Abstract
The number of corticotrophs increases in the anterior pituitary (AP) gland in adrenalectomized (AdX) rats. In this study, aimed at identifying the growth factor implicated in this proliferation, we analyzed proteins secreted from a cDNA library of the AP of AdX rats, using the signal sequence trap method. A PCR analysis of several cDNAs that coded for insulin-like growth factor binding protein (IGFBP) 5, IGFBP7, and vacuolar H+-ATPase accessory subunit Ac45 revealed an increased and decreased expression level of IGFBP7 mRNA in the AP of AdX rats and AdX rats injected with dexamethasone, respectively. IGFBP7 mRNA was predominately expressed in the corticotrophs of the APs of both sham-operated and AdX rats. The AP of AdX rats contained an increased number of IGFBP7 mRNA–expressing cells and corticotrophs compared with that of sham-operated rats, but the ratio of IGFBP7 mRNA–positive corticotrophs per total number of corticotrophs did not significantly change in either group. Histochemical analysis of labeled proliferating cell nuclear antigen (PCNA) and sex-determining region Y box-2 (SOX2) revealed the presence of several PCNA-positive signals and the absence of SOX2 cells among the corticotrophs, suggesting that IGFBP7 mRNA–expressing corticotrophs are derived from in situ corticotrophs and that they increase in number as corticotrophs increase. The possible roles of IGFBP7 in the corticotrophs are also discussed. (J Histochem Cytochem 58:969–978, 2010)
Keywords: IGFBP7, signal sequence trap method, anterior pituitary, adrenalectomy, rat
The controlling center of endocrine systems in vertebrates is the hypothalamic–pituitary axis. In this system, the functions of the peripheral tissues are regulated by hormones secreted from the hypothalamic–pituitary axis, and the functions of the hypothalamic–pituitary axis are regulated by the negative feedback of signals from the peripheral tissues, such as steroid hormones. In the anterior pituitary (AP) of adrenalectomized (AdX) rats, the synthesis and the secretion of adrenocorticotropin and the proliferation of corticotrophs are induced as a result of a breakdown in the negative feedback mechanism owing to the depletion of glucocorticoids (Gulyás et al. 1991; Taniguchi et al. 1995). However, the actual mechanism by which corticotroph proliferation is induced by adrenalectomy is not fully understood. One possibility is that the proliferation of corticotrophs is elicited by corticotrophin-releasing hormone and epidermal growth factor (EGF) (Childs et al. 1995). As EGF receptor is expressed not only in corticotrophs but also in other hormone-secreting cells of the AP (Fan and Childs 1995), this proposed mechanism leads to the possibility that novel growth factors induce the proliferation of corticotrophs.
The insulin-like growth factor (IGF) signaling pathway plays a crucial role in regulating the proliferation, differentiation, and apoptosis of cells in mammalian tissues, such as brain, liver, kidney, and muscle (Pollak et al. 2004). IGFs, which are produced mainly in the liver, regulate various endocrine effects, including energy metabolism, body size, aging, and cancer risk. They have also been reported to stimulate cell proliferation via a paracrine/autocrine system (Hwa et al. 1999; Pollak et al. 2004). IGF signaling, mediated by interaction with type I and type II IGF receptors, is modulated by IGF-binding proteins (IGFBPs) and their regulators (Sepp-Lorenzino 1998; Hwa et al. 1999). Consequently, IGF bioactivity is not only dependent on the interaction with IGF receptors but is also influenced by the multiregulator family of IGFBPs. Sixteen isoforms of IGFBPs have been identified to date; IGFBP1–6 bind with high affinity to IGFs, whereas IGFBP7/IGFBP-rP1–10 show low-affinity binding with IGFs (Schmid 1995; Hwa et al. 1999; Firth and Baxter 2002; Burger et al. 2005). IGFBP7, referred to as IGFBP-related protein 1, has a high affinity for insulin, namely ∼100-fold higher than that of other IGFBPs, indicating its important role in modifying metabolism, modulating insulin binding to the insulin receptor, and inhibiting insulin action (Yamanaka et al. 1997). In experiments with cancer cells, IGFBP7 has been found to induce the proliferation of glioma cells, whereas it inhibits the proliferation of colorectal carcinoma cells, inducing apoptosis (Ruan et al. 2007; Jiang et al. 2008). IGFBP7 protein is also expressed in a wide range of human tissues, including the adrenal gland and pituitary. However, the pituitary cells expressing this protein have not been identified yet (Degeorges et al. 2000).
In this study, we analyzed secreted proteins, obtained using the signal sequence trap strategy, by the retrovirus-mediated expression screening (SST-REX) method using the cDNA library of the AP of AdX rats (Kitamura et al. 1995; Kojima and Kitamura 1999; Nakakura et al. 2009). Because SST-REX is the retrovirus-mediated expression system with the ability of the thrombopoietin receptor myeloproliferative leukemia virus oncogene to promote factor-independent growth of Ba/F3 cells to efficiently screen for signal sequence–containing cDNA (Kojima and Kitamura 1999), we could comprehensively analyze proteins with the signal sequence. We also investigated the function of IGFBP7 in the AP once it had been determined that the expression of IGFBP7 mRNA increased in the AP of AdX rats.
Materials and Methods
Animals
Normal adult male rats of the Wistar strain (8–9 weeks of age) were housed in a temperature-controlled room (22 ± 2C) with automatically controlled lighting (lights on from 6:00 am to 6:00 pm daily) and supplied with food and water ad libitum. All animal experiments were conducted in compliance with the Guide for Care and Use of Laboratory Animals established by Shizuoka University.
Experimental Procedure
Bilateral adrenalectomy was carried out using a dorsal approach under Somnopentyl (Kyoritsu Seiyaku; Tokyo, Japan) anesthesia (Taniguchi et al. 1995). Sham-operated rats were subjected to the same surgical procedure without removal of the adrenals. Rats were given 0.9% saline to drink following surgery. Seven days after surgery, AdX and sham-operated rats (n = 12 in each group) were decapitated after ether anesthesia, and their pituitary glands were removed for further analysis.
