
- Zammit PS (2008) All muscle satellite cells are equal, but are some more equal than others? J Cell Sci 121:2975–2982 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR (2004) Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]