Beginning on postsurgery day and for the following 4 days (five injections in total), the rats were each given a once-daily IP injection that consisted of either dexamethasone (DEX, 1 mg/kg body weight; Wako, Osaka, Japan) in oil or solely oil (equal volume as DEX injection). All injections were given between 9:00 am and 10:00 am. To study the effect of the adrenalectomy and DEX on the expression levels of IGFBP7 mRNA, the rats were divided into four experimental groups (n = 6): sham plus oil (control group; SO), sham plus DEX (SD), adrenalectomy plus oil (AO), and adrenalectomy plus DEX (AD). Following the fifth injection, all animals were decapitated after ether anesthesia and their pituitary glands were quickly removed and used for semiquantitative RT-PCR analysis.
SST-REX Method
To identify cDNAs encoding both secreted and membrane proteins from the AP of the AdX rats (n = 5), we constructed a cDNA library from the AP of AdX rats and screened it using the SST-REX method (Kojima and Kitamura 1999; Nakakura et al. 2009). Briefly, poly-A+-RNA was isolated from the AP of AdX rats, using the FastTrack 2.0 Kit (Invitrogen; Carlsbad, CA). cDNA was synthesized from poly-A+-RNA with random hexamers by using a SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen) and then inserted into the BstXI sites of the pMXs-SST vector (Kojima and Kitamura 1999) using BstXI adapters (Invitrogen). The ligated DNA was then introduced into ElectroMAX DH10B Cells (Invitrogen), and the plasmid DNA was prepared using a QIAGEN Plasmid Maxi Kit (QIAGEN; Hilden, Germany). High-titer retroviruses representing the SST-REX library were produced using PlatE cells (Kojima and Kitamura 1999) and infected into Ba/F3 cells as previously described (Kitamura et al. 1995). One day after the infection, the process of selecting factor-independent Ba/F3 cells in the absence of interleukin-3 was initiated using 96-well plates. Integrated cDNA was isolated from factor-independent Ba/F3 clones by genomic PCR using PrimeSTAR polymerase (Takara; Shiga, Japan). The PCR fragments were labeled by Dye Terminator Cycle Sequencing with a CEQ2000 Quick Start kit (Beckman Coulter; Fullerton, CA) and analyzed using an automatic sequencer (model CEQ2000; Beckman Coulter).
Semiquantitative RT-PCR
Total RNAs of the rat AP and mouse pituitary corticotroph tumor cell line, AtT-20 cells (five culture dishes), were isolated and prepared using the TRIzol Reagent (Invitrogen), then treated with DNase I (4 U; Takara), and cDNA was synthesized by M-MLV reverse transcriptase (Invitrogen), as described previously (Nakakura et al. 2007). The RT-PCR was performed using sense and antisense primers, based on IGFBP5 (M62781), IGFBP7 cDNA (BC086582), vacuolar H+-ATPase accessory subunit Ac45 (Ac45) cDNA (BC07937), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA (AF106860) as shown in Table 1. PCR cycling conditions consisted of 24 cycles (IGFBP5, IGFBP7, Ac45) or 23 cycles (GAPDH) of 94C for 30 sec, 55C for 30 sec, and 72C for 60 sec, in a thermal cycler (ASTEC; Fukuoka, Japan). The RT-PCR products were analyzed on a 2% agarose gel containing ethidium bromide (0.5 μg/ml) with Marker 6 (λ/Sty1 digest; Wako) as molecular weight markers. After band data were obtained, the signal intensity was quantified with ImageJ software (National Institutes of Health; Bethesda, MD). The relative density was determined as the ratio of the signal intensity of the IGFBP5, IGFBP7, and Ac45 bands to that of the GAPDH band in order to normalize any variation in the amplification efficiency of the cDNA.
Table 1.
Primers used for RT-PCR analysis
| Name | Primer sequence (5′–3′) | bp | Accession number |
|---|---|---|---|
| IGFBP5 | M62781 | ||
| Sense | ACATGGAAGCTTCCCTCCAGG | 1131–1151 | |
| Antisense | CGTCACTCAACGTTACTGCTG | 1348–1368 | |
| IGFBP7 | BC086582 | ||
| Sense | AAGGTCCTTCCATAGTGACG | 489–508 | |
| Antisense | AGGAATATGTCAGGCAGGAG | 929–948 | |
| Ac45 | BC070937 | ||
| Sense | TATTACCAGCGATATGCAGC | 185–204 | |
| Antisense | TACTAGGTCCTGTGACTTGG | 1061–1080 | |
| GAPDH | AF106860 | ||
| Sense | GACAAGATGGGTGAAGGTCGG | 844–863 | |
| Antisense | TCCCATTCTCAGCCTTGACT | 1020–1039 |
In Situ RT-PCR Protocol and IHC
Sham-operated and AdX rats (n = 5 in each group) were perfused through the heart with MEMFA (3.7% formaldehyde, 2 mM EGTA, 1 mM MgSO4, 0.1 M MOPS; pH7.4), and their pituitary glands were excised and further fixed by immersion in the same fixative overnight. After fixation, the tissues were dehydrated through a graded ethanol series, embedded in Paraplast (McCormick Scientific; St Louis, MO), cut into 4-μm sections, mounted on silane-coated slides, and pretreated with 1 U/μl DNase I (Takara) in 1× DNase buffer containing 2 U/μl RNasin (Promega; Madison, WI) at 37C overnight. Following the DNase I treatment, the sections were washed two times for 1 min with RNase-free PBS and Milli-Q (Millipore; Tokyo, Japan). One-step in situ RT-PCR was performed with the RT-PCR kit containing rTth DNA polymerase (Toyobo; Osaka, Japan) and digoxigenin-11-dUTP (Roche Molecular Biochemicals; Meylan, France), as described previously (Nakakura et al. 2006), in a thermal cycler (PCR Express Thermo Hybaid; ASTEC). The same sequence primers were used in both the in situ RT-PCR analyses. The cDNA was synthesized at 60C for 30 min. PCR cycling consisted of an initial step of 90C for 1 min, followed by 20 reaction cycles of denaturation (94C, 30 sec), annealing (55C, 30 sec), and extension (72C, 60 sec), with a final extension reaction at 72C for 5 min. The samples were fixed with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) for 10 min at 4C and washed two times in 0.1× standard saline citrate for 20 min at 45C and one time in washing buffer for 7 min. After a blocking step, the sections were incubated with alkaline phosphatase–conjugated sheep anti-digoxigenin Fab antibody (Roche Molecular Biochemicals) for 2 hr at 37C. The label was detected with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate (Roche Molecular Biochemicals) by incubating the sections for the specified time. To check the specificity of the staining, we carried out in situ RT-PCR using a reaction solution lacking digoxigenin-11-dUTP.
Following staining of the mRNA, the sections were washed in 10 mM Tris–HCl containing 1 mM EDTA, fixed with 4% PFA in 0.1 M PB for 10 min, and washed two times with Milli-Q (5 min each) and three times with PBS (5 min each). The sections were blocked with 1% BSA–PBS for 1 hr and immunolabeled by the immunofluorescence method described by Tanaka et al. (1997). They were then incubated with guinea pig anti-amidated joining peptide (JP), which can identify corticotrophs (ST-3; 1:2000; Tanaka and Kurosumi 1992), for 21 h, followed by incubation with indocarbocyanine (Cy3)-labeled donkey anti-guinea pig IgG (1:400; Jackson ImmunoResearch, West Grove, PA) for 2 hr. Finally, the sections were washed with PBS, mounted in PermaFluor (Immunon; Pittsburgh, PA), and examined with an Olympus BX61 microscope equipped with a BX-epifluorescence attachment (Olympus Optical; Tokyo, Japan). The numbers of IGFBP7 mRNA–expressing cells and corticotrophs were counted, and the ratio of IGFBP7 mRNA–positive corticotrophs per total number of corticotrophs was used as an index of each cell population in the midfrontal plane of the gland. For quantitative analysis, data were randomly selected from five sections obtained from five animals, in each of which five sections were used to count the cells.
Adjacent serial sections that had been treated for antigen retrieval by heating in a retrieval solution (1 mM EDTA in Milli-Q) at 90C for 20 min in a pan were immunostained to identify sex-determining region Y (SRY) box-2 (SOX2)–positive cells and proliferating cell nuclear antigen (PCNA)–positive cells, using the same method with guinea pig anti-amidated JP (ST-3; 1:2000) and goat anti-human SOX2 (1:80; R&D Systems, Minneapolis, MN), or mouse monoclonal antibody against rat PCNA (1:2000; DAKO Japan, Tokyo, Japan), followed by fluorescein isothiocyanate-labeled donkey anti-guinea pig IgG (1:200; Jackson ImmunoResearch) and Alexa 546-labeled donkey anti-goat IgG (1:400; Molecular Probes, Eugene, OR), or Cy3-labeled donkey anti-mouse IgG (1:400; Jackson ImmuoResearch), respectively.
Statistical Analysis
All data are presented as the mean ± standard error. For statistical analysis, differences between groups were evaluated by Student's t test or the Steel–Dwass' test. p<0.05 was considered to be significant.
Results
Identification of the IGFBP Family Expressed in the cDNA Library of the AP of AdX Rats by the SST-REX Method
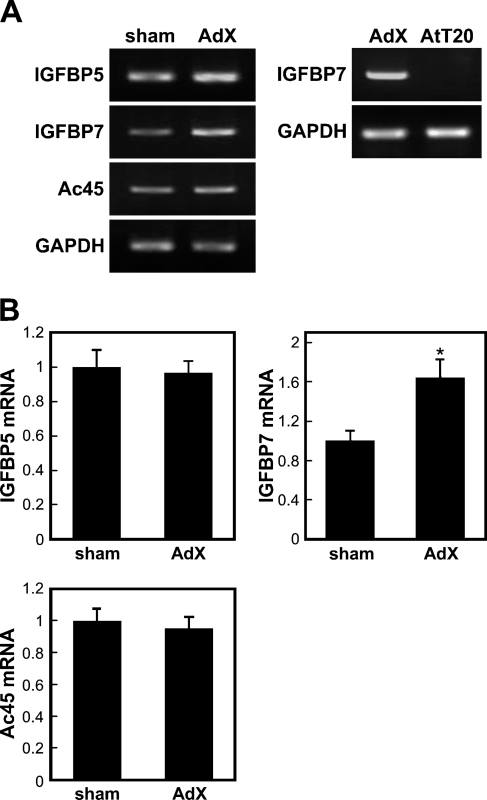
The SST-REX method was used to perform an extensive search for proteins secreted at higher levels in the AP of AdX rats compared with the control. A total of 167 positive clones were identified in the cDNA library of the AP of AdX rats (Table 2), of which three, namely, IGFBP5 and IGFBP7, belonging to the IGFBP family of proteins, and Ac45, which is involved in acidification within secretory granules containing pro-opiomelanocortin (Tanaka et al. 1997; Holthuis et al. 1999; Jansen et al. 2008), attracted our attention and were subsequently cloned. To confirm the effect of the adrenalectomy, we examined the expression levels of mRNA for IGFBP5, IGFBP7, and Ac45 in the APs of both sham-operated and AdX rats by semiquantitative RT-PCR (Figure 1). As shown in Figure 1B, the expression level of IGFBP7 mRNA increased by 1.65-fold (p<0.05) in the AP of the AdX rats compared with the sham-operated ones, but there was no significant difference in the expression levels of IGFBP5 and Ac45 mRNA between the two groups. In addition, IGFBP7 mRNA was not expressed in the AtT-20 cells (Figure 1A).
Table 2.
Clones identified by SST-REX method using the cDNA library of the anterior pituitary in the adrenalectomized rat
| Gene name | Accession number | Number of identical clones |
|---|---|---|
| δ-like protein 1 | D84336 | 40 |
| Chromogranin A | X06832 | 19 |
| Amyloid β (A4) precursor-like protein 1 | BC161904 | 9 |
| Pro-opiomelanocortin | AF51039 | 9 |
| Seizure related 6 homolog like 2 | BC096615 | 6 |
| Epithelin 1 and 2 | X62322 | 5 |
| Insulin-like growth factor binding protein 5 (IGFBP5) | M62781 | 4 |
| Myeloproliferative leukemia virus oncogene | AB235197 | 4 |
| Prosaposin | BC061759 | 4 |
| Collagen α1 type I | Z78279 | 3 |
| Guanine nucleotide-binding protein G-s, α subunit | M12673 | 3 |
| Pro-α-2 (I) collagen | AF121217 | 3 |
| Secretogranin II | X13618 | 3 |
| Chromogranin B | AF019974 | 2 |
| Dystroglycan 1 | AF357216 | 2 |
| Immunoglobulin superfamily, member 3 | BC052892 | 2 |
| Protein disulphide isomerase | X02918 | 2 |
| Acrosin binding protein | BC079212 | 1 |
| Peroxisome proliferator-activated receptor γ1 | AF156665 | 1 |
| α-1 type III collagen | BC087039 | 1 |
| α-1 type IV collagen | J04694 | 1 |
| α-2 collagen VI | BC089923 | 1 |
| Amyloid precursor-like protein 1 | BC161904 | 1 |
| Amyloid precursor-like protein 2 | X77934 | 1 |
| Apolipoprotein E | BC060313 | 1 |
| Cactin | AY934505 | 1 |
| CD47 antigen | BC012667 | 1 |
| Collagen α-2(IV) | X04647 | 1 |
| Complement component factor H | BC083174 | 1 |
| Core protein of HSPG | M81687 | 1 |
| Cysteine-rich with EGF-like domains 1 | BC097951 | 1 |
| EGF receptor | X59698 | 1 |
| Fasciclin II GPI-linked protein isoform | AY495696 | 1 |
| Fibrillin | AF135059 | 1 |
| Strain CD IGS fos-related 2 | AY622611 | 1 |
| Frizzled homolog 7 | BC063077 | 1 |
| γ-glutamyl transpeptidase type-IV | AF062641 | 1 |
| IGFBP7 | BC086582 | 1 |
| Interleukin 6 signal transducer | BC058679 | 1 |
| Prolyl 4-hydroxylase, β polypeptide | BC061857 | 1 |
| Keratin-associated protein 9-2 | A5A6P5 | 1 |
| LGP 107 mRNA for a 107 kDa sialoglycoprotein | X14765 | 1 |
| Low-density lipoprotein receptor-related protein 11 precursor | AK299599 | 1 |
| Membrane-type metalloproteinase | X91785 | 1 |
| mKIAA1131 protein | AK173106 | 1 |
| MG-160 | U08136 | 1 |
| Neural proliferation, differentiation and control 1 | AF263513 | 1 |
| Neuroglycan C precursor | U33553 | 1 |
| Notch 1 | AF508809 | 1 |
| 5'-nucleotidase | J05214 | 1 |
| Podocalyxin-like 2 | BC161970 | 1 |
| Possible OmpA family member precursor | Q6ND96 | 1 |
| Procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), α1 polypeptide | AK030574 | 1 |
| Procollagen, type III, α1 | AK019448 | 1 |
| Protocadherin β11 | AF326304 | 1 |
| Protocadherin γ-a2 | AY574012 | 1 |
| Ribophorin II | X55298 | 1 |
| Serine/arginine repetitive matrix 1 | BC166715 | 1 |
| Serine protease inhibitor, Kunitz type 2 | BC061768 | 1 |
| Similar to MIR-interacting saposin-like protein precursor | BC12610 | 1 |
| Solute carrier family 1 | BC080242 | 1 |
| Tail fibroblast receptor for feline leukemia virus subgroup C | AK036315 | 1 |
| Transmembrane 4 superfamily member 13 | BC089906 | 1 |
| Vacuolar H+–ATPase accessory subunit Ac45 | BC070937 | 1 |
| Total | 167 |
SST-REX, signal sequence trap strategy, by retrovirus-mediated expression screening.
Figure 1.
Expression of insulin-like growth factor binding protein (IGFBP) 5, IGFBP7, and vacuolar H+-ATPase accessory subunit Ac45 (Ac45) in the anterior pituitary (AP) of sham-operated (sham) and adenalectomized (AdX) rats and of AtT-20 cells by semiquantitative RT-PCR. (A) RT-PCR products obtained using primers as described in Table 1 were separated on a 2% agarose gel and stained with ethidium bromide. (B) The data obtained after RT-PCR were quantified using ImageJ software. The densitometry intensity was normalized with the expression levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each bar represents the mean ± standard error (SE) of five independent experiments. *p<0.05 vs sham-operated rats.
Expression Levels of IGFBP7 mRNA in the Rat AP by Semiquantitative RT-PCR
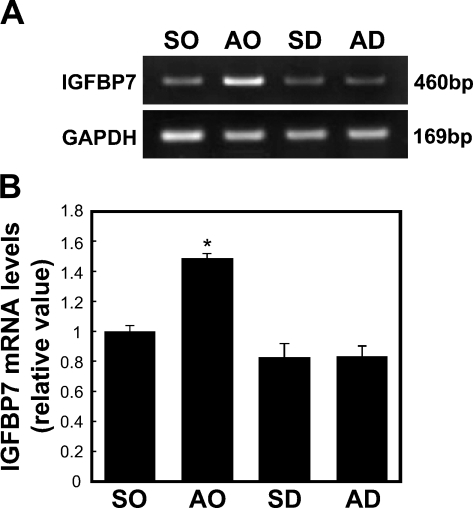
To investigate whether the expression level of IGFBP7 mRNA was directly regulated by glucocorticoids, we analyzed the expression levels of IGFBP7 mRNA in the APs of the four rat treatment groups (SO, AO, SD, and AD) using a semiquantitative RT-PCR. The expression level of IGFBP7 mRNA increased by 1.5-fold (p<0.05) in the AP of the AO rats compared with the SO rats and decreased by 0.55-fold (p<0.05) in the APs of the SD and AD rats compared with the AO rats (Figure 2). There was no significant difference in the expression level of IGFBP7 mRNA between the SD and the SO rats (Figure 2).
Figure 2.
Semiquantitative RT-PCR for IGFBP7 mRNA in the AP obtained from the different treatment groups: sham plus oil group (SO), adrenalectomy plus oil (AO), sham plus dexamethasone (DEX; SD), and adrenalectomy plus DEX (AD). (A) Representative RT-PCR bands. (B) Data obtained after RT-PCR were quantified using ImageJ software. The densitometry intensity was normalized with the expression levels of GAPDH. Each bar represents the mean ± SE of six independent experiments. *p<0.05 vs the SO, SD, and AD groups.
Identification of IGFBP7 mRNA–expressing Cells in the Rat Pituitary
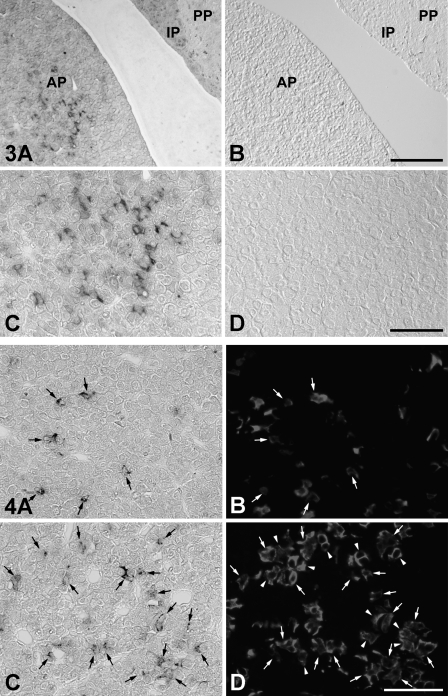
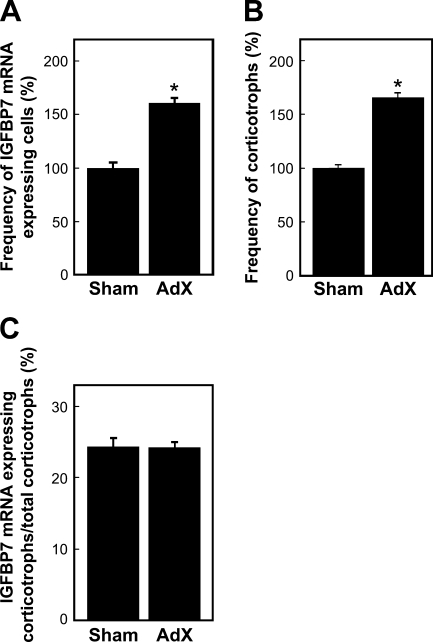
To identify IGFBP7 mRNA–expressing cells in the AP, we performed a double-staining method using in situ RT-PCR for detecting IGFBP7 mRNA and immunohistochemical methods using anti-amidated JP for identifying corticotrophs (Tanaka and Kurosumi 1992). In the normal (control) rat sections, signals indicating the expression of IGFBP7 mRNA were observed only in the AP (Figures 3A and 3C). However, no positive reaction was detected when the digoxigenin-11-dUTP was omitted from the reaction mixture, indicating the validity of the results of the in situ RT-PCR method (Figures 3B and 3D). Moreover, signals for IGFBP7 mRNA were predominantly detected in corticotrophs of the AP of both sham-operated and AdX rats (Figure 4). In addition, most of the IGFBP7 mRNA–negative corticotrophs were observed in the vicinity of IGFBP7 mRNA–positive corticotrophs, especially in the AP of AdX rats (Figures 4C and 4D). The numbers of IGFBP7 mRNA–expressing cells and corticotrophs were counted, and the ratio of IGFBP7 mRNA–expressing corticotrophs per total number of corticotrophs in the AP of sham-operated and AdX rats was determined. The numbers of IGFBP7 mRNA–expressing cells and corticotrophs were significantly increased by 1.6- to 1.66-fold (p<0.001) in the AP of AdX rats relative to those of the sham-operated rats (Figures 5A and 5B). However, there was no significant difference in the ratio of IGFBP7 mRNA–expressing corticotrophs per total corticotrophs between sham-operated (24.4 ± 1.16%) and AdX (24.2 ± 0.76%) rats (Figure 5C).
Figure 3.
Expression site of IGFBP7 mRNA in the AP of normal rats using the in situ RT-PCR method. (A,C) IGFBP7 mRNA–expressing cells are predominately observed in the AP. (B,D) No positive label for IGFBP7 mRNA is visible when the digoxigenin-11-dUTP label is omitted from the reaction. IP, intermediate pituitary; PP, posterior pituitary. Bars: A,B = 100 μm; C,D = 50 μm.
Figure 4.
Expression site of IGFBP7 mRNA in corticotrophs of the AP of sham-operated (A,B) and AdX (C,D) rats, using a double-staining method combined with in situ RT-PCR for IGFBP7 mRNA and IHC for amidated joining peptide. IGFBP7 mRNA is detected in the corticotrophs of the AP of sham-operated and AdX rats. Arrows indicate the same cells. Arrowheads indicate the IGFBP7 mRNA–negative corticotrophs observed in the vicinity of IGFBP7 mRNA–positive corticotrophs. Bar = 50 μm.
Figure 5.
Frequency of IGFBP7 mRNA–expressing cells (A) and corticotrophs (B), and the ratio of IGFBP7 mRNA–expressing corticotrophs per total number of corticotrophs (C) in the AP of sham-operated and AdX rats. The areas that were randomly selected from five sections obtained from each of five animals were examined. *p<0.001 vs sham-operated rats.
Localization of SOX2-positive Cells and PCNA-positive Cells in the Vicinity of IGFBP7 mRNA–expressing Corticotrophs
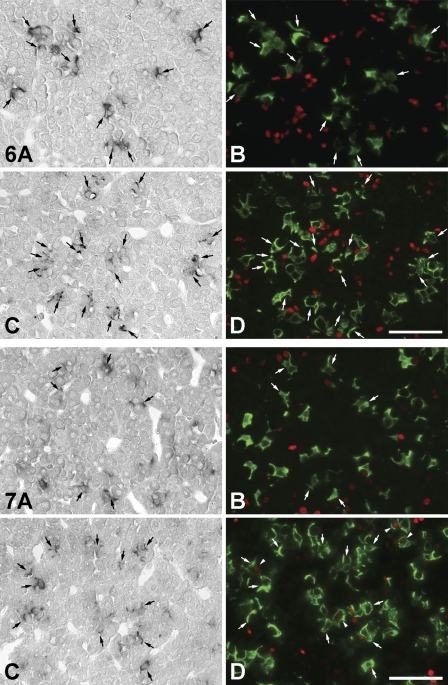
There have been recent reports of stem cells expressing SOX2 and nestin existing in the adult mouse pituitary (Fauquier et al. 2008; Gleiberman et al. 2008). On the other hand, IGFBP7 is involved in cell differentiation and cell proliferation in various tissues and cells (Jiang et al. 2008; Kutsukake et al. 2010). To elucidate the role of IGFBP7 in increasing the number of corticotrophs following adrenalectomy, we investigated the histological distribution of IGFBP7 mRNA–expressing corticotrophs, SOX2-positive cells, and PCNA-positive cells. SOX2 protein was not expressed in the IGFBP7 mRNA–expressing corticotrophs and other IGFBP7 mRNA–expressing cells in the APs of sham-operated and AdX rats (Figure 6), although several SOX2-positive cells were predominately located near IGFBP7 mRNA–expressing corticotrophs in both groups (Figure 6). PCNA signals were not observed in IGFBP7-positive and -negative corticotrophs of the AP of the sham-operated rats (Figures 7A and 7B), whereas PCNA signals were seen in a very few IGFBP7-negative corticotrophs, but not in IGFBP7-positive corticotrophs of the AP of AdX rats (Figures 7C and 7D). In addition, a small number of PCNA-positive cells were distributed in the close vicinity of IGFBP7 mRNA–expressing corticotrophs in the APs of both sham-operated and AdX rats (Figure 7).
Figure 6.
Localization of IGFBP7 mRNA–expressing cells (A,C), corticotrophs, and SOX2-positive cells (B,D) in the AP. IGFBP7 mRNA is predominantly expressed in corticotrophs (green), but SOX2 signals (red) are not present in the corticotrophs in the AP of sham-operated (A,B) and AdX (C,D) rats. A few SOX2-positive cells are closely situated near IGFBP7 mRNA–expressing corticotrophs in both groups. Arrows indicate the same cells. Bar = 50 μm.
Figure 7.
Localization of IGFBP7 mRNA–expressing cells (A,C), corticotrophs, and PCNA-positive cells (B,D) in the AP. In the AP of sham-operated rats (A,B), the PCNA signal (red) is not observed in IGFBP7 mRNA–expressing cells and corticotrophs (green; A,B). In the AP of AdX rats (C,D), PCNA is expressed in only a few corticotrophs (arrowheads), but there are no IGFBP7-positive corticotrophs (C,D). A few PCNA-positive cells are in close proximity to IGFBP7 mRNA–expressing corticotrophs in both the groups. Arrows indicate the same cells. Bar = 50 μm.
Discussion
We have identified a new factor, IGFBP7, in the AP of rats by using the SST-REX method and demonstrated that IGEBP7 mRNA levels increased in the AP of AdX rats. The SST-REX method is able to identify the proteins secreted to the outside of cells or inserted into the plasma membranes. Consequently, the proteins identified by this method may be involved in cell-to-cell communication, including cell division. It is also known that IGFBP7 protein sequence contains a “follistatin module,” and follistatin has been localized in a number of cell types (Lee et al. 1993; Kato 2000). However, because we used the specific primers for IGFBP7 in RT-PCR and further confirmed the sequence of amplified cDNA fragments by sequencing (data not shown), we consider that IGFBP7 mRNA was specifically detected in this study. The results of the semiquantitative RT-PCR analysis indicated that IGFBP7 mRNA levels increased in the rat AP following adrenalectomy but decreased in the AP of AdX rats treated with DEX. However, there was no significant difference between the SO and SD groups even though the SD group comprised the AP of sham-operated rats treated with DEX. Furthermore, the number of IGFBP7 mRNA–positive cells and corticotrophs significantly increased in a similar ratio in the AP following adrenalectomy. On the basis of these results, we suggest that IGFBP7 may increase and decrease in direct correlation with the changes in the number of corticotrophs. Our data also show that corticosterone secreted from the adrenal gland does not affect IGFBP7 mRNA level.
The number of corticotrophs has been reported to increase in the AP of AdX rats (Taniguchi et al. 1995) and to decrease by apoptosis in rats treated with DEX following an adrenalectomy (Nolan and Levy 2006a,b). It is a well-known fact that RKO and SW620 colorectal adenocarcinoma cell lines die as a result of apoptosis when IGFBP7 is overexpressed in these cells (Ruan et al. 2007). The results of our study, however, suggest that expression of IGFBP7 mRNA does not depend on the presence or absence of DEX. Consequently, IGFBP7 may not participate in the induction of apoptosis induced with DEX and may have roles/functions other than apoptosis in the AP.
It has been reported recently that SOX2- and nestin-positive stem cells are present in the AP of the adult mouse pituitary, raising the possibility that these could differentiate into all types of hormone-secreting cells (Fauquier et al. 2008; Gleiberman et al. 2008). These stem cells predominantly exist in the marginal cell layer in the AP, but several stem cells are scattered among the hormone-secreting cells (Fauquier et al. 2008; Gleiberman et al. 2008). One hypothesis that has been proposed to account for the increase in the number of corticotrophs following adrenalectomy is that the increase in the number of corticotrophs in the AP of AdX animals is derived not only from the division of preexisting corticotrophs but also from the differentiation of stem cells (Taniguchi et al. 1995; Nolan and Levy 2006a,b; Subburaju and Aguilera 2007). In our study, we examined immunolabeling for SOX2 and PCNA to determine the origin of the cells that could account for the observed increase in the number of corticotrophs in the AP of AdX rats. However, we found no SOX2-positive corticotrophs in the AP of AdX rats. However, we did find several PCNA-positive corticotrophs in the AP of AdX rats. On the basis of these results, we therefore suggest that the increase in the number of corticotrophs induced by AdX is derived from the division of preexisting corticotrophs. However, we also found that a small number of SOX2- and PCNA-positive cells were situated near IGFBP7 mRNA–expressing corticotrophs in the AP of sham-operated and AdX rats. This observation raises the possibility that IGFBP7 is secreted from corticotrophs in the AP of AdX rats and acts on SOX2-positive cells close to IGFBP7 mRNA–expressing corticotrophs, thereby differentiating SOX2-positive stem cells into corticotrophs. Thus, IGFBP7 may stimulate the proliferation of corticotrophs in a manner similar to its effect in the induction of cell proliferation of glioma cells and in the formation of a mesh-like structure of an endometrial glandular epithelial cell line (Jiang et al. 2008; Kutsukake et al. 2010).
IGFBP7 is also a factor identical to tumor-derived adhesion factor (TAF), which has been identified as a cell adhesion molecule secreted by human bladder carcinoma cell line EJ-1 (Akaogi et al. 1994). Similarly, IGFBP7 has also been identified by subtractive hybridization as being differentially expressed in a meningioma cell line and normal leptomeningeal cell, referred as to Mac25 (Murphy et al. 1993). Burger et al. (1998) showed that both IGFBP7 mRNA and protein disappear when non-tumorigenic breast cells are transformed to tumorigenic ones. IGFBP7 has been identified in numerous lines of cancer cells, suggesting that this factor may be involved in tumorigenesis. It has also been reported that IGFBP7 inhibits the proliferation of various tumor cells in in vitro and in vivo models (Ruan et al. 2007; Chen et al. 2010). In this regard, it is interesting that IGFBP7 mRNA is not expressed in AtT-20 cell line, which is derived from the pituitary corticotroph tumors. However, why IGFBP7 mRNA is not expressed remains unknown at present. On the other hand, bone morphogenetic protein-4 (BMP-4) is expressed in the corticotrophs of the normal human AP, and its expression has been found to decrease in corticotroph adenomas obtained from Cushing's patients relative to the normal pituitary (Giacomini et al. 2006). BMP-4 treatments have also been found to inhibit the proliferation of AtT-20 cells (Giacomini et al. 2006). Taken together, this evidence suggests that IGFBP7 and BMP-4 have a similar effect on the proliferation of corticotrophs and their derived tumor cells, such as AtT-20 cells.
In conclusion, the results of this study show that IGFBP7 mRNA is specifically expressed in corticotrophs of the rat AP and that the expression level increases with increasing number of corticotrophs following adrenalectomy.
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan (to ST) and by a grant-in-aid from the Japan Society for the Promotion of Science (to TN).
We are grateful to Prof. Toshio Kitamura, Institute of Medical Science, University of Tokyo, for his gift of vectors and cells and for his advice on the signal sequence trap method.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Akaogi K, Okabe Y, Funahashi K, Yoshitake Y, Nishikawa K, Yasumitsu H, Umeda M, et al. (1994) Cell adhesion activity of a 30-kDa major secreted protein from human bladder carcinoma cells. Biochem Biophys Res Commun 198:1046–1053 [DOI] [PubMed] [Google Scholar]
- Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK (2005) Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer 41:1515–1527 [DOI] [PubMed] [Google Scholar]
- Burger AM, Zhang X, Li H, Ostrowski JL, Beatty B, Venanzoni M, Papas T, et al. (1998) Down-regulation of T1A12/mac25, a novel insulin-like growth factor binding protein related gene, is associated with disease progression in breast carcinomas. Oncogene 16:2459–2467 [DOI] [PubMed] [Google Scholar]
- Chen RY, Chen HX, Lin JX, She WB, Jiang P, Xu L, Tu YT (2010) In-vivo transfection of pcDNA3.1-IGFBP7 inhibits melanoma growth in mice through apoptosis induction and VEGF downexpression. J Exp Clin Cancer Res 29:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV, Rougeau D, Unabia G (1995) Corticotropin-releasing hormone and epidermal growth factor: mitogens for anterior pituitary corticotropes. Endocrinology 136:1595–1602 [DOI] [PubMed] [Google Scholar]
- Degeorges A, Wang F, Frierson HF Jr, Seth A, Sikes RA (2000) Distribution of IGFBP-rP1 in normal human tissues. J Histochem Cytochem 48:747–754 [DOI] [PubMed] [Google Scholar]
- Fan X, Childs GV (1995) Epidermal growth factor and transforming growth factor-alpha messenger ribonucleic acids and their receptors in the rat anterior pituitary: localization and regulation. Endocrinology 136:2284–2293 [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth SM, Baxter RC (2002) Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23:824–854 [DOI] [PubMed] [Google Scholar]
- Giacomini D, Páez-Pereda M, Theodoropoulou M, Labeur M, Refojo D, Gerez J, Chervin A, et al. (2006) Bone morphogenetic protein-4 inhibits corticotroph tumor cells: involvement in the retinoic acid inhibitory action. Endocrinology 147:247–256 [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, et al. (2008) Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA 105:6332–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás M, Pusztai L, Rappay G, Makara GB (1991) Pituitary corticotrophs proliferate temporarily after adrenalectomy. Histochemistry 96:185–189 [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Jansen EJ, Schoonderwoert VT, Burbach JP, Martens GJ (1999) Biosynthesis of the vacuolar H+-ATPase accessory subunit Ac45 in Xenopus pituitary. Eur J Biochem 262:484–491 [DOI] [PubMed] [Google Scholar]
- Hwa V, Oh Y, Rosenfeld RG (1999) The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 20:761–787 [DOI] [PubMed] [Google Scholar]
- Jansen EJ, Scheenen WJ, Hafmans TG, Martens GJ (2008) Accessory subunit Ac45 controls the V-ATPase in the regulated secretory pathway. Biochim Biophys Acta 1783:2301–2310 [DOI] [PubMed] [Google Scholar]
- Jiang W, Xiang C, Cazacu S, Brodie C, Mikkelsen T (2008) Insulin-like growth factor binding protein 7 mediates glioma cell growth and migration. Neoplasia 10:1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato MV (2000) A secreted tumor-suppressor, mac25, with activin-binding activity. Mol Med 6:126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Onishi M, Kinoshita S, Shibuya A, Miyajima A, Nolan GP (1995) Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci USA 92:9146–9150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Kitamura T (1999) A signal sequence trap based on a constitutively active cytokine receptor. Nat Biotechnol 17:487–490 [DOI] [PubMed] [Google Scholar]
- Kutsukake M, Tamura K, Yoshie M, Tachikawa E (2010) Knockdown of IGF-binding protein 7 inhibits transformation of the endometrial gland in an in vitro model. Mol Reprod Dev 77:265–272 [DOI] [PubMed] [Google Scholar]
- Lee BL, Unabia G, Childs G (1993) Expression of follistatin mRNA by somatotropes and mammotropes early in the rat estrous cycle. J Histochem Cytochem 41:955–960 [DOI] [PubMed] [Google Scholar]
- Murphy M, Pykett MJ, Harnish P, Zang KD, George DL (1993) Identification and characterization of genes differentially expressed in meningiomas. Cell Growth Differ 4:715–722 [PubMed] [Google Scholar]
- Nakakura T, Sato M, Suzuki M, Hatano O, Takemori H, Taniguchi Y, Minoshima Y, et al. (2009) The spatial and temporal expression of delta-like protein 1 in the rat pituitary gland during development. Histochem Cell Biol 131:141–153 [DOI] [PubMed] [Google Scholar]
- Nakakura T, Suzuki M, Watanabe Y, Tanaka S (2007) Possible involvement of brain-derived neurotrophic factor (BDNF) in the innervation of dopaminergic neurons from the rat periventricular nucleus to the pars intermedia. Zoolog Sci 24:1086–1093 [DOI] [PubMed] [Google Scholar]
- Nakakura T, Yoshida M, Dohra H, Suzuki M, Tanaka S (2006) Gene expression of vascular endothelial growth factor-A in the pituitary during formation of the vascular system in the hypothalamic-pituitary axis of the rat. Cell Tissue Res 324:87–95 [DOI] [PubMed] [Google Scholar]
- Nolan LA, Levy A (2006a) The effects of testosterone and oestrogen on gonadectomised and intact male rat anterior pituitary mitotic and apoptotic activity. J Endocrinol 188:387–396 [DOI] [PubMed] [Google Scholar]
- Nolan LA, Levy A (2006b) A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J Neuroendocrinol 18:655–661 [DOI] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4:505–518 [DOI] [PubMed] [Google Scholar]
- Ruan W, Xu E, Xu F, Ma Y, Deng H, Huang Q, Lv B, et al. (2007) IGFBP7 plays a potential tumor suppressor role in colorectal carcinogenesis. Cancer Biol Ther 6:354–359 [DOI] [PubMed] [Google Scholar]
- Schmid C (1995) Insulin-like growth factors. Cell Biol Int 19:445–457 [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L (1998) Structure and function of the insulin-like growth factor I receptor. Breast Cancer Res Treat 47:235–253 [DOI] [PubMed] [Google Scholar]
- Subburaju S, Aguilera G (2007) Vasopressin mediates mitogenic responses to adrenalectomy in the rat anterior pituitary. Endocrinology 148:3102–3110 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kurosumi K (1992) A certain step of proteolytic processing of proopiomelanocortin occurs during the transition between two distinct stages of secretory granule maturation in rat anterior pituitary corticotrophs. Endocrinology 131:779–786 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Yora T, Nakayama K, Inoue K, Kurosumi K (1997) Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J Histochem Cytochem 45:425–436 [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Tamatani R, Yasutaka S, Kawarai Y (1995) Proliferation of pituitary corticotrophs following adrenalectomy as revealed by immunohistochemistry combined with bromodeoxyuridine-labeling. Histochem Cell Biol 103:127–130 [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Wilson EM, Rosenfeld RG, Oh Y (1997) Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J Biol Chem 272:30729–30734 [DOI] [PubMed] [Google Scholar